Abstract
Background
There are limited data on the efficacy of adjuvant therapy in ampullary cancer. The aim of this study was to determine whether adjuvant therapy was associated with improved survival for patients with ampullary cancer.
Methods
From the National Cancer Database, we identified ampullary cancer patients who underwent resection between 2004–2013. We performed 1:1 propensity score matching, comparing patients who had postoperative observation to patients who received adjuvant chemotherapy (ACT) or adjuvant chemoradiotherapy (ACRT).
Results
We identified 4190 patients who fit our inclusion criteria; 63% had postoperative observation, 21% received ACT, and 16% underwent ACRT. In the matched cohorts, the use of ACT was associated with improved overall survival (HR = 0.82, 95% CI = 0.71 to 0.95). The median overall survival was 47.2 months for the ACT group and 35.5 months for the observation group. In a separate matched analysis, ACRT was also associated with improved survival (HR = 0.84, 95% CI = 0.72 to 0.98) as compared to observation. The median overall survival was 38.1 months for the ACRT group and 31.0 months for the observation group. The benefit was more pronounced in high risk patients, such as ones with higher T and N categories.
Conclusions
In this retrospective study, the use of adjuvant therapy in ampullary cancer was associated with significantly improved overall survival. The benefit of adjuvant therapy for this disease should be confirmed in a more rigorous fashion via randomized controlled trials.
Keywords: Adjuvant therapy, ampullary cancer, ampullary carcinoma, chemotherapy, chemoradiation
Introduction
Five-year survival for resected ampullary cancer ranges from 38% to 68%.1 While randomized controlled trials have demonstrated the efficacy of adjuvant therapy for pancreatic adenocarcinoma, which is the most common type of periampullary tumor, no such high-quality data exists for ampullary cancer. The results of clinical trials are inconclusive due to flawed methodology, and the only data supporting adjuvant therapy originates from single-institution studies with inherent limitations due to small sample sizes and the nature of a retrospective design. As a result, neither the National Comprehensive Cancer Network nor the European Society for Medical Oncology provide recommendations for the postoperative management of ampullary cancer.2–7
To evaluate the effects of adjuvant therapy for ampullary cancer, we used the National Cancer Database to perform a propensity-matched study comparing the overall survival of patients who had postoperative observation to patients who received adjuvant chemotherapy (ACT) or chemoradiotherapy (ACRT).
Materials and Methods
Database and Patient Population
This was a retrospective study using the National Cancer Database (NCDB). The NCDB is a national cancer registry that receives information from over 1500 Commission on Cancer–accredited cancer programs in the United States, and captures approximately 70% of cancer cases in the United States.8
We identified patients with ampullary malignancies (International Classification of Diseases for Oncology, third edition [ICD-O-3], topographical code C24.1) diagnosed between 2004 and 2013 who had surgical resection within 90 days of diagnosis. We included patients who were diagnosed with carcinoma and excluded all other histology types. We excluded patients who had metastatic disease, underwent palliative surgery, received neoadjuvant therapy, had macroscopic margin status, or had missing information (Supplementary Figure 1).
The following variables were abstracted: gender, age, ethnicity, insurance status, median household income of each patient's area of residence, Charlson/Deyo score, tumor grade, year of diagnosis, facility type, margin status, length of stay, 30-day readmission, 90-day mortality, pathological T (pT) and N (pN) categories based on the seventh edition of the American Joint Committee on Cancer TNM staging manual, and receipt of adjuvant therapy, which we divided into ACT and ACRT. We selected only patients who initiated adjuvant therapy within 90 days following their surgery to exclude patients who had most likely received therapy for recurrence. We also abstracted follow-up and vital status data.
Statistical Analysis
In two separate analyses, we used propensity scores to match patients having postoperative observation to patients who received ACT or ACRT. We estimated the propensity score using a multivariable logistic regression model that included the following variables: gender, age, insurance status, median income of residence, Charlson/Deyo score, pT category, pN category, tumor grade, year of diagnosis, facility type, resection margin status, length of stay, and 30-day readmission. Patients in the two groups were then matched without replacement through a greedy 8-1 digit-matching algorithm.9 We excluded patients who died within 90 days postoperatively to minimize the immortal time bias as patients who died in the immediate postoperative period would not receive adjuvant therapy.10 The choice of the landmark time at 90 days corresponds to the postoperative time during which most of the surgery-related mortality occurs.11, 12 Standardized differences between groups were assessed to establish whether adequate balance was achieved using a cutoff value ≥ 0.1 for imbalance.
Overall survival was estimated using Kaplan-Meier curves and compared with log-rank tests on the matched patient pairs. The hazard ratios were calculated using a Cox proportional hazards model. We evaluated the proportional hazards assumption by examining the Martingale residuals.
In this study, two-sided P values of ≤.05 were considered statistically significant. Analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC) and SPSS version 24.
Results
Baseline Characteristics of the Cohorts
We identified 4190 patients who met the inclusion criteria of the study; 63% (2651 patients) were observed after resection, 21% (870 patients) received ACT, and 16% (669 patients) underwent ACRT. Over the study time period, there was increased use of adjuvant therapy (Supplementary Figure 2). Notably, there was a shift away from ACRT in favor of ACT, which increased from 9% in 2004–2005 to 32% in 2012–2013, while ACRT utilization decreased from 20% in 2004–2005 to 12% in 2012–2013.
The baseline characteristics of the unmatched cohorts are presented in Table 1. Patients who received ACT or ACRT were more likely to have higher pT and pN categories, poorly/undifferentiated tumors, positive resection margins, and private insurance. They were younger, less likely to have comorbidities, had a lower mean length of stay after operation, and had a lower rate of 30-day readmission.
Table 1.
Patient-, tumor-, and treatment-related factors of the entire cohort.
| Surgery + observation n (%) |
Surgery + ACT n (%) |
Surgery + ACRT n (%) |
P | |
|---|---|---|---|---|
| Number of patients | 2651 | 870 | 669 | |
| Gender | .16 | |||
| Male | 1480 (55.8) | 504 (57.9) | 355 (53.1) | |
| Female | 1171 (44.2) | 366 (42.1) | 314 (46.9) | |
| Age (mean +/− SD years) | 68.6 (10.6) | 63.9 (10.3) | 62.6 (9.9) | <.01 |
| Ethnicity | .85 | |||
| Non-Hispanic | 2430 (91.6) | 794 (91.3) | 616 (92.1) | |
| Hispanic | 221 (8.3) | 76 (8.7) | 53 (7.9) | |
| Insurance status | <.01 | |||
| Not insured | 101 (3.8) | 41 (4.8) | 24 (3.6) | |
| Private | 857 (32.3) | 403 (46.3) | 325 (48.6) | |
| Medicaid | 123 (4.6) | 42 (4.8) | 53 (7.9) | |
| Medicare | 1538 (58.0) | 380 (43.7) | 257 (38.4) | |
| Other government | 32 (0.8) | 4 (0.5) | 10 (1.5) | |
| Median income of residence | .02 | |||
| Below median | 1084 (40.9) | 309 (35.5) | 273 (40.8) | |
| Above median | 1567 (59.1) | 561 (64.5) | 396 (59.2) | |
| Charlson/Deyo score | .04 | |||
| 0 | 1849 (69.7) | 645 (74.1) | 480 (71.8) | |
| ≥1 | 802 (30.3) | 225 (25.9) | 189 (28.3) | |
| pT | <.01 | |||
| 1 | 570 (21.5) | 64 (7.3) | 43 (6.4) | |
| 2 | 950 (35.8) | 239 (27.5) | 156 (23.3) | |
| 3 | 665 (25.1) | 314 (36.1) | 250 (37.4) | |
| 4 | 466 (17.6) | 253 (29.1) | 220 (32.9) | |
| pN | <.01 | |||
| N0 | 1755 (66.2) | 268 (30.8) | 160 (23.9) | |
| N+ | 896 (33.8) | 602 (69.2) | 509 (76.1) | |
| Grade | <.01 | |||
| Well/moderately differentiated | 1910 (72.1) | 523 (60.1) | 388 (58.0) | |
| Poorly/undifferentiated | 741 (27.9) | 347 (39.9) | 281 (42.0) | |
| Year | <.01 | |||
| 2004–2005 | 491 (18.5) | 62 (7.1) | 136 (20.3) | |
| 2006–2007 | 506 (19.1) | 104 (12.0) | 133 (19.9) | |
| 2008–2009 | 470(17.7) | 162 (18.6) | 129 (19.3) | |
| 2010–2011 | 573 (21.6) | 193 (22.2) | 146 (21.8) | |
| 2012–2013 | 611 (23.1) | 349 (40.1) | 125 (18.7) | |
| Facility type | <.01 | |||
| Academic | 1566 (59.1) | 540 (62.1) | 347 (51.9) | |
| Non-academic | 1085 (40.9) | 330 (37.9) | 322 (48.1) | |
| Margin status | <.01 | |||
| Negative | 2584 (97.5) | 829 (95.3) | 614 (91.8) | |
| Positive | 67 (2.5) | 41 (4.7) | 55 (8.2) | |
| Length of stay (mean +/− SD days) | 14.8 (12.6) | 10.8 (7.4) | 11.0 (9.2) | <.01 |
| 30-day readmission | <.01 | |||
| No | 2350 (88.7) | 816 (93.8) | 628 (93.9) | |
| Yes | 301 (11.4) | 54 (6.2) | 41 (6.1) |
ACRT = adjuvant chemoradiotherapy; ACT = adjuvant chemotherapy; SD = standard deviation.
Survival Comparison of Observation Versus Adjuvant Chemotherapy
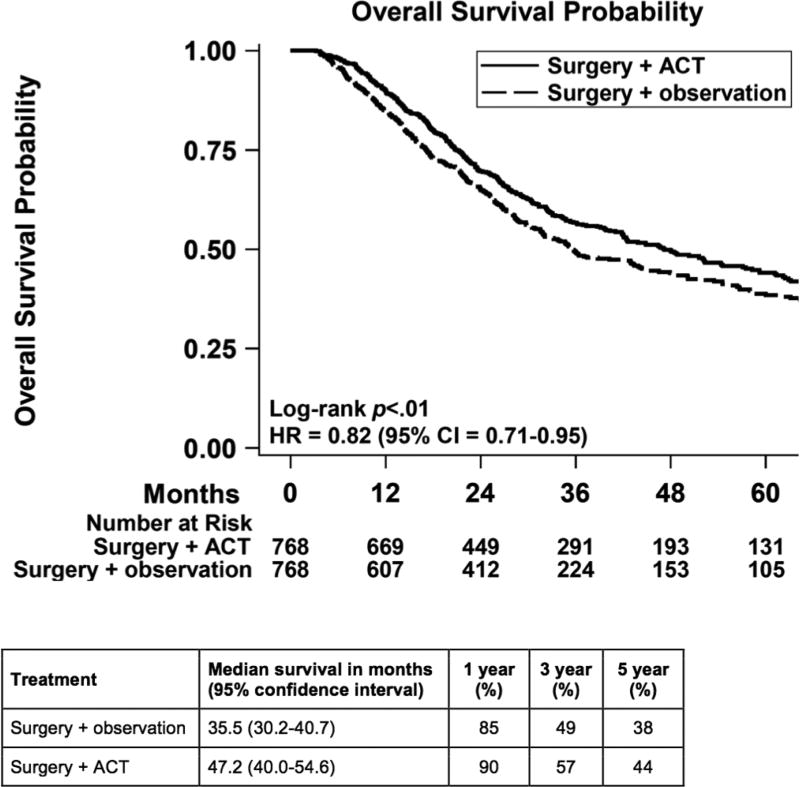
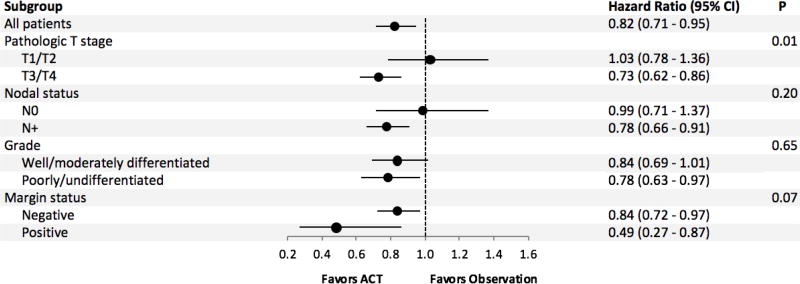
After 1:1 matching, we compared 768 patients who had observation to 768 patients who received ACT. The groups were well-balanced (Table 2). The median follow-up was 25.1 months for the observation group and 28.3 months for the ACT cohort. The receipt of ACT was associated with improved overall survival (HR = 0.82, 95% CI = 0.71 to 0.95; Figure 1). The median overall survival was 47.2 months for the ACT group and 35.5 months for the observation group. The 1-, 3-, and 5-year survival were 90%, 57%, and 44% for the ACT group and 85%, 49%, and 38% for the observation group. Subgroup analysis showed that the test of interaction was significant for T stage disease with T3/T4 disease benefiting more from the treatment compared to T1/T2 disease (Figure 2).
Table 2.
Patient-, tumor-, and treatment-related factors of patients who underwent surgery and observation matched to patients who underwent surgery and adjuvant chemotherapy.
| Surgery + observation n (%) |
Surgery + ACT n (%) |
P | S diff | |
|---|---|---|---|---|
| Number of patients | 768 | 768 | ||
| Gender | .96 | <0.01 | ||
| Male | 440 (57.3) | 439 (57.2) | ||
| Female | 328 (42.7) | 329 (42.8) | ||
| Age (mean +/− SD years) | 65.0 (11.2) | 64.9 (10.2) | .78 | 0.01 |
| Ethnicity | .13 | 0.07 | ||
| Non-Hispanic | 712 (92.7) | 696 (90.6) | ||
| Hispanic | 56 (7.3) | 72 (9.4) | ||
| Insurance status | .44 | |||
| Not insured | 36 (4.7) | 38 (5.0) | 0.01 | |
| Private | 318 (41.4) | 328 (42.7) | 0.02 | |
| Medicaid | 41 (5.3) | 28 (5.0) | 0.02 | |
| Medicare | 370 (48.2) | 360 (46.9) | 0.03 | |
| Other government | 3 (0.4) | 4 (0.5) | 0.02 | |
| Median income of residence | .87 | <0.01 | ||
| Below median | 282 (36.7) | 285 (37.1) | ||
| Above median | 486 (63.3) | 483 (62.9) | ||
| Charlson/Deyo score | .69 | 0.02 | ||
| 0 | 551 (71.7) | 558 (72.7) | ||
| ≥1 | 217 (28.3) | 210 (27.3) | ||
| pT | .53 | |||
| 1 | 54 (7.0) | 63 (8.2) | 0.04 | |
| 2 | 234 (30.5) | 224 (29.2) | 0.03 | |
| 3 | 275 (35.8) | 272 (35.4) | <0.01 | |
| 4 | 205 (26.7) | 209 (27.2) | 0.01 | |
| pN+ | .79 | 0.01 | ||
| N0 | 262 (34.1) | 266 (34.6) | ||
| N+ | 506 (65.9) | 502 (65.4) | ||
| Grade | .96 | <0.01 | ||
| Well/moderately differentiated | 478 (62.2) | 477 (62.1) | ||
| Poorly/undifferentiated | 290 (37.8) | 291 (37.9) | ||
| Year | .92 | |||
| 2004–2005 | 64 (8.3) | 62 (8.1) | <0.01 | |
| 2006–2007 | 105 (13.7) | 98 (12.8) | 0.03 | |
| 2008–2009 | 148 (19.3) | 146 (19.0) | <0.01 | |
| 2010–2011 | 169 (22.0) | 171 (22.3) | <0.01 | |
| 2012–2013 | 282 (36.7) | 291 (37.9) | 0.02 | |
| Facility type | .72 | 0.09 | ||
| Academic | 472 (61.5) | 465 (60.6) | ||
| Non-academic | 296 (38.5) | 303 (39.4) | ||
| Margin status | .90 | <0.01 | ||
| Negative | 737 (96.0) | 736 (95.8) | ||
| Positive | 31 (4.0) | 32 (4.2) | ||
| Length of stay (mean +/− SD days) | 11.3 (7.7) | 11.3 (7.6) | .89 | <0.01 |
| 30-day readmission | .84 | 0.01 | ||
| No | 713 (92.8) | 715 (93.1) | ||
| Yes | 55 (7.2) | 53 (6.9) |
ACT = adjuvant chemotherapy; S diff = standardized differences; SD = standard deviation.
Figure 1.
Overall survival of matched cohorts comparing patients who had surgery and observation versus surgery and adjuvant chemotherapy. ACT = adjuvant chemotherapy; CI = confidence interval; HR = hazard ratio.
Figure 2.
Subgroup analysis of patients treated with adjuvant chemotherapy. ACT = adjuvant chemotherapy; CI = confidence interval.
Survival Comparison of Observation Versus Adjuvant Chemoradiotherapy
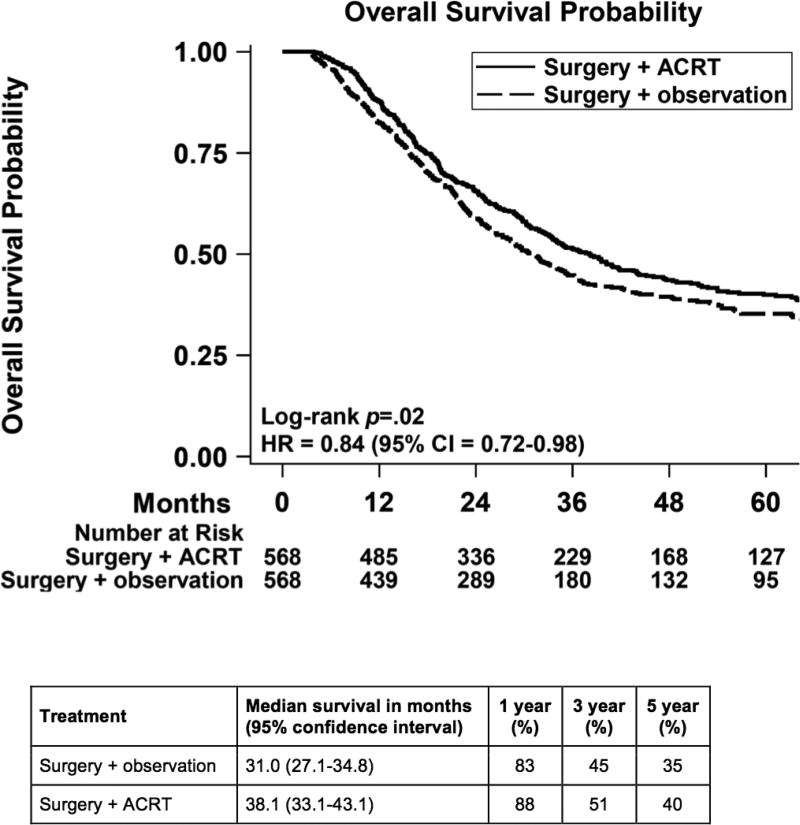
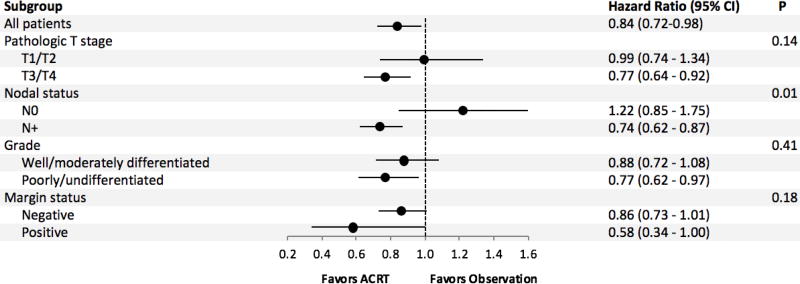
After 1:1 matching, we compared 568 observation patients to 568 ACRT patients. The groups were well-balanced (Table 3). The median follow-up was 24.4 months for the observation group and 29.4 months for ACRT. The receipt of ACRT was associated with improved overall survival (HR = 0.84, 95% CI = 0.72 to 0.98; Figure 3). The median overall survival was 38.1 months for the ACRT group and 31.0 months for the observation group. The 1-, 3-, and 5-year survival were 88%, 51%, and 40% for the ACRT group and 83%, 45%, and 35% for the observation group. Subgroup analysis showed that the test of interaction was significant for nodal stage disease, with positive nodal disease benefiting more from the treatment compared to negative nodal disease (Figure 4).
Table 3.
Patient-, tumor-, and treatment-related factors of patients who underwent surgery and observation matched to patients who underwent surgery and adjuvant chemoradiotherapy.
| Surgery + observation n (%) |
Surgery + ACRT n (%) |
P | S diff | |
|---|---|---|---|---|
| Number of patients | 568 | 568 | ||
| Gender | .76 | 0.02 | ||
| Male | 301 (53.0) | 306 (53.9) | ||
| Female | 267 (47.0) | 262 (46.1) | ||
| Age (mean +/− SD years) | 63.9 (10.8) | 63.9 (9.8) | .86 | <0.01 |
| Ethnicity | .91 | <0.01 | ||
| Non-Hispanic | 525 (92.4) | 524 (92.3) | ||
| Hispanic | 43 (7.6) | 44 (7.7) | ||
| Insurance status | .83 | |||
| Not insured | 19 (3.4) | 21 (3.7) | 0.02 | |
| Private | 245 (43.1) | 252 (44.4) | 0.02 | |
| Medicaid | 36 (6.3) | 40 (7.0) | 0.03 | |
| Medicare | 259 (45.6) | 247 (43.5) | 0.04 | |
| Other government | 9 (1.6) | 8 (1.4) | 0.01 | |
| Median income of residence | .64 | 0.03 | ||
| Below median | 249 (43.8) | 241 (42.4) | ||
| Above median | 319 (56.2) | 327 (57.6) | ||
| Charlson/Deyo score | .41 | 0.05 | ||
| 0 | 391 (68.8) | 404 (71.1) | ||
| ≥1 | 177 (31.2) | 164 (28.9) | ||
| pT | .78 | |||
| 1 | 38 (6.7) | 42 (7.4) | 0.03 | |
| 2 | 146 (25.7) | 151 (26.6) | 0.02 | |
| 3 | 220 (38.7) | 200 (35.2) | 0.07 | |
| 4 | 164 (28.9) | 175 (30.8) | 0.04 | |
| pN | .33 | 0.04 | ||
| N0 | 149 (26.2) | 160 (28.2) | ||
| N+ | 419 (73.8) | 408 (71.8) | ||
| Grade | ||||
| Well/moderately differentiated | 336 (59.2) | 341 (60.0) | ||
| Poorly/undifferentiated | 232 (40.9) | 227 (40.0) | ||
| Year | .62 | |||
| 2004–2005 | 111 (19.5) | 113 (19.9) | <0.01 | |
| 2006–2007 | 118 (20.8) | 110 (19.4) | 0.04 | |
| 2008–2009 | 91 (16.0) | 108 (19.0) | 0.08 | |
| 2010–2011 | 121 (21.3) | 124 (21.8) | 0.01 | |
| 2012–2013 | 127 (22.4) | 113 (19.9) | 0.06 | |
| Facility type | >.99 | <0.01 | ||
| Academic | 303 (53.4) | 303 (53.4) | ||
| Non-academic | 265 (46.6) | 265 (46.6) | ||
| Margin status | .79 | 0.01 | ||
| Negative | 533 (93.8) | 535 (94.2) | ||
| Positive | 35 (6.2) | 33 (5.8) | ||
| Length of stay (mean +/− SD days) | 12.1 (8.7) | 11.4 (9.7) | .08 | 0.09 |
| 30-day readmission | .91 | <0.01 | ||
| No | 530 (93.3) | 529 (93.1) | ||
| Yes | 38 (6.7) | 39 (6.9) |
ACRT = adjuvant chemoradiotherapy; S diff = standardized differences; SD = standard deviation.
Figure 3.
Overall survival of matched cohorts comparing patients who had surgery and observation versus surgery with adjuvant chemoradiotherapy. ACRT: adjuvant chemoradiotherapy; CI = confidence interval; HR = hazard ratio.
Figure 4.
Subgroup analysis of patients treated with adjuvant chemoradiotherapy. ACRT = adjuvant chemoradiotherapy; CI = confidence interval.
Discussion
In this retrospective study of the NCDB, we found that adjuvant therapy is associated with improved overall survival for patients with ampullary cancers, and may be more effective for patients with tumors of higher T and N categories. Evidence supporting the use of adjuvant therapy for ampullary cancer has been equivocal. In previous large randomized clinical trials, ampullary cancers have been grouped with other periampullary tumors, making it difficult to ascertain the true benefit of adjuvant therapy for ampullary cancer patients. The ESPAC-3 trial randomized 434 patients with periampullary cancer to adjuvant chemotherapy or observation and showed no difference in overall survival between the groups.13 A subgroup analysis of the 304 ampullary adenocarcinoma patients published in abstract form showed that the median survival of patients who received adjuvant therapy was 57 months as compared to 34 months for patients underwent observation. However, the difference did not reach statistical significance.14 When only the 276 patients who received R0 resections were evaluated, the median survival was 58 months for patients who received adjuvant therapy and 45 months for patients who underwent observation, with a Cox proportional hazards of P = 0.057. However, since this was a subgroup analysis, the data has to be interpreted with appropriate caution. The EORTC 40891 trial that evaluated adjuvant chemoradiotherapy in 93 resected periampullary cancers also demonstrated no survival benefit.15 However, detailed pathologic review that differentiated true ampullary cancers from other subtypes was not performed. Finally, a phase III randomized trial of a heterogeneous population of patients with pancreaticobiliary tumors compared adjuvant 5-fluorouracil and mitomycin-C to surgery alone and showed no survival improvement in the 48 patients with ampullary cancers.16
In contrast, several retrospective reports suggest that adjuvant therapy for ampullary cancer is associated with improved survival.2–7 Most of these studies are single-institution reports limited by small sample size, uncontrolled analysis, and selection bias, making the interpretation of the results challenging. A meta-analysis of ten retrospective studies that included 3361 patients found adjuvant chemoradiation was associated with a lower risk of death (HR = 0.75; P = .001) compared to surgery alone.17 This report was limited by the fact that all of the studies included were retrospective, some of which presented only unadjusted outcomes, and there was significant heterogeneity between the included studies.
The strength of our report is based on the large sample size of the NCDB that allowed us to mitigate biases that are inherent to all retrospective reviews. First, we were able to perform adjusted survival analyses controlling for various patient and tumor factors known to be associated with survival. Second, we generated large, well-balanced cohorts via propensity matching to diminish selection bias. Next, we decreased the effect of immortal time bias, which weakened many previous studies evaluating the use of adjuvant therapy, by excluding patients who died within the first 90 days. In addition, we matched patients based on length of stay and 30-day readmission as a surrogate for postoperative complications that may preclude the receipt of adjuvant therapy, as patients who have major postoperative complications requiring prolonged length of stay or readmissions in the early postoperative period are less likely to get adjuvant therapy.18 Finally, we were able to analyze chemotherapy and chemoradiotherapy as separate variables because the NCDB does not indicate if chemotherapy used with radiation was a radiosensitizer or a full-course regimen. Finally, the NCDB gathers information from across the nation and thus provides information that is widely applicable.
Due to the lack of granular data in the NCDB, several important questions remain unaddressed by our study. First, we could not determine if adjuvant therapy was effective for both pancreaticobiliary and intestinal subtypes of ampullary cancer since detailed pathology information was not available. Previous studies have shown that pancreaticobiliary subtype tumors had more aggressive biology and worse outcomes compared to intestinal tumors.19, 20 In an attempt to address this bias, we included tumor grade as a covariate in the analysis since pancreaticobiliary is more likely to be poorly differentiated.21
Second, we could not discern the chemotherapy regimens that were associated with improved survival. Going forward, ampullary cancer will likely be treated with a fluoropyrimidine-based regimen as extrapolated using data from other periampullary cancers. The ESPAC-4 study established 5-FU and gemcitabine as the standard of care for adjuvant therapy for pancreas cancer while the BILCAP trial, which was recently presented in abstract form, demonstrated that adjuvant capecitabine improved survival for biliary tract cancer patients.22, 23 These results likely will be generalized to patients with pancreaticobiliary subtype while patients with intestinal subtype likely will be treated with FOLFOX, as based on the colon cancer treatment paradigm.
The role of radiation as an adjuvant modality for ampullary cancer will need to be clarified. The largest clinical trials evaluating adjuvant radiotherapy for periampullary and pancreatic malignancies, such as the EORTC trial and ESPAC-1, respectively, have shown no survival benefit for radiation.15, 24 In addition, the recent LAP07 study also showed that radiation provided no survival advantage in locally advanced pancreas cancer.25 While we found that adjuvant chemoradiotherapy was associated with improved survival compared to observation alone, the lack of detailed chemotherapy information made it impossible to determine if the systemic component was a radiosensitizer or full-dose chemotherapy. Thus, the associated survival advantage may be due to the chemotherapy component.
Finally, we could not perform an intent-to-treat analysis since the NCDB does not determine which patients were selected a priori for adjuvant therapy. However, the inclusion of length of stay and readmission rate as covariate in our matching analysis, and the exclusion of patients who died within 90 days of resection, may mitigate this bias.
In conclusion, we found that the receipt of adjuvant therapy is associated with improved survival in patients with resected ampullary cancer in this propensity-matched, retrospective, hospital-based study. The benefits of therapy appeared to be especially valuable in patients with high risk disease such as ones with T3/T4 tumors and positive nodal involvement. Although our study is subject to the known limitations of a retrospective study, it provides treating physicians another source of data to use while discussing adjuvant therapy with their patients. Finally, our findings provide equipoise to study the role of adjuvant therapy in ampullary cancers in a randomized fashion.
Supplementary Material
Supplementary Figure 1. CONSORT diagram. ACRT = adjuvant chemoradiotherapy; ACT = adjuvant chemotherapy.
Supplementary Figure 2. Changes in the utilization of adjuvant therapy from 2004 to 2013. ACRT = adjuvant chemoradiotherapy; ACT = adjuvant chemotherapy.
Acknowledgments
Source of funding: The National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
The authors would like to thank Dave Primm for his help in editing this manuscript and Helen Mayo from the UT Southwestern Health Sciences Digital Library and Learning Center for assistance with literature searches.
Footnotes
Conflict of interest disclosures: All authors have no disclaimers.
Author contributions: Ibrahim Nassour, Linda Hynan, Alana Christie, and Sam Wang: Study design, data analysis and interpretation, writing initial draft, revising and approving final draft. Rebecca Minter, Adam Yopp, Michael Choti, John Mansour, Matthew Porembka: data interpretation, revising and approving final draft.
Rebecca M. Minter is the Alvin Baldwin, Jr. Chair in Surgery. Matthew R. Porembka is the Dedman Family Scholar in Clinical Care. Sam C. Wang is a UT Southwestern Disease-Oriented Clinical Scholar and American College of Surgeons Faculty Research Fellow.
References
- 1.O’Connell JB, Maggard MA, Manunga J, et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820–1827. doi: 10.1245/s10434-008-9886-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514–519. doi: 10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Palta M, Patel P, Broadwater G, et al. Carcinoma of the ampulla of Vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol. 2012;19:1535–1540. doi: 10.1245/s10434-011-2117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narang AK, Miller RC, Hsu CC, et al. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol. 2011;6:126. doi: 10.1186/1748-717X-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiergens TS, Reu S, Neumann J, et al. Histomorphologic and molecular phenotypes predict gemcitabine response and overall survival in adenocarcinoma of the ampulla of Vater. Surgery. 2015;158:151–161. doi: 10.1016/j.surg.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Whittington R, Williams NN, et al. Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 2000;47:945–953. doi: 10.1016/s0360-3016(00)00537-x. [DOI] [PubMed] [Google Scholar]
- 7.Ha HR, Oh DY, Kim TY, et al. Survival outcomes according to adjuvant treatment and prognostic factors including host immune markers in patients with curatively resected ampulla of Vater cancer. PLoS One. 2016;11:e0151406. doi: 10.1371/journal.pone.0151406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Data Base. [Accessed Jul 2016]; https://www.facs.org/quality%20programs/cancer/ncdb.
- 9.Parson LS. Performing a 1: N case-control match on propensity score: Proceedings of the 29th Annual SAS Users Group International Conference; 2004. [Google Scholar]
- 10.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149–1154. doi: 10.1002/bjs.8813. [DOI] [PubMed] [Google Scholar]
- 12.Talsma AK, Lingsma HF, Steyerberg EW, Wijnhoven BP, Van Lanschot JJ. The 30-day versus in-hospital and 90-day mortality after esophagectomy as indicators for quality of care. Ann Surg. 2014;260:267–273. doi: 10.1097/SLA.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 13.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Moore MJ, Cox TF, et al. Ampullary cancer ESPAC-3 (v2) trial: a multicenter, international, open-label, randomized controlled phase III trial of adjuvant chemotherapy versus observation in patients with adenocarcinoma of the ampulla of Vater. J Clin Oncol. 2016;29:LBA4006. [Google Scholar]
- 15.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999 Dec;230(6):776–82. doi: 10.1097/00000658-199912000-00006. discussion 782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 17.Kwon J, Kim BH, Kim K, Chie EK, Ha SW. Survival benefit of adjuvant chemoradiotherapy in patients with ampulla of Vater cancer. Ann Surg. 2015;262:47–52. doi: 10.1097/SLA.0000000000001182. [DOI] [PubMed] [Google Scholar]
- 18.Xia B, Vikrom D, Levinsky N. Early recurrence and omission of adjuvant therapy after pancreaticoduodenectomy: an argument against a surgery-first approach. Ann Surg Oncol. 2016;23(13):4156–4164. doi: 10.1245/s10434-016-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Li D, Wu L, Si X. The histopathologic type predicts survival of patients with ampullary carcinoma after resection: a meta-analysis. Pancreatology. 2017 Mar-Apr;17(2):273–278. doi: 10.1016/j.pan.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of Vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008;207:210–218. doi: 10.1016/j.jamcollsurg.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Williams JL, Chan CK, Toste PA, et al. Association of histopathologic phenotype of periampullary adenocarcinomas with survival. JAMA Surg. 2017;152:82–88. doi: 10.1001/jamasurg.2016.3466. [DOI] [PubMed] [Google Scholar]
- 22.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 23.Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. J Clin Oncol. 2017 [Google Scholar]
- 24.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 25.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. CONSORT diagram. ACRT = adjuvant chemoradiotherapy; ACT = adjuvant chemotherapy.
Supplementary Figure 2. Changes in the utilization of adjuvant therapy from 2004 to 2013. ACRT = adjuvant chemoradiotherapy; ACT = adjuvant chemotherapy.