Abstract
Influential models of schizophrenia suggest that patients experience incoming stimuli as excessively novel and motivating, with important consequences for hallucinatory experience and delusional belief. However, whether schizophrenia patients exhibit excessive novelty value and whether this interferes with adaptive behaviour has not yet been formally tested. Here, we employed a three-armed bandit task to investigate this hypothesis. Schizophrenia patients and healthy controls were first familiarised with a group of images and then asked to repeatedly choose between familiar and unfamiliar images associated with different monetary reward probabilities. By fitting a reinforcement-learning model we were able to estimate the values attributed to familiar and unfamiliar images when first presented in the context of the decision-making task. In line with our hypothesis, we found increased preference for newly introduced images (irrespective of whether these were familiar or unfamiliar) in patients compared to healthy controls and this to correlate with severity of hallucinatory experience. In addition, we found a correlation between value assigned to novel images and task performance, suggesting that excessive novelty value may interfere with optimal learning in patients, putatively through the disruption of the mechanisms regulating exploration versus exploitation. Our results suggest excessive novelty value in patients, whereby even previously seen stimuli acquire higher value as the result of their exposure in a novel context – a form of ‘hyper novelty’ which may explain why patients are often attracted by familiar stimuli experienced as new.
Keywords: schizophrenia, aberrant salience, decision-making, novelty, psychosis, novelty-seeking
1. Introduction
As humans, we are often faced with the dilemma of choosing between a familiar and novel option; whether to order ‘the usual’ or try a new dish in a restaurant, book last year’s holiday destination or go somewhere new are all examples where the values of the known and the unknown are weighted and compared with each other. The choice is not inconsequential as the two options hold different motivational value and satisfy different goals: exploring novel options permits the acquisition of new information in order to optimise behaviour in the long run, whereas pursuing options with known values facilitates the efficient exploitation of available information. It has been argued that humans have developed mechanisms aimed at increasing the value of novel stimuli as a way to promote exploration of unknown options (Kakade & Dayan, 2002). However, high novelty value and novelty-seeking behavior are only appropriate if the balance between exploration and exploitation is kept at optimal levels (Pezzulo et al., 2013), as dysfunctions in this regulatory mechanism may lead to motivational disturbance and maladaptive behavior (Averbeck, 2015; Friston et al., 2015).
In schizophrenia, several considerations hint that there may be an important deficit in the balance between the value attributed to old and novel stimuli. Within a Bayesian inference framework, influential models of schizophrenia propose that patients give relatively excessive weight to incoming sensory evidence compared to prior beliefs, resulting in heightened sense of novelty and an on-going state of surprise (Adams et al., 2013; Fletcher & Frith, 2009). Other theories have also emphasised patients’ alterations in novelty processing as central to the disorder, suggesting that key symptoms, such as delusions and hallucinations, may be consequent upon aberrant salience attribution associated with novelty processing. This is supported by observations of patients with psychosis perceiving routine stimuli as novel-like and excessively engaging, with a consequent elaboration of the importance of this sense of novelty into delusional belief and hallucinatory experience (Kapur, 2003; Kapur & Li, 2005). Hence, such theories propose that patients may exhibit motivational dysfunctions as the result of aberrant novelty processing, but the nature of this aberrance remains unclear.
That there is an imbalance in the proper allocation of novelty value in schizophrenia is further supported by evidence pointing towards a link between novelty-seeking traits and several behaviours such as excessive drug (Kim et al., 2007) and alcohol consumption (Dervaux et al., 2010), impulsivity (Ouzir, 2013) and violent behaviour (Fresan et al., 2007). Novelty-seeking has also been associated with non-adherence to medication (Margetic et al., 2011) and increased hospital admissions (albeit only in males; Miralles et al., 2014). In line with models of motivational regulation, these findings suggest that excessive attraction towards novel stimuli may disrupt motivational regulation and thus interfere with optimal decision-making and behaviour in patients affected by schizophrenia.
However, whether patients exhibit higher novelty-seeking traits relative to healthy controls remains unclear, as effects in both directions have been reported (Ohi et al., 2012). It is important to clarify that that the existing studies relied on self-report measures, which suffer from well-recognised limitations (Martinelli et al., 2013; Wilson & Dunn, 2004). In the context of novelty value investigations, the use of self-reports may be particularly misleading, as many of the questions used to assess novelty-seeking behaviour are not relevant to the life style of most patients. Thus, occasional observations of reduced novelty-seeking in patients may be merely reflecting the lack of their engagement in the activities used to assess this trait. It would be much more informative to have a direct on-line behavioural assessment in determining the presence of novelty-seeking alterations in the illness.
Additional support for the involvement of novelty processing in schizophrenia comes from evidence that dopamine, known to play a crucial role in the neurobiological substrate of schizophrenia, may crucially modulate novelty value in healthy individuals. In line with this, a recent study found a correlation between brain activity in the midbrain and ventral striatum, and value attributed to novelty while engaging in a decision-making task, as well as between the same brain areas and novelty-seeking traits (Wittmann et al., 2008). Moreover, pharmacological manipulation studies observed a correlation between increase in dopamine levels and novelty detection in humans (Rangel-Gomez et al., 2013) as well as novelty-seeking in animals (Costa et al., 2014). Furthermore, genetic studies have emphasised the importance of D2 receptors in the regulation of exploration versus exploitation behaviour (Frank & Hutchison, 2009). In schizophrenia, evidence of dopamine unbalance playing a key role in the illness (Howes & Kapur, 2009) suggests that patients may experience excessive novelty value as a consequence of their dopaminergic dysregulation.
Despite evidence described above, novelty salience dysfunctions in schizophrenia have not yet been formally tested. In the present study, we studied novelty value in schizophrenia with an armed bandit task (Daw et al., 2006), which has been successfully employed for the investigation of novelty-seeking behaviour in healthy individuals (Wittmann et al., 2008) and those affected by Parkinson’s disease (Djamshidian et al., 2011). We tested the hypotheses that: a) patients would exhibit enhanced novelty-seeking compared to healthy controls; b) altered novelty processing in patients would interfere with their decision-making leading to sub-optimal performance; and c) altered novelty value would be associated with severity of positive symptoms.
2. Materials and methods
2.1 Participants
On the basis of a previous study (Djamshidian et al., 2011) using the same task on a clinical population and reporting an effect size of d = 0.91, we estimated we needed at least 20 subjects per group to ensure a power of 0.80. We thus recruited 24 patients, with a diagnosis of schizophrenia (based on assessment using the ICD-10 criteria; WHO, 1992), being treated with stable doses of atypical antipsychotic medication, and 24 controls without a personal or family history of mental illness, screened with the MINI International Neuropsychiatric Interview (Sheehan et al., 1998). Due to initial technical difficulties, data from 4 patients were lost, thus the overall sample included 20 patients and 24 controls. The study was approved by the London Chelsea Research Ethics Committee and all participants provided written informed consent before testing. Participants met the following inclusion criteria: 1) capacity to consent; 2) age between 18–60 years; 3) sufficient command of the English language to follow the experimental tasks and 4) having an IQ above 80. Participants were excluded if they had: 1) current drug or alcohol dependence; 2) brain disease or damage or if they 3) used psychotropic medication (except patients). Brain disease or damage was assessed by asking participants if they had ever experienced loss of consciousness for longer than 30 s, head injury resulting in loss of consciousness, had any neurological condition, or had ever been referred to a neurologist. All participants underwent IQ assessment through the vocabulary and matrix reasoning scales of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1992). The diagnosis of schizophrenia was confirmed by an experienced clinician and symptom severity was assessed using the Positive and Negative Syndrome Scale (Kay et al., 1987).
2.2 Three-armed bandit task
Before the start of the task, subjects underwent a familiarisation phase in which were shown 18 images, eight times each, and asked to judge via button press whether the image depicted a building or a natural landscape. This was done to consolidate encoding by forcing participants to process images semantically. Subsequently, all participants were asked to verbally describe the images one at the time in order to further aid their consolidation in memory.
After the familiarization phase, participants performed the three-armed bandit task (programmed with the Cogent 2000 in Matlab and administered to participants on a laptop computer). The task comprised of 180 trials lasting approximately 30 minutes. During the study, each trial consisted of three black and white post-cards (Fig 1) presented on screen, each associated to a different probability of winning money, varying on three levels (i.e., 0%, 40% and 80%), which remained constant throughout the course of the task. Participants were instructed to select the most rewarded image as many times as possible in order to maximise their earnings, which were paid to participants at the end of the task. Each positive outcome was 0.10p and each negative outcome was 0p. Both visual (image framed in green for gains and in red for no-gains) and auditory feedback (tones at 5 Khz for gains and 2.5 Khz for no-gains) was provided to reinforce feedback learning. Location of pictures was randomised across trials in order to prevent habituation.
Figure 1.
Sequence of events in three-armed bandit task.
During the task, images on the screen were replaced with novel ones on approximately 20% of trials, (i.e., every five trials on average). These newly introduced images could be of two kinds: either unfamiliar or familiar. The unfamiliar images were images that subjects had never seen before, whereas familiar images were those that had been shown to participants during the familiarisation phase preceding the three-armed bandit task. Thus, we will name “familiar” the images shown to participants during the familiarisation phase, “unfamiliar” those never seen before, and “novel” when they are presented for the first time in the bandit task (irrespective of whether these were seen during the familiarization phase or not). Participants could find out images’ reward probabilities only by repeatedly sampling them, thus facing the classic exploration versus exploitation dilemma (Wittmann et al., 2008). Pay-off probabilities associated to the images were equal across the two sets, so that familiar and unfamiliar images did not differ per average value.
To ensure that all participants properly encoded images during the familiarisation phase we performed the following memory checks. First, the experimenter asked participants to identify the unfamiliar image on the first trial of the bandit task (one unfamiliar image was always present on the first trial). Second, at the end of the task, all images showed during the task were presented to participants who were required to discriminate the ones seen during the familiarisation phase from those seen during the task only.
2.3 Behavioural analyses
Statistical analyses were conducted using the Statistical Package for the Social Sciences Version 21.0 (SPSS 21.0; IBM Corp, 2012) and MATLAB (The Mathworks, 2013). Demographic and clinical variables were collected and appropriate statistics were run to test for group differences. A two-tailed significance level of α = 0.05 was adopted for all analyses, which could be grouped in: (i) based on our a-priori hypotheses (see Introduction), (ii) exploratory analyses and (iii) sanity-check analyses. For a-priori analyses, our results should be interpreted as confirmations or disconfirmations of our initial hypotheses. Results of exploratory analyses require further research to be confirmed. Results of our sanity-check analyses rule out possible confounds. More specifically, to investigate performance on task, we first fitted a reinforcement learning model (see below) to participants’ choices in order to estimate parameters reflecting the value assigned to novel stimuli. We then ran a two-way ANOVA on the initial value attributed to the images with group as a between-subjects variable and familiarity of image as a within-subjects variable. Further post-hoc analyses were conducted using independent samples t-tests. Bivariate correlations were conducted between the estimated parameters and both medication dosage and severity of positive symptoms.
2.4 Reinforcement learning model
To characterise the mechanisms underlying choice behaviour, we estimated separately for each subject a reinforcement learning model which learns trial by trial the value of the images (associated with the choice options in the bandit task) based on reward feedback. At trial t+1, the value V of the chosen option i is updated based on its value at the preceding trial Vi(t) plus the reward prediction error obtained at trial t (corresponding to the difference between the reward collected R(t) and the expected value Vi(t)) multiplied by the learning rate parameter α:
Crucially, when a novel image is presented in the task at trial t = x, its initial value Vi(x) (i.e., before any experience of reward with the image) is cast as a free parameter of the model. More specifically, the model distinguishes familiar and unfamiliar images and associates a different initial value to each group of images. The model includes two free parameters relative to the initial value of images, one associated to familiar (i.e., presented already in the bandit task; Vi(x) = VF) and the other to unfamiliar (i.e., never presented in the bandit task; Vi(x) = VU) images. A large initial value Vi(x) indicates the tendency to pick a novel image (i.e., newly introduced) relative to images presented in previous trials thus with a reward history. Other free parameters of the model are the learning rate (α) and the inverse temperature (β), which indicates choice randomness by influencing the probability Pi(t) of choosing the image i over the other images available k and z, obtained through a softmax function:
Parameters were estimated for each subject separately using fminsearchbound function in Matlab. We ascertained that parameters assumed plausible values by setting the following boundaries: α between zero and one, β between zero and four, and VF and VU between zero and one (given that in the task the maximum possible value of images is one – i.e., certainty of receiving reward).
We performed an analysis comparing the full model (Model 1) against Model 2, where a single parameter for novel images regardless of whether they were familiar or unfamiliar was introduced, and Model 3, including learning rate and inverse temperature parameters only. Given that models are nested (i.e., Model 3 is a subset of Model 2 which in turn is a subset of Model 1), model comparison was performed using chi-square (χ2) statistics.
3. Results
Demographic and clinical characteristics are shown in Table 1.
Table 1.
| Demographic and clinical characteristics | SCZ (n = 20) | HC (n = 24) | Test statistic | P value |
|---|---|---|---|---|
| Age: mean (SD) | 41.50 (6.76) | 40.50 (7.58) | t(42) = −0.46 | 0.65 |
| Gender/male: n (%) | 17 (85) | 21 (87.5) | FET | |
| WASI (IQ): mean (SD) | 97.20 (16.69) | 110.29 (14.11) | t(41) = 2.82 | < 0.01 |
| PANSS Positive: mean (SD) | 19 (4.7) | -- | -- | -- |
| PANSS Negative: mean (SD) | 20.50 (4.95) | -- | -- | -- |
| PANSS General: mean (SD) | 38.45 (7.99) | -- | -- | -- |
| PANSS Total: mean (SD) | 78.21 (14.5) | -- | -- | -- |
| Medication: mean (SD)a | 562.90 (422.1) | -- | -- | -- |
SCZ: volunteers diagnosed with schizophrenia, HC: healthy controls, SD: standard deviation, WASI: Wechsler Abbreviated Scale of Intelligence, PANSS: Positive and Negative Syndrome Scale, FET: Fisher’s Exact Test.
Chlorpromazine equivalent (mg per day) – all volunteers were on stable atypical medication.
With regards to the three-armed bandit task, we used parameters derived from the model to assess putative group differences in novelty-seeking, which in our study correspond to the initial values of the novel images (separately for familiar and unfamiliar items), the learning rate and the inverse temperature.
We compared different models of choice behaviour by summing negative log-likelihoods for all subjects and using chi-square statistics. Model comparison favoured Model 1 over Model 2 (SCZ: χ2(20) = 77.72, p < 0.001; HC: χ2(24) = 86.54, p < 0.001) and over Model 3 (SCZ: χ2(40) = 180.42, p < 0.001; HC: (48) = 226, p < 0.001), supporting the idea that all four parameters are actually necessary to describe participants’ behaviour. We further measured how well the model fit the data with the use of pseudo-r2, a standard measure used to quantify the fit of the model compared to chance and bounded between 0 and 1 (Daw, 2011; pseudo-r2 = 0.287).
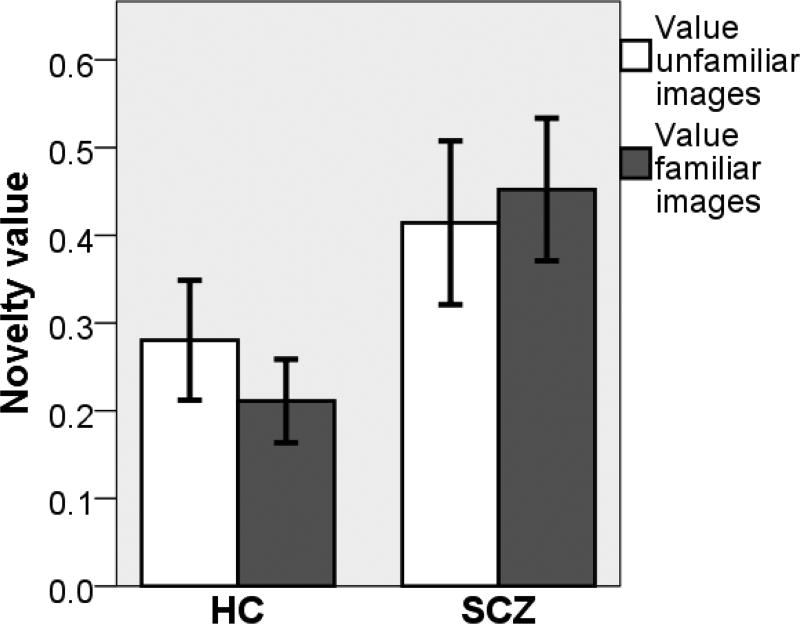
Next, we compared patients and controls based on the parameters estimated with Model 1. This model allowed us to independently analyse two forms of novelty value: the value dependent on being never seen before (absolute novelty) and the value dependent on being newly introduced in the bandit task (contextual novelty). To investigate these, we carried out a two-way ANOVA on the initial value attributed to images in the bandit task with group as a between-subjects variable and familiarity of image as a within-subjects variable. A main effect of image was predicted by the hypothesis of value being dependent on absolute novelty, prescribing a larger initial value to unfamiliar compared to familiar images. A main effect of group driven by larger initial values in patients would suggest that patients evaluate more contextual novelty than controls (i.e., given that contextual novelty corresponds to the average of the initial values assigned to familiar and unfamiliar images). An interaction effect would suggest, for instance, that patients compared to controls attribute heightened value to absolute than contextual novelty. We found a main effect of group (F(1,42) = 4.27, p = 0.045, ηp2 = 0.092), such that schizophrenia patients selected newly presented images more often than controls irrespective of whether these were familiar or unfamiliar (Fig. 2). However, we found no main effect of image familiarity (F(1,42) = 0.107, p = 0.745, ηp2 < 0.01) or interaction between image familiarity and group (F(1,42) = 1.249, p = 0.270, ηp2 = 0.02). These results suggest that patients affected by schizophrenia are more influenced by contextual novelty compared to healthy individuals and tend to assign an higher value to newly presented images irrespective of whether these had been seen before or not.
Figure 2.
Group differences in novelty value. Patients affected by schizophrenia assign higher value to both familiar and unfamiliar images when first presented in the context of the task compared to other images, and thus exhibit higher contextual novelty value compared to healthy controls.
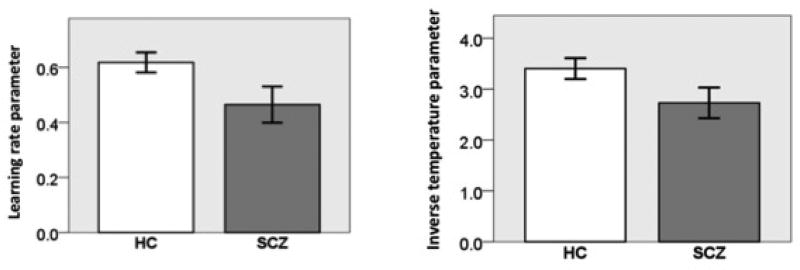
We further assessed between groups differences on the learning rate parameter, measuring the extent to which subjects updated their value representations based on reward feedback, and the inverse temperature parameter, measuring participants’ randomness in decision-making (Fig. 3). The schizophrenia patients were found to exhibit a statistically significant reduction in learning rate (t(42) = 2.129; p = 0.039; d = 0.63) and a trend towards reduced inverse temperature (t(42) = 1.897, p = 0.065; d = 0.57) compared to the healthy controls.
Figure 3.
Group differences on learning rate and inverse temperature parameters.
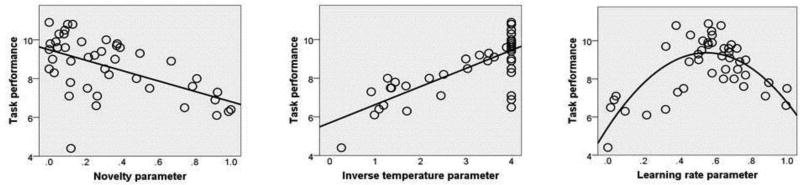
Performance on the task differed between groups as schizophrenia patients won significantly less money than healthy controls (t(42) = 2.914, p = 0.006; d = 0.88). Interestingly, we found a significant negative correlation between participants’ task performance (i.e., amount of money won) and value assigned to novel images (Fig. 4A; r(44) = −0.555, p < 0.001; we used the mean between VF and VU as estimated by the full model), suggesting that patients’ excessive novelty-seeking may interfere with optimal reward learning. We also found that participants’ task performance positively correlated with the inverse temperature parameter (Fig. 4B; r(44) = 0.758, p < 0.001). From visual inspection, we observed an inverted u-shaped relationship between task performance and learning rate; we thus tested a quadratic model of this relationship which was significantly better than the linear one (Fig. 4C; F(2,41) = 27.87, p < 0.001). This indicates that optimal learning rate was an intermediate score (around 0.5). Also, we observed an inverse correlation between novelty-seeking and learning rate (r(44) = −0.338, p = 0.025) and between novelty-seeking and inverse temperature (r(44) = −0.526, p < 0.001), suggesting that these variables may be partially linked to a common factor. No correlation was observed between learning rate and inverse temperature.
Figure 4.
Correlations between task performance and model parameters.
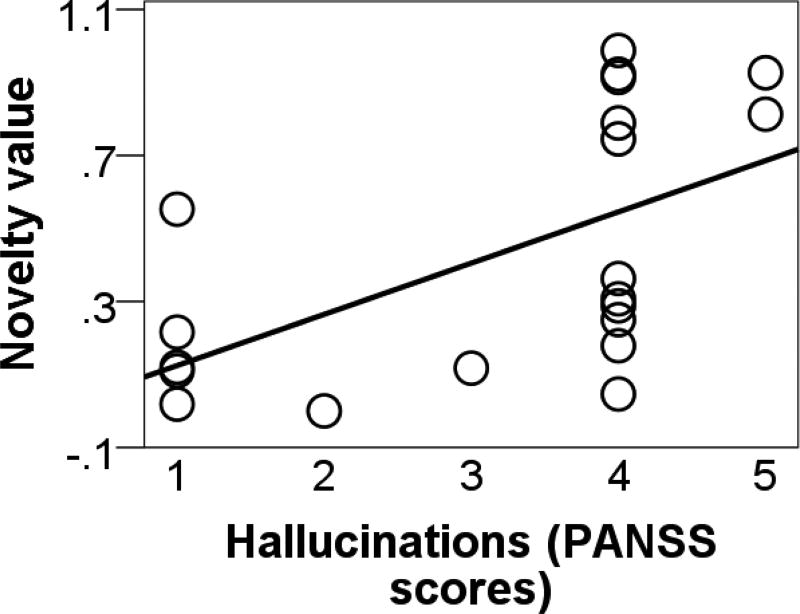
We found no group differences for memory of familiarised images, as evidenced by identification of unfamiliar image during the first trial of task (t(40) = −1.013, p =0.277; data missing in 2 subjects) and discrimination of familiarised and non-familiarised images at the end of the testing session (t(33) = −1.447, p =0.157; data missing in 9 subjects). We found a positive correlation between novelty value and severity of hallucinatory experience (Fig. 5; r(20) = 0.567, p < 0.01), but not between novelty value and delusional ideation. Our further exploration of the relationship between novelty value and PANSS positive and negative scales were also non-significant. We also found no correlation between estimated parameters and medication dosage. Lastly, we found no correlation between IQ and novelty value, suggesting that there was no association between cognitive abilities and contextual novelty-seeking. However, we found a trend between IQ and amount of money won (r(44) = 0.294, p +0.055). To check wehtehr group differences on task performance were attributable to IQ, we ran a mediation analysis with group as the independent variable, monetary amount won as the dependent variable, and IQ as a putative mediator. This analysis revealed that IQ did not fully mediate the relationship between group and task performance, as the effect of group on monetary amount won (r(44) = −0.41, p < 0.01) remained significant even when controlling for WASI scores (r(44) = −0.334, p = 0.02), suggesting that group differences on overall task performance are not merely attributable to cognitive abilities.
Figure 5.
Correlation between novelty value and severity of hallucinatory symptoms
4. Discussion
Recent theoretical models in schizophrenia emphasise the role of novelty processing deficits in the pathophysiology of the disorder (Adams et al., 2013; Fletcher & Frith, 2009; Friston et al., 2015). Such frameworks have been supported by both neural and clinical evidence and have been fruitful in linking central dopaminergic dysfunctions in schizophrenia to disturbances of motivation and value. In particular, theories and empirical research converge towards predicting excessive novelty processing in patients, potentially as the result of a dysregulation in the mechanisms maintaining proper motivational balance. However, despite its clinical relevance, and tight links with the neurological substrate of the illness, the motivational value of novelty in schizophrenia has not been formally investigated.
To address this, we compared the performance of schizophrenia patients and healthy controls in a novelty-seeking task requiring participants to choose images associated with different reward probabilities. The task was arranged in a way that in 20% of the trials, novel images were introduced – these could be images seen during a pre-task familiarisation phase (familiar) or not (unfamiliar). By fitting a reinforcement learning model, we were able to estimate the value of the options, and thus to derive the value assigned to images presented for the first time in the task. The model further differentiated between the value attributed to images never seen before (absolute novelty) and the value attributed to images newly introduced in the task regardless of previous exposure (contextual novelty).
Compared to healthy controls, we found that schizophrenia patients were more attracted to newly introduced images, regardless of whether these had been previously seen in the pre-task familiarisation phase. Our results suggest excessive (contextual) novelty-seeking in patients, whereby even previously seen stimuli acquire higher value as the result of their exposure in a novel context – this could be conceived as a form of ‘hyper novelty’ and may describe why patients often report being attracted by stimuli, which are experienced as new but are nevertheless familiar.
This finding provides the first experimental evidence of aberrant novelty value in schizophrenia and thus clarifies previous mixed results based on self-reports, which may be limited for this particular clinical population. The observation of exaggerated novelty-seeking in patients is all the more important in light of recent research showing a link between novelty-seeking traits and a wide range of motivational and decision-making impairments in the disorder. It is indeed plausible that excessive attraction towards novel stimuli may underlie many of patients’ dysfunctional behaviours reported in the literature.
It should be noted that previous experimental work on novelty in the disorder focused on memory (e.g., Martinelli and Shergill, 2015) and attentional deficits such as mismatch negativity, namely the event-related potential generated by unexpected violations of auditory regularities (Umbricht and Krljes, 2005). This violation may include change in the location, frequency or duration of the stimulus and its detection has been found to be diminished in schizophrenia (Naatanen and Kahkonen, 2009). Mismatch negativity has been interpreted as reflecting automatic, low-level auditory processes and has been shown to be free from motivational factors. Here, we have investigated a higher-order, motivational aspect of novelty processing, namely the one related to novelty value and related decision-making. Although these two processes are largely different, it would be interesting to know whether they share some underlying mechanisms; this could be the focus of future investigations.
Several studies showed reinforcement learning impairments in the illness (Strauss et al., 2014), particularly in patients' ability to update values on the basis of feedback (e.g., Waltz et al., 2007) or prediction error signalling (e.g., Gradin et al., 2011). Here, we extended these findings by investigating reward-related decision-making in the context of a novelty-seeking task, and by showing that excessive novelty-value may directly interfere with reward learning. Indeed, we found that schizophrenia patients won significantly less money on the task compared to healthy participants. Crucially, task performance was inversely correlated with novelty-seeking, so that the higher the tendency to pick novel images, the lower the ability to reach optimum decision-making. This result accords with the proposal that excessive novelty value interferes with reward learning in the illness and may provide evidence for maladaptive regulation between exploration and exploitation in patients.
No robust group differences were evident for the inverse temperature parameter, although patients exhibited lower levels of consistency in their decision-making. One might at first raise the possibility that an increased (contextual) novelty-seeking parameter in patients might be an artefact actually explained by a decreased inverse temperature or learning rate, which would bias also the estimate of the novelty-seeking parameter. However, this is highly unlikely as, if this was the case, model comparison would have favoured a simple model without any novelty-seeking parameter, while for both groups the full model was favoured by model comparison, indicating that novelty-related parameters (i.e., those indicating the values of options when initially presented) are distinguished from other parameters.
Lastly, we found that novelty value correlated with current hallucinatory experience. This is in line with the idea that excessive salience and value attributed to incoming stimuli may lead to abnormal perceptions, perhaps as the result of reduced weight of prior beliefs over sensory data or reduced sensory attenuation mechanisms (Fletcher & Frith, 2009). Contrary to proposals of an association between aberrant novelty processing and delusional ideation, we found no link between novelty value in our task and severity of delusions as measured by the PANSS. This raises the possibility of a specific role of aberrant value attribution to novel stimuli in the development or maintenance of hallucinatory experiences. One intriguing possibility is that hallucinations may be triggered by stimuli that are novel or surprising within a context. Future research should focus on deepening our understanding of the relation between hallucinations and novelty value in schizophrenia. Furthermore, the association between novelty value and hallucinations observed in patients raises the question of whether excessive novelty value in patients is stable (a trait) or fluctuates in relation to clinical state. This is particularly interesting as novelty-seeking is considered to be a stable trait in healthy individuals (e.g., Cloninger et al., 1991). Future studies may provide further insight into the matter by assessing novelty-seeking in groups of patients stratified according to symptoms' severity, by evaluating the stability of novelty-seeking behaviour across time and by investigating novelty-seeking in preclinical conditions.
Contrary to previous data (Wittmann et al., 2008), healthy individuals did not exhibit increased tendency to choose unfamiliar over familiar images. One possibility is that that the familiarisation procedure did not work optimally; however, this is unlikely because healthy individuals (and patients) performed well on recollection of familiar images both pre- and post-task. Another study using a paradigm similar to the one used by Wittmann and colleagues (2008) also failed to replicate increased seeking behaviour for unfamiliar versus familiar images in healthy control subjects (Djamshidian et al., 2011), suggesting that this may not be a ubiquitous finding.
This study presents a number of limitations. First, all patients were on stable antipsychotic treatment at the time of the experiment, possibly affecting our results. This is particularly relevant here, as novelty seeking has been repeatedly shown to be influenced by dopaminergic regions targeted by current antipsychotics. However, we here found no association between medication dosage and estimated parameters, suggesting that our results were not due to antipsychotic medication. It is nevertheless possible that medication dampened novelty seeking in our patients and that the effect would have been stronger in drug naïve patients, possibly even extending to forms of novelty other than contextual. Future studies would benefit from directly addressing the role of medication in novelty value attribution. Second, we performed multiple exploratory analyses (not associated with our a-priori hypotheses), which should be taken with caution.
In sum, in line with the proposal of aberrant novelty processing playing an important role in the pathophysiology of schizophrenia, we observed excessive motivational value attributed to novel stimuli in patients. We further found evidence suggesting that excessive novelty-seeking may have maladaptive consequences on learning and decision-making in a reward context. In line with theories of motivational regulation, this mechanism might mirror aberrant regulation of the exploitation versus exploration system and at such may underlie some of the disturbances previously linked with novelty-seeking traits in schizophrenia patients. The observed association between novelty value and hallucinatory experience in patients suggests that excessive attraction towards novel stimuli may be an important mechanism underlying symptomatology in the illness. These findings contribute to the clarification of motivational deficits in the illness and provide support to those models proposing aberrant value processing as an important mechanism in the disorder.
References
- Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB. Theory of Choice in Bandit, Information Sampling and Foraging Tasks. PLoS Comput Biol. 2015;11(3) doi: 10.1371/journal.pcbi.1004164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Tran VL, Turchi J, Averbeck BB. Dopamine modulates novelty seeking behavior during decision making. Behav Neurosci. 2014;128(5):556. doi: 10.1037/a0037128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441(7095):876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND. Trial-by-trial data analysis using computational models. Decision making, affect, and learning: Attention and performance XXIII. 2011;23:3–38. [Google Scholar]
- Dervaux A, Laqueille X, Bourdel MC, Olié JP, Krebs MO. Impulsivity and sensation seeking in alcohol abusing patients with schizophrenia. Front Psychiatry. 2010;1 doi: 10.3389/fpsyt.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamshidian A, O'Sullivan SS, Wittmann BC, Lees AJ, Averbeck BB. Novelty seeking behaviour in Parkinson's disease. Neuropsychologia. 2011;49(9):2483–2488. doi: 10.1016/j.neuropsychologia.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nature Reviews Neuroscience. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Hutchison K. Genetic contributions to avoidance-based decisions: striatal D2 receptor polymorphisms. Neuroscience. 2009;164(1):131–140. doi: 10.1016/j.neuroscience.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresán A, Apiquian R, Nicolini H, Cervantes JJ. Temperament and character in violent schizophrenic patients. Schizophr Res. 2007;94(1):74–80. doi: 10.1016/j.schres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Friston K, Rigoli F, Ognibene D, Mathys C, Fitzgerald T, Pezzulo G. Active inference and epistemic value. Cogn Neurosci. 2015;1:28. doi: 10.1080/17588928.2015.1020053. [1] [DOI] [PubMed] [Google Scholar]
- Kakade S, Dayan P. Dopamine: generalization and bonuses. Neural Networks. 2002 Jul 31;15(4):549–59. doi: 10.1016/s0893-6080(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am Jour Psychiat. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophrenia research. 2005 Nov 1;79(1):59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim D, Park SH, Lee HB, Chung EK. Novelty-seeking among schizophrenia patients with comorbid alcohol abuse. Journ Nerv Ment Dis. 2007;195(7):622–624. doi: 10.1097/NMD.0b013e318093f425. [DOI] [PubMed] [Google Scholar]
- Margetić BA, Jakovljević M, Ivanec D, Tošić G, Margetić B. Novelty seeking and medication adherence in patients with schizophrenia. Psychiatr Res. 2011;186(1):141–143. doi: 10.1016/j.psychres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Miralles C, Alonso Y, Verge B, et al. Personality dimensions of schizophrenia patients compared to control subjects by gender and the relationship with illness severity. BMC Psychiatry. 2014;14(1):151. doi: 10.1186/1471-244X-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Hashimoto R, Yasuda Y, et al. Personality traits and schizophrenia: evidence from a case–control study and meta-analysis. Psychiatr Res. 2012;198(1):7–11. doi: 10.1016/j.psychres.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Ouzir M. Impulsivity in schizophrenia: a comprehensive update. Aggress Violent Behav. 2013;18(2):247–254. [Google Scholar]
- Pezzulo G, Rigoli F, Chersi F. The Mixed Instrumental Controller: Using Value of Information to Combine Habitual Choice and Mental Simulation. Front Psychol. 2013;4(92):1–15. doi: 10.3389/fpsyg.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Gomez M, Hickey C, Van Amelsvoort T, Bet P, Meeter M. The detection of novelty relies on dopaminergic signaling: Evidence from apomorphine’s impact on the novelty N2. Plos One. 2013;8 doi: 10.1371/journal.pone.0066469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of clinical psychiatry. 1998 [PubMed] [Google Scholar]
- The Mathworks, MATLAB version 12. Natick, Massachusetts: The MathWorks Inc; 2013. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company; New York, NY: 1992. [Google Scholar]
- Wilson TD, Dunn EW. Self-knowledge: Its limits, value, and potential for improvement. Annu Rev Psychol. 2004;55 doi: 10.1146/annurev.psych.55.090902.141954. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Daw ND, Seymour B, Dolan RJ. Striatal activity underlies novelty-based choice in humans. Neuron. 2008;58(6):967–973. doi: 10.1016/j.neuron.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization; 1992. [Google Scholar]