Abstract
Alpha-Synuclein (α-syn) is by far the most highly vetted pathogenic and therapeutic target in Parkinson’s disease. Aggregated α-syn is present in sporadic Parkinson’s disease, both in the central nervous system (CNS) and peripheral nervous system (PNS). The enteric division of the PNS is of particular interest because 1) gastric dysfunction is a key clinical manifestation of Parkinson’s disease, and 2) Lewy pathology in myenteric and submucosal neurons of the enteric nervous system (ENS) has been referred to as stage zero in the Braak pathological staging of Parkinson’s disease. The presence of Lewy pathology in the ENS and the fact that patients often experience enteric dysfunction before the onset of motor symptoms has led to the hypothesis that α-syn pathology starts in the periphery, after which it spreads to the CNS via interconnected neural pathways. Here we sought to directly test this hypothesis in rodents and non-human primates (NHP) using two distinct models of α-syn pathology: the α-syn viral overexpression model and the preformed fibril (PFF) model. Subjects (rat and NHP) received targeted enteric injections of PFFs or adeno-associated virus overexpressing the Parkinson’s disease associated A53T α-syn mutant. Rats were evaluated for colonic motility monthly and sacrificed at 1, 6, or 12 months, whereas NHPs were sacrificed 12 months following inoculation, after which the time course and spread of pathology was examined in all animals. Rats exhibited a transient GI phenotype that resolved after four months. Minor α-syn pathology was observed in the brainstem (dorsal motor nucleus of the vagus and locus coeruleus) 1 month after PFF injections; however, no pathology was observed at later time points (nor in saline or monomer treated animals). Similarly, a histopathological analysis of the NHP brains revealed no pathology despite the presence of robust α-syn pathology throughout the ENS which persisted for the entirety of the study (12 months). Our study shows that induction of α-syn pathology in the ENS is sufficient to induce GI dysfunction. Moreover, our data suggest that sustained spread of α-syn pathology from the periphery to the CNS and subsequent propagation is a rare event, and that the presence of enteric α-syn pathology and dysfunction may represent an epiphenomenon.
Keywords: Alpha-synuclein, Enteric nervous system, Parkinson’s disease, Enteric dysfunction, Prion spread
1. Introduction
Alpha-Synuclein (α-syn) is currently the most vetted participant in Parkinson’s disease pathology. Mutations in, and multiplications of, the gene encoding α-syn are associated with familial forms of the disease, and polymorphisms in the gene confer increased risk in developing Parkinson’s disease (Polymeropoulos et al., 1997; Singleton et al., 2003; Ibanez et al., 2004; Simon-Sanchez et al., 2009). In both familial and sporadic Parkinson’s disease, α-syn is a major component of Lewy bodies and Lewy neurites, the pathological hallmark of Parkinson’s disease (Spillantini et al., 1997). Finally, ectopic overexpression of α-syn in rodents and non-human primates results in a dose-dependent and stereotypic pattern of neurodegeneration that is relatively specific to nigrostriatal neurons (Kirik et al., 2002; Eslamboli et al., 2007; Gombash et al., 2013). Although the molecular etiology underlying α-syn-mediated neurotoxicity is yet to be defined, it is clear that oligomerization and fibrillization of the protein play a central role in the pathology. As such, it has been argued that α-syn aggregates, or intermediate forms of aggregates, are directly toxic to cellular organelles and/or processes. The alternate viewpoint contends that the role of α-syn in neurodegeneration is that of a toxic loss of function as a result of sequestration of the monomeric (and presumptive functional) form of α-syn in to insoluble aggregates (Benskey et al., 2016a; Dettmer et al., 2016).
Regardless of the modality by which aggregates cause neurotoxicity, recent advances in the field suggest that pathological, aggregated, forms of α-syn can spread throughout the nervous system via cycles of transmission, templating, and aggregation of α-syn in recipient cells, in a process that propagates along the neuraxis (Volpicelli-Daley et al., 2011; Luk et al., 2012a; Paumier et al., 2015). The first indication that α-syn pathology can spread was observed in patients receiving fetal mesencephalic neuronal transplants (Kordower et al., 2008; Li et al., 2008; Chu and Kordower, 2010; Li et al., 2010; Li et al., 2016; Kordower et al., 2017). Post-mortem histological analyses revealed that a portion of the transplanted (presumably healthy) tissue contained Lewy pathology, with the degree of Lewy pathology being time-dependent. These data suggested that α-syn pathology had spread from the host to the graft; a finding supported by preclinical experiments (Hansen et al., 2011; Kordower et al., 2011). This finding was followed up experimentally with research suggesting that recombinant α-syn pre-formed α-syn fibrils (PFF) can transmit from cell to cell. Stereotaxic injections of PFFs in to various regions of the central nervous system (CNS) results in the aggregation of endogenous α-syn in a wide range of anatomically distinct loci throughout the brain, with concomitant neuronal loss in areas such as the substantia nigra (Luk et al., 2012a; Paumier et al., 2015). Although unequivocal evidence of actual in vivo neuron-to-neuron transmission is still lacking, interneuronal transmission has been observed in microfluidic devices between cultured neurons in vitro (Volpicelli-Daley et al., 2011; Brahic et al., 2016) and these processes are dependent upon the binding of pathological α-syn to the lymphocyte-activation gene 3 (LAG3) (Mao et al., 2016) and transmembrane protein 9 superfamily member 2 (TM9SF2; preliminary data from the laboratory of Mark Cookson (Wadman, 2016)) receptors.
The potential propagation of α-syn pathology throughout the CNS holds great explanatory power as it applies to the varying staging schemes of Parkinson’s disease progression (Braak et al., 2003a). However, it has become increasingly recognized that α-syn pathology is not limited to the CNS; rather, α-syn (i.e. Lewy) pathology has been observed throughout the peripheral nervous system (PNS) as well. α-syn pathology has been observed in Parkinson’s disease patients in organs such as skin (Ikemura et al., 2008), heart (Iwanaga et al., 1999), and the enteric nervous system (ENS) (Wakabayashi et al., 1988; Wakabayashi et al., 1990; Braak et al., 2006; Beach et al., 2010). The ENS is of particular interest, because gastric dysfunction is a key component of Parkinson’s disease symptomology (Edwards et al., 1991; Pfeiffer, 2011; Park et al., 2015). Moreover, symptoms such as constipation are present years prior to an established diagnosis (Ross et al., 2012; Sung et al., 2014; Chen et al., 2015). Indeed, Lewy pathology in myenteric and submucosal neurons of the ENS is sometimes referred to as stage zero in the classic Braak staging of Parkinson’s disease (Braak et al., 2003b). Although a direct correlation between enteric Lewy pathology and gastrointestinal (GI) dysfunction in Parkinson’s disease is yet to be made, a GI phenotype has been observed in transgenic animals overexpressing α-syn (Wang et al., 2008; Kuo et al., 2010; Hallett et al., 2012; Farrell et al., 2014).
The presence of Lewy pathology in the ENS and the fact that patients experience ENS dysfunction before the onset of motor symptoms has led to the hypothesis that α-syn pathology originates in peripheral neurons, specifically within neurons of the ENS, from where it spreads to the CNS. Congruent with this idea is the fact that enteric α-syn pathology has been detected in patients prior to a Parkinson’s disease diagnosis as demonstrated in several independent studies (Shannon et al., 2012a; Hilton et al., 2014; Stokholm et al., 2016), but see Beach et al., 2016 (Beach et al., 2016). Moreover, the presumptive route of transmission is thought to be via vagal innervation, due to the fact that brainstem nuclei such as the dorsal motor nucleus of the vagus display Lewy pathology early in the course of the disease. Further, direct delivery of adeno-associated virus (AAV) overexpressing human α-syn (hα-syn) into the vagus nerve results in spread of exogenous α-syn extending rostrally throughout the brainstem (Ulusoy et al., 2013). Moreover, injection of human Parkinson’s disease brain lysate in to the wall of the intestine of rodents results in the transport of exogenous α-syn in the vagus nerve to the dorsal motor nucleus of the vagus (Holmqvist et al., 2014). Thus, ample scientific evidence exists in support of the “synuclein-spreading” hypothesis of Parkinson’s disease.
Herein we took a multi-modal approach in order to determine whether α-syn pathology can be initiated in the periphery (i.e. ENS) and spread to the CNS over time. We injected PFFs directly in to the myenteric layer of either the rat colon, or the non-human primate (NHP) colon and stomach. In addition, a separate cohort of non-human primates received localized enteric injections with AAV overexpressing the Parkinson’s disease associated A53T α-syn mutant form. Rodents were monitored for colonic dysmotility for up to one year and sacrificed at intermediate time points in order to monitor the progression of α-syn pathology. Similarly, NHPs were sacrificed one year after α-syn or vector delivery and assessed for CNS and ENS pathology.
2. Materials and methods
2.1. Vector production
Recombinant AAV (rAAV) genomes were the same as those used in previous studies investigating α-syn overexpression and neurodegeneration in the CNS (Gombash et al., 2013): Coding sequences for human α-syn (hα-syn) A53T or GFP were under the control of the chicken beta-actin (CBA)/cytomegalovirus (CMV) promoter hybrid. Genomes were packaged in to AAV5 capsids as previously described (Benskey et al., 2016b). HEK 293T cells were transfected with plasmids encoding the respective genomes together with plasmids encoding helper functions. 72 h following transfection cells were harvested and media was collected and concentrated using tangential flow filtration. Viral particles were purified using an iodixanol gradient followed by column chromatography. Titers were determined via dot-blot (Zolotukhin et al., 1999) and normalized to 1 × 1013 vector genomes/ml (vg/ml) using balanced salt solution (Alcon, Fort Worth, TX).
2.2. Generation of PFFs
Production of recombinant mouse α-syn (for rat studies) and human α-syn (for NHP studies) and in vitro fibril assembly was performed as described (Luk et al., 2012a; Volpicelli-Daley et al., 2014). Mouse PFFs were identical to those previously used within the mouse and rat CNS (Luk et al., 2012a; Paumier et al., 2015). Prior to the surgeries PFFs were thawed, diluted in dPBS, and sonicated at room temperature using an ultrasonic homogenizer (300VT; Biologics, Inc., Manassas, VA; pulser at 20%, power at 30%, 60 pulses at 1 s each). Recombinant α-syn monomer was thawed on ice, diluted in ice-cold sterile saline, and kept on ice throughout the surgical session. Sonicated fibrils were visualized using electron microscopy (EM). Formvar/carbon coated copper grids (EMSDIASUM: FCF300-Cu) were washed twice with ddH2O and floated for 1 min on a 10 μl drop of sonicated α-syn fibrils diluted 1:20 with DPBS. Grids were stained for 1 min on a drop of 2% uranyl acetate aqueous solution; excess uranyl acetate was wicked away with filter paper, and grids were allowed to dry before imaging. Grids were imaged on a JEOL JEM-1400 transmission electron microscope. The length of over 500 fibrils per sample was measured and the data was analyzed and graphed in GraphPad Prism 7 and the average fibril length from each sample was determined. A majority of the fibril species was at a length considered to be ideal for seeding (mean = 55.9 nm ± 1.1 nm for mouse fibrils (n = 641) and 75.8 nm ± 2.2 nm for human fibrils (n = 520) (Tarutani et al., 2016; Abdelmotilib et al., 2017); (Sup. Fig. 1).
2.3. Surgeries-rat
Young adult (220 g) Sprague-Dawley male rats were utilized in accordance with the Michigan State University Institutional Animal Care & Use Committee (AUF 08/12-150-00) guidelines. Injections directly targeted to the enteric neurons of the descending colon were performed as previously described (Benskey et al., 2015b; Benskey and Manfredsson, 2016). Briefly, rats were anesthetized using isoflurane and a full laparotomy was performed in order to isolate and expose the descending colon. The proximal border of the area to be injected was demarcated with a tattoo, and 6 × 5 μl injections of PFFs (2 μg/μl; n = 15, recombinant monomeric α-syn (2 μg/μl; n = 15), or a saline vehicle control (n = 14) were administered distal to the tattoo. Injections were performed with an automated micropump at 1 μl/s. Following the injection the needle was left in place for an additional 10–20 s to allow the injection pressure to dissipate and prevent reflux.
2.4. Fecal output assay
Animals in the longest survival group (12 months) were evaluated monthly for changes in colonic motility (Devries et al., 2010). Rats were food deprived for 24 h prior to the start of the experiment. Rats were housed singly for a 12-h period, during which time fecal pellets were collected, counted, and weighed every 3 h. Following the final collection, the total fecal matter collected from each animal was dried overnight at 60 °C to remove water and weighed a final time in order to calculate water content of feces.
2.5. NHP surgeries
All non-human primate work was conducted in accordance with Rush University Medical Center Institutional Animal Care & Use Committee. NHPs (Macaca fascicularis) were tranquilized with ketamine (10 mg/kg) and then anesthetized with isoflurane (1–2%). Under sterile conditions, a midline incision was made and the stomach and intestines were exteriorized. Injections of PFF’s (n = 4), monomer (n = 2), AAV2/5-alpha syn A53T (n = 4), AAV2/5-GFP (n = 3), or saline vehicle (n = 2) were made both into the colon (9 injections) and stomach (1 injection). All injections were made in 10 μl volumes (2 μg/μl). Non-absorbable sutures were placed at the rostral and caudal margins of the injection sites to facilitate identification of the injection sites post-sacrifice. The organs were replaced into their original positions and the incision was closed in anatomical layers. All monkeys recovered uneventfully.
2.6. Tissue collection and processing-rat
1, 6, or 12 months following injections, rats were euthanized with a lethal injection of sodium pentobarbital and transcardially perfused with Tyrode’s solution. Whole mount colon tissue was collected and prepared as previously described (Benskey et al., 2015b), and small portions of the injected colon was dissected by separating the myenteric and submucosal layers. The brains were harvested and post-fixed in 4% paraformaldehyde (in TBS) for 72 h followed by cryoprotection in 30% sucrose. Brains were sectioned coronally in to 40 μm sections using a sliding stage microtome.
2.7. Tissue collection and processing-NHP
Twelve months post-surgery, all monkeys were anesthetized with pentobarbital (25 mg/kg, iv) and perfused with warm followed by ice cold saline. The stomach and intestines were removed and immersion fixed in 4% paraformaldehyde. The brain was removed and placed in ice cold saline, slabbed on a calibrated brain slice apparatus and immersion fixed in 4% paraformaldehyde.
2.8. Immunohistochemistry and proteinase K treatment
Monkey colon and stomach samples were collected near the injection site (demarcated by a suture in the mesentery). The collected monkey tissue was embedded in paraffin and sectioned in to 8 μm sections. Prior to immunohistochemical detection, paraffin embedded tissue was dewaxed and processed for antigen retrieval and removal of connective tissue (Fried and Gulbransen, 2015). Briefly, sections were rinsed in H2O and incubated in 10 mM sodium citrate buffer (pH 6.0) for 30 min at 80 °C, then cooled to room temperature for 30 min. Tissue was then rinsed in H2O and incubated in 150 U/ml (0.7 mg/ml) collagenase (Life Technologies, Grand Island, NY) and 1 U/ml (0.6 mg/ml) dispase II (Sigma, St. Louis, MO) in DMEM for 15 min.
All immunohistochemical detection in rats and monkey was performed as follows for colon and brain: sections were washed in TBS containing 0.5% Triton-× 100, incubated in 3% peroxide solution and blocked in 10% normal goat serum. Neurons within enteric ganglia were identified using the pan-neuronal marker HuC/D (1:2000; Invitrogen/Life Technologies, Grand Island, NY). Sections were also probed for α-synuclein using both a pan α-synuclein antibody (1:1000; BDbioscience, San Jose, CA) and an antibody that recognizes α-synuclein phosphorylated at serine 129 (1:10,000) (Waxman and Giasson, 2008; Volpicelli-Daley et al., 2011). Secondary antibodies used were Goat anti-mouse IgG2a Alexa Fluor 488(1:500; Invitrogen/Life Technologies, Grand Island, NY), Goat anti-mouse IgG1 Alexa Fluor 488(1:500; Invitrogen), Goat anti-mouse IgG2b Alexa Fluor 594(1:500; Invitrogen). In rAAV-GFP treated animals Alexa Fluor 350 was used in lieu of 488 (however, native GFP fluorescence was quenched due to the tissue processing). Sections were coverslipped using Vectorshield hard set mounting medium (VectorLabs, Burlingame, Ca).
Proteinase K treatment was performed on a subset of rat and monkey tissue prior to immunohistochemistry. Enteric tissue or free floating brain tissue sections (40 μm) was washed in PBS and treated in 1 mg/ml Proteinase K (Fungal, Invitrogen #25530015) in TE buffer (1 M Tris, 0.5 M EDTA at pH 7.5) at 25 °C for 10 min. Tissue was then processed for immunohistochemical detection of α-synuclein as described above.
Sections were imaged on a Nikon eclipse 90i fluorescence microscope connected to a Q-imaging fast 1394 camera (fluorescence microscopy) or a Nikon D-1 camera (brightfield microscopy). Confocal images were captured using a Nikon Ti Eclipse microscope and images were processed using the NIS elements software. Figures were assembled using Canvas 7SE (ACD Systems, Canada).
2.9. Quantitation of enteric α-syn immunoreactivity-rat
To quantify enteric α-syn immunoreactivity, whole mount tissue was analyzed using densitometric imaging. High magnification images of individual ganglia were obtained as described above using identical parameters. Images were thereafter exported to image J where individual ganglia were outlined for measurements of mean pixel intensity per area and subject to background subtraction. No statistical difference was observed between saline treated animals from different time points, thus, these data points were grouped together. Data is presented as mean intensity/ganglia and normalized against saline treatment.
2.10. Quantitation of enteric α-syn immunoreactivity-NHP
To quantify the fluorescence intensity of α-syn tissue was processed for immunofluorescent detection (as described above) of pan α-syn or S129+ α-syn using an alexafluor 488 secondary antibody and the neuronal marker HuC/D using an alexafluor 594 secondary antibody. High magnification images of enteric ganglia were obtained from several sections of stomach and colon. Images were acquired using a Nikon eclipse 90i fluorescence microscope connected to a Q-imaging fast 1394 camera using standardized detection settings that were optimized to avoid saturation in all channels. Ganglia with at least 5 visible neurons were included in the analysis. Individual neurons were outlined and Nikon NIS elements software was used to perform automated-unbiased pixel fluorescence intensity analysis in the 488 channel. Background measurements were performed in an area immediately adjacent to each ganglion. Data is presented as mean fluorescent intensity per ganglia, normalized to saline treatment.
2.11. Manual cell counting of DMN neurons
To quantify the number of neurons in the DMN, brainstem tissue was processed for immunohistochemical detection (as described above) of the pan-neuronal marker HuC/D. High magnification images of the DMN were obtained bilaterally, at the level of the central canal, from 3 to 4 adjacent sections per animal. The Image J cell counter plugin was used to manually count the number of HuC/D positive neurons within the anatomical boundaries of the DMN. The mean number of HuC/D+ DMN neurons per section was averaged over all sections within a single animal and presented as HuC/D+ neurons per section.
2.12. Statistical analysis
Colonic motility assays were performed by an experimenter blind to all experimental conditions. All statistical tests were performed using Statview and graphed using GraphPad Prism software (Version 7, GraphPad, La Jolla, CA). One-way analysis of variance (ANOVA) was used to detect differences in one independent variable between treatment groups. When appropriate, post-hoc comparisons were performed using the Bonferroni method. Statistical significance was set at p ≤ .05.
3. Results
3.1. Enteric inoculations of PFFs result in a transient reduction in colonic motility in the rat
Enteric dysfunction is a key component of Parkinson’s disease and here we wanted to determine whether pathological α-syn located solely in the ENS is sufficient to alter colonic motility, and whether colonic dysmotility would progress over time. Rats received injections to the descending colon with PFFs, monomeric α-syn, or saline, and colonic motility was assessed monthly for one year using total fecal pellet output and fecal water content as an indirect measure of colonic motility (Raffa et al., 1987). Both treatment with monomeric α-syn and PFFs resulted in a significant reduction in fecal water content one month after the inoculations as compared to saline treated animals at the same time point (Fig. 1A). Moreover, PFF treated rats also exhibited decreased fecal output; however, this was only significant 3 months following the injections (Fig. 1B). The effect of α-syn on colonic motility was transient, however, and no impact of treatment on colonic motility was seen with either treatment for the remainder of the study (1 year).
Fig. 1.
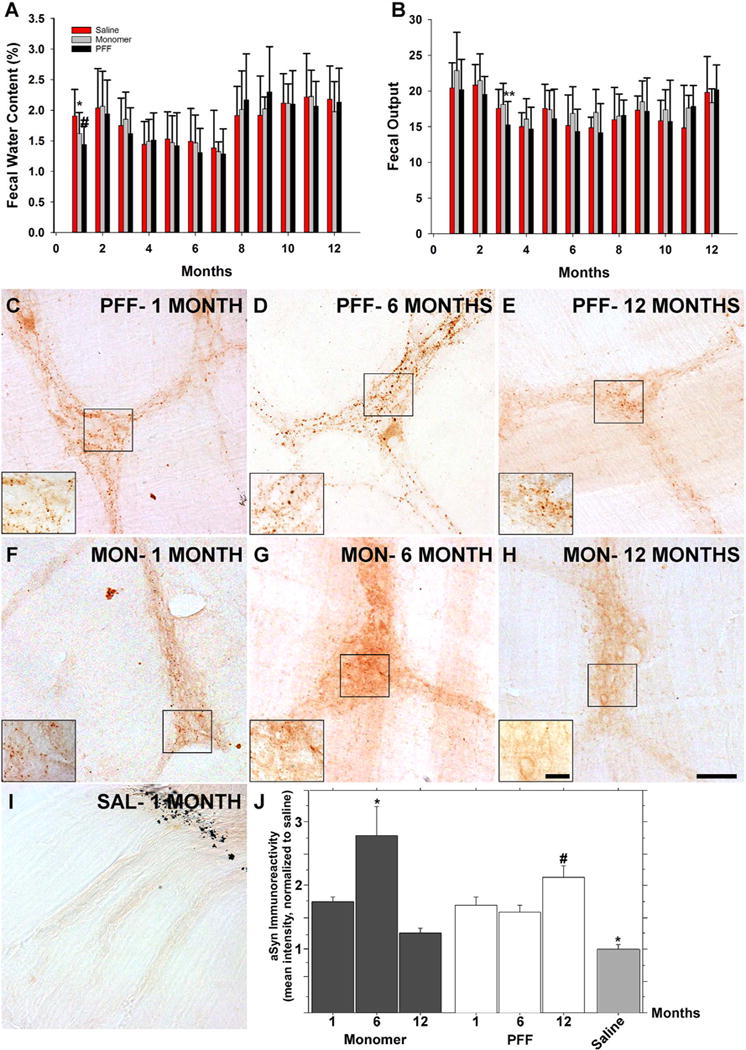
Colonic inoculations of α-syn results in a transient gastrointestinal phenotype but persistent gastrointestinal pathology Gastrointestinal motility was evaluated monthly in rats following treatment by measuring fecal water content (A) and total fecal output (B). A) Both PFF (black bars, n = 15) and monomer (grey bars; n = 15) treated animals exhibited a significant decrease in fecal water content one month after the treatment as compared to saline treated controls (red bars; n = 14), and reduced fecal output was seen in PFF treated animals three months after the inoculation (B). However, GI dysfunction normalized between groups over time (A,B). Endogenous α-syn immunoreactivity following PFF (C–E), monomer (F–H), or saline treatment (I) reveal a persistent punctate staining pattern that persists over 6 months in PFF treated animals, and with a decline between 6 (D) and 12 (E) months. F–H. The same pattern of immunoreactivity was observed to a lesser degree in monomer treated animals. I. No significant α-syn immunoreactivity was seen in saline treated controls. Black markings in upper right corner indicate the tattoo that was placed proximally to the injection zone. J. Quantitation of α-syn immunoreactivity. Error bars in A and B represents the mean + SD. Top graphs: *p = .012 versus saline, #p < .05 versus saline, **p < .05 versus all groups. Scale bar in H = 100 μm and applies to C-I. Scale bar in inset H = 20 μm and applies to insets C-H. MON = monomer. SAL = saline. Bottom graphs: *p < .05 versus 1 and 12 months, #p < .05 versus monomer 12-month time point, **p < .05 versus all groups except monomer 12-month time point.
3.2. Enteric inoculations of PFF results in persistent ENS α-syn pathology in the rat
We then wanted to evaluate the histopathological consequences following enteric α-syn delivery, and to assess whether the normalization of colonic motility was due to clearance of pathological α-syn from enteric neurons over time. A subset of rats were sacrificed 1, 6 or 12 months following the enteric inoculations, after which colonic whole mount preparations containing myenteric neurons were assessed for α-syn immunoreactivity. Rats inoculated with PFF exhibited a significant degree of punctate α-syn immunoreactivity (i.e. aggregated α-syn) within myenteric ganglia as well as throughout axon tracts radiating from these ganglia (Fig. 1C). As α-syn is typically presynaptically enriched (Iwai et al., 1995; Benskey et al., 2016a) it is likely that many of these distal aggregates coincide with myenteric varicosities. Roughly the same degree and distribution of α-syn immunoreactivity was seen in myenteric ganglia of subjects sacrificed 6 and 12 months after the inoculation (Fig. 1D, E, J). Monomer injected animals also exhibited pronounced punctate α-syn immunoreactivity at 1, 6, and 12 months following the injection (Fig. 1F–H). However, α-syn immunoreactivity showed a significant increase 6 months following the injection (Fig. 1G, J), but at 12 months levels returned to levels similar to that of the 1 month time point (Fig. 1H, J), and no different from saline injected controls. All other points analyzed demonstrated significantly higher α-syn immunoreactivity than that of control, suggesting that PFF and monomer treatment is capable of (at least temporarily) inducing endogenous α-syn expression (Fig. 1J). To confirm that the punctate α-syn immunoreactivity indeed represented insoluble inclusions, a subset of whole mount preparations were processed with Proteinase K and thereafter processed for α-syn immunohistochemistry. A significant degree of Proteinase K resistant α-syn was observed in both PFF (Fig. 2A–C, H) and monomer (Fig. 2D–F, H) treated animals, and these inclusions persisted for 12 months. Interestingly, the degree of proteinase K resistant α-syn immunoreactivity declined over time (Fig. 2H). However, at all time points the level of proteinase K resistant α-syn was higher than that of saline treated controls which exhibited very little α-syn immunoreactivity at any time point examined (Fig. 1I; Fig. 2G, H).
Fig. 2.
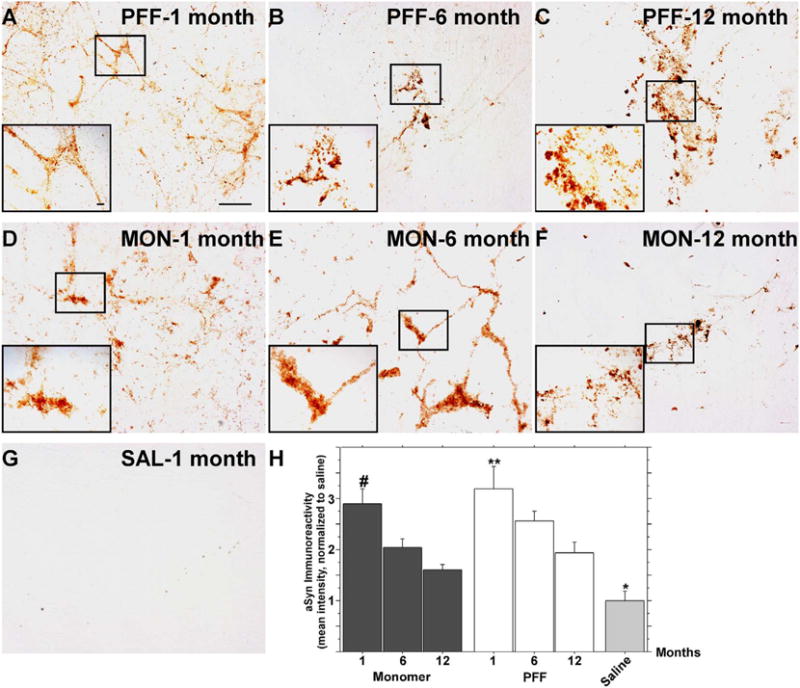
A single inoculation of α-syn fibrils and monomers in the rat ENS results in the persistence of insoluble, Proteinase-K resistant, α-syn aggregates. Rats received injections of either α-syn PFFs (A–C), α-syn monomers (D–F), or saline (G) to the descending colon. In order to determine whether the α-syn immunoreactivity shown in Fig. 1 represents “lewy-like” insoluble aggregates, enteric tissue was also analyzed for Proteinase-K resistant α-syn immunoreactivity. Proteinase-K resistant α-syn aggregates persisted within the ENS for up to one year following PFF (A-C) or α-syn monomer (D-F) delivery. No proteinase-K resistant α-syn was seen in saline treated controls (G). B. Quantitation of proteinase-K resistant α-syn immunoreactivity. #p < .05 versus monomer 6 and 12 month time points, **p < .005 versus PFF 12 month time point. *p < .05 versus all other points. Scale bar in A = 100 μm and applies to A–G. Scale bar in inset A = 10 μm and applies to insets A–G.
3.3. Enteric inoculations of PFF results in transient brainstem α-syn pathology in the rat
The presence of enteric (pathological) α-syn is observed in most Parkinson’s disease patients (Wakabayashi et al., 1990; Braak et al., 2006) and is thought to arise prior to the onset of motor symptoms (Shannon et al., 2012b). Accordingly, it has been postulated that certain forms of α-syn (e.g. fibrils) can spread from the ENS to the central nervous system where propagation occurs and pathology ensues. Thus, a key aim in this study was to assess whether enteric α-syn pathology would spread to the CNS. Our PFF injections were targeted to the descending colon where approximately 20% of ganglia are innervated by the vagus nerve (Berthoud et al., 1991; Altschuler et al., 1993). In order to determine whether pathological α-syn could spread from the ENS to the brainstem and beyond, a subset of rats were sacrificed 1, 6 or 12 months following the enteric inoculations with α-syn and evaluated for CNS pathology as measured by the detection of α-syn phosphorylated at serine 129 (S129); a modification commonly associated with pathological, Lewy body-associated, α-syn (Hasegawa et al., 2002). S129 phosphorylated α-syn was consistently observed in PFF treated subjects sacrificed one month after the enteric injection in a small number of neurons in the dorsal motor nucleus of the vagus (DMN; Fig. 3C). Moreover, we observed the presence of phosphorylated α-syn in a few cells in the locus coeruleus (Fig. 3J) at the same time point. It is worth noting that the degree of α-syn pathology was minor, and the extent of pathology did not resemble that seen with intracerebral administration of PFF (e.g. (Paumier et al., 2015); see Fig. 2K for an example of nigral pathology following striatal administration. No pathology was observed in monomer or saline treated animals (Fig. 3A, B). Importantly, this pathology was not observed at later time points (Fig. 3D–I). In order to verify the observed pathology, we utilized a second measure of pathological α-syn proteinase K treated tissue was processed for α-syn immunoreactivity. As seen with the S129 immunoreactivity, proteinase K resistant α-syn aggregates in the brain stem were present, but rather sparse (Sup. Fig. 2), and not detected anywhere else in the brain. The lack of abundant α-syn pathology at the 1-month time point, and the loss of α-syn pathology over time, was also consistent with the absence of overt cell loss in the DMN. Quantification of HuC/D+ neurons within the DMN did not reveal any change in the number of neurons within saline or PFF treated animals at any time point examined (Fig. 3L–N). Moreover, no neuronal loss was observed in the substantia nigra of PFF or monomer treated animals (Sup. Fig. 2).
Fig. 3.
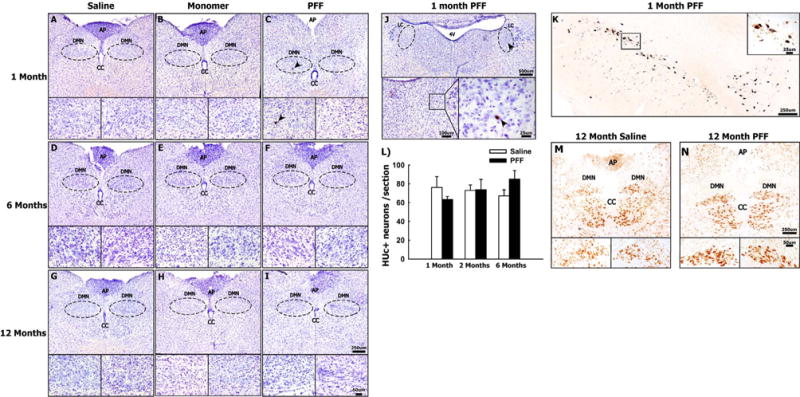
Colonic Injections of α-syn PFFs results in transient and minor α-syn accumulation in the brainstem Panels A-I show representative photomicrographs of serine 129 phosphorylated α-syn staining at the level of the DMN in 1, 6, and 12 month animals treated with saline (A,D,G), α-syn monomer (B, E, H) or α-syn PFF (C,F, I), respectively. Arrowheads in panel C indicates aggregated α-syn (S129+) within the DMN at one month following colonic injections of α-syn PFFs. Arrowheads in Panel J indicate positive detection of S129 phosphorylated α-syn within the locus coeruleus one month following injection of α-syn PFF. Panel K shows tissue used as a positive control for serine 129 phosphorylated α-syn immunoreactivity. Representative photomicrographs show S129+ aggregated α-syn within neurons of the SNc, one month following striatal injections of α-syn PFFs. Panel L shows quantification of neurons in the DMN using the pan-neuronal marker HUc. Columns in Panel L represent the mean number of HUc + neurons (normalized per sections counted) + 1 SEM (n = 3–4/group). Panels M&N show representative HUc staining at the level of the DMN in saline and PFF treated animals, 12 months post-injection (representing the time point at which we would expect to see the greatest degree of toxicity). Abbreviations: dorsal motor nucleus of the vagus (DMN), Area postrema (AP), central canal (CC), locus coeruleus (LC). Scale bar in the low and high magnification images of Panel I represent 250 μm and 50 μm respectively, and apply to panels A-H and L &M. Scale bars in low and high magnification images in Panel J represent 500 μm and 25 μm respectively. Scale bars in low and high magnification images in Panel K represent 250 μm and 25 μm, respectively.
3.4. Enteric inoculations of PFF does not result in spinal cord α-syn pathology in the rat
In addition to vagal innervation (Berthoud et al., 1991; Altschuler et al., 1993), the descending colon is also innervated by the lumbar splanchnic nerve. Accordingly, we wanted to determine whether α-syn PFFs were internalized by axon terminals originating within the intermediolateral nucleus of the spinal cord. Lumbar segments 1 and 2 of the rat spinal cord (sacrificed 1 month post injection) were processed for S129 immunoreactivity. However, no S129 immunoreactivity was seen in any sections (Sup. Fig. 3).
3.5. Enteric overexpression of α-syn in non-human primates results in neuronal α-syn pathology
Viral vector-mediated overexpression of α-syn is a popular means to model nigrostriatal neurodegeneration, wherein long-term overexpression results in aggregate formation, altered neuronal function, and ultimately neurodegeneration (Kirik et al., 2002; Lundblad et al., 2012; Gombash et al., 2013). Similarly, herein we utilized targeted delivery of rAAV directly to the ENS of the NHP stomach and colon (Benskey et al., 2015b; Benskey and Manfredsson, 2016) in order to overexpress the A53T disease mutant form of α-syn specifically in enteric neurons without the confound of transgene expression in other tissues. Importantly, this direct delivery method does not result in detectable vector genomes in the brainstem (as measured by qRT-PCR) (Benskey et al., 2015b). Titer matched rAAV expressing a GFP trans-gene was used as a control. One year following the vector delivery there was a clear presence of aggregated α-syn in enteric neurons throughout the ganglia of both the colon (Fig. 4P–T) and stomach (Fig. 5P–T) as indicated by the presence of S129 phosphorylated α-syn. No S129+ α-syn was detected in rAAV-GFP treated subjects (Fig. 4U–Z; Fig. 5U–Z)).
Fig. 4.
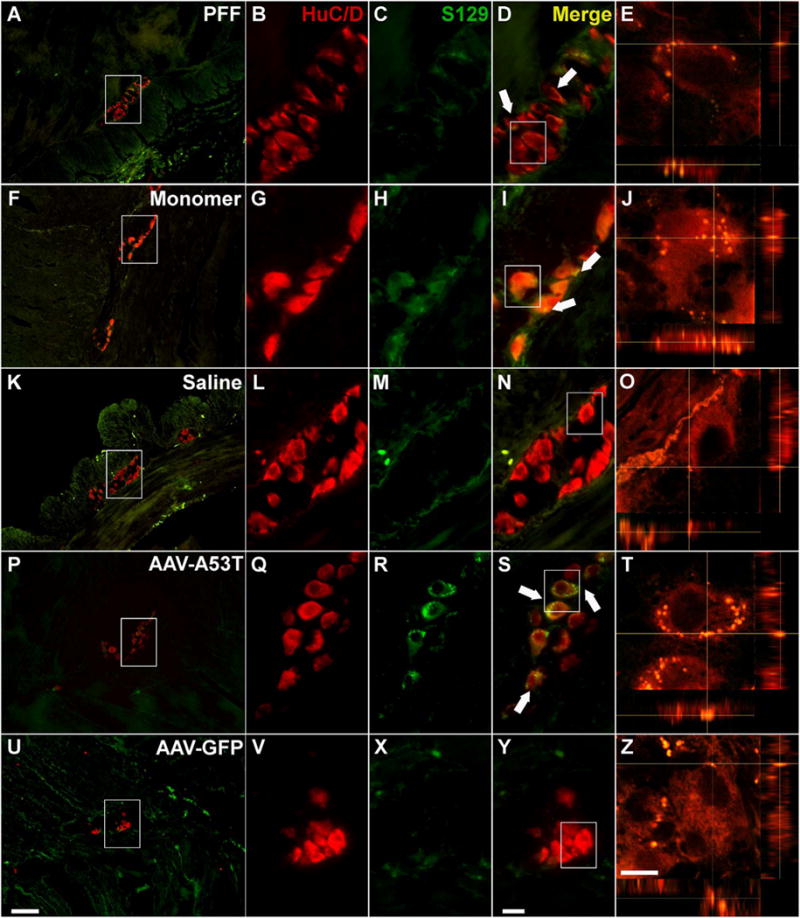
Alpha-synuclein pathology persists in the non-human primate colon one year following the inoculation Sections from the colon of NHPs treated with PFF (A–E), α-syn monomer (F–J), Saline (K–O), rAAV-A53T (P–T), and rAAV-GFP (U-Z) where double-stained with the pan-neuronal marker HuC/D (red) and serine 129 phosphorylated α-syn (green). A–E. PFF treatment was associated with the presence of intraneuronal S129+ α-syn inclusions, which was also observed in monomer treated subjects (F–J). No S-129+ α-syn was observed in enteric neurons in saline treated animals (K–O). P–T. rAAV-mediated overexpression of human α-syn A53T gave rise to a high degree of α-syn aggregates in enteric neurons; no S129 α-syn was seen in the AAV-GFP treated controls (U–Z). White arrows (D, I, S) indicate examples of S129+ neurons. Box in A, F, K, P, U outlines the area of magnification in B–D, G–I, L–N, Q–S, V–Y, respectively. Box in D, I, N, S, Y outlines the cell analyzed using confocal imaging in E, J, O, T, and Z, respectively. Scale bar in U = 100 μm and also applies to A,F,K, and P. Scale bar in Y = 20 μm and also applies to B-D, G-I, L-N, Q-S, V-Y. Scale bar in Z = 10 μm and also applies to E, J, O, T.
Fig. 5.
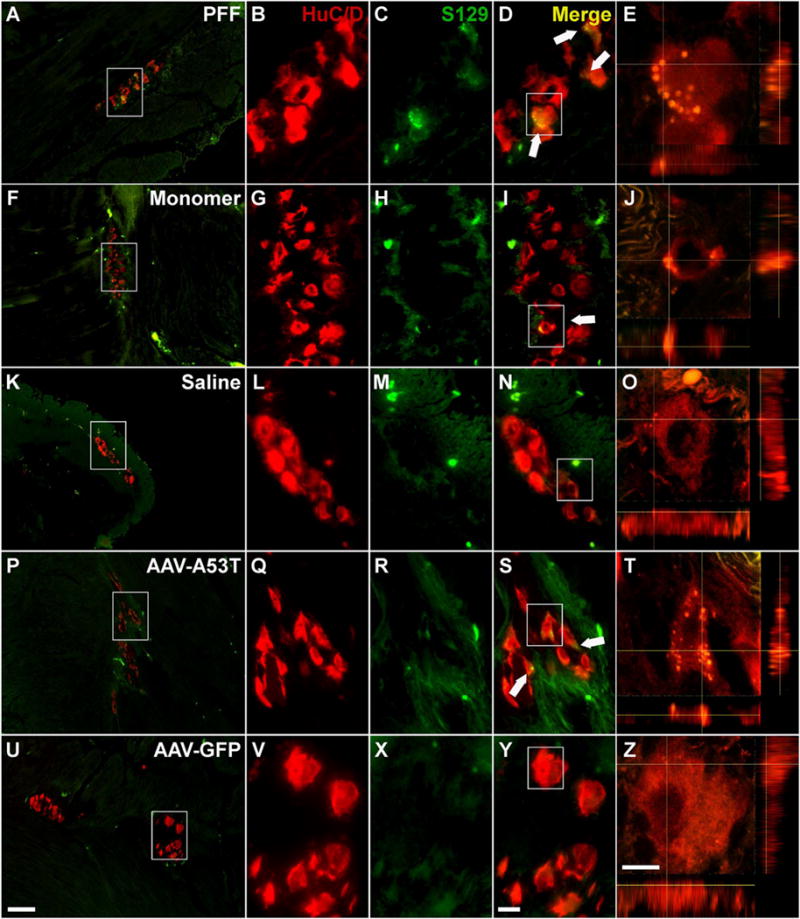
Alpha-synuclein pathology persists in the non-human primate stomach one year following the inoculation Representative photomicrographs of sections from the stomach from NHPs treated with PFF (A–E), α-syn monomer (F–J), Saline (K–O), rAAV-A53T (P–T), and rAAV-GFP (U–Z). Dual label fluorescence of the pan-neuronal marker HuC/D (red) and serine 129 phosphorylated α-syn (green) reveal a significant degree of intraneuronal S129+ α-syn aggregates with PFF treatment (A–E) and α-syn A53T overexpression (P–T), and to a lesser degree in subjects injected with α-syn monomeric protein (F–J). No S129+ synuclein was observed in either saline (K–O) or rAAV-GFP (U–Z) treated controls. Box in A, F, K, P, U outlines the area of magnification in B–D, G–I, L–N, Q–S, V–Y, respectively. Box in D, I, N, S, Y outlines the cell analyzed using confocal imaging in E, J, O, T, and Z, respectively. Scale bar in U = 100 μm and also applies to A,F,K, and P. Scale bar in Y = 20 μm and also applies to B–D, G–I, L–N, Q–S, V–Y. Scale bar in Z = 10 μm and also applies to E, J, O, T.
Immunofluorescent imaging of enteric tissue is inherently difficult due to the strong background fluorescence of the tissue(Smith, 2011). Consequently, additional tissue was processed for brightfield imaging using immunohistochemical development in order to confirm the findings. In congruence with the fluorescent imaging, A53T overexpression correlated with significant α-syn S129 immunoreactivity throughout the treated ENS (Sup. Fig. 4A, B). Similarly, evaluating the immunoreactivity of endogenous α-syn showed that a portion of α-syn appeared to be associated with aggregates with a punctate staining pattern observed in many cells (Sup. Fig. 4C, D, Sup. Fig. 5M–P, Sup. Fig. 6M–P). However, it was also clear that a detectable degree of diffuse α-syn was present in the soma of neurons of the stomach and colon in subjects treated with AAV-A53T (e.g. Sup. Fig. 4N–P); perhaps reflecting endogenous expression of α-syn. Interestingly, both the fluorescent and the brightfield analysis of Ser129 immunoreactivity in A53T expressing subjects demonstrated that a significant degree of phosphorylated α-syn was diffusely distributed throughout the soma and not limited to dense aggregate structures (e.g. Fig. 4Q–S).
3.6. Confirmation of vector transduction
Studies using rAAV in the CNS have demonstrated stable expression for decades following the delivery(Marks et al., 2016). However, such data for transduction of enteric neurons does not exist and up to this point we have only analyzed expression one month following vector delivery (Benskey et al., 2015b). Accordingly, vector transduction was confirmed by immunohistochemistry for GFP (control subjects). Numerous transgene positive neurons were observed in all ganglia surveyed (Sup. Fig. 7). Moreover, because of the degree of endogenous expression levels of α-syn it was not possible to detect the A53T transgene per se. Rather, we utilized PCR to confirm transduction (i.e. the presence of viral genomes) in all vector treated tissue (data not shown).
3.7. A single inoculation of α-syn PFFs or monomers into the NHP ENS result in persistent enteric pathology
As with α-syn overexpression, the inoculation of either monomeric recombinant mouse α-syn or PFFs correlated with the presence of aggregated (S129+ α-syn) in neurons throughout enteric ganglia of injected portions of the colon (Fig. 4) and the stomach (Fig. 5) 12 months following the inoculations Aggregated α-syn was not observed in saline treated control animals (Fig. 4K–O, Fig. 5K–O). Brightfield imaging confirmed these findings as S129+ neurons were observed throughout the enteric tissue (Sup. Fig. 4). Again, as was seen in A53T treated animals, following PFF injections a significant amount of S129+ α-syn was found in a punctate (i.e. aggregate) pattern and no S129 immunoreactivity was seen in saline controls. We also observed diffuse S129 α-syn immunoreactivity throughout aggregate containing cells (e.g. Fig. 4B–D, Fig. 5B–D). Immunofluorescent staining with a pan-α-syn antibody confirmed what was observed using the S129 antibody; Both PFF and monomer treatment was associated with both diffuse and punctate staining patterns throughout the neurons (Sup. Figs. 5, 6). This was also confirmed using brightfield immunohistochemistry as PFF and monomer treatment (Sup. Fig. 4G, H, K, L) was associated with a significant degree of both punctate and diffuse immunoreactivity. In contrast, endogenous α-syn expression was much less pronounced in saline treated animals, and no aggregated α-syn was seen in these animals using DAB immunohistochemistry (Sup. Fig. 4M–P). To confirm that the observed pathology indeed represents insoluble α-syn aggregates we also analyzed the tissue for proteinase K insoluble α-syn immunoreactivity; dense aggregates was found in ganglia throughout tissue from all α-syn treatments, but not in saline treated tissue (Sup. Fig. 8).
3.8. Comparison between treatments
In order to better gauge the extent of pathology with each treatment, a quantitative histological analysis was performed. As expected, a comparison between the various treatment groups demonstrated an increase in α-syn expression as a result of A53T vector delivery in both the colon (Sup. Fig. 9A; p < .005 versus all groups) and the stomach (Sup. Fig. 9B; p < .05 versus monomer and saline, p = .07 versus PFF). However, no difference was seen between PFF/monomer treatment and saline control, suggesting that the protein treatment had no effect on endogenous expression at the 12 month time point. No difference in overall α-syn expression was found between stomach and colon. The same analysis was performed with the S129 antibody. In the colon, A53T overexpression was associated with significantly higher S129 immunoreactivity as compared to both PFF and saline treatment (Sup. Fig. 9C; p < .01; p = .07 versus monomer). Moreover, both PFF and monomer injections resulted in significant α-syn pathology as compared to saline control (Sup. Fig. 9C; p < .01). Similarly, all synuclein treatments correlated to elevated S129 immunoreactivity in stomach neurons (Sup. Fig. 9D; p < .05).
3.9. CNS histology-NHP
As for the rat 12-month time-point, no S129 α-syn immunoreactivity was seen in any nucleus across the neuraxis in the NHP, including the DMN (Fig. 6). In contrast, robust staining was seen in positive control tissues from other studies in which rAAV-α-syn or PFF’s were injected into the monkey CNS (Sup. Fig. 10).
Fig. 6.
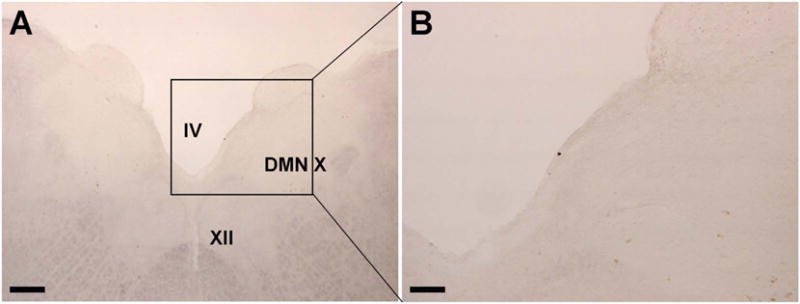
Lack of brainstem alpha-synuclein pathology in the non-human primate brain 12 months after the enteric inoculations. Low (A) and medium (B) power photomicrographs demonstrating the lack of serine 129 phospho-α-syn staining in a subject that received PFF injections in to the ENS of the stomach and colon 12 months prior. IV = fourth ventricle; XII = hypoglossal nucleus; DMN X = dorsal motor nucleus of the vagus nerve. Scale bar in A = 400 μm. Scale bar in B = 100 μm.
4. Discussion
Here we demonstrate that the inoculation of pathological α-syn into the enteric nervous system of the rat results in a transient GI phenotype, with persistence of α-syn pathology in enteric neurons for as long as one year following the treatment in both rats and NHPs. Moreover, we observed some α-syn pathology in the brainstem of PFF treated rats one month following the injection. However, this pathology was not observed at later time points in either species, suggesting clearance of pathological α-syn and a lack of any significant spread.
4.1. Enteric α-syn pathology causes GI dysfunction
Numerous reports have described the presence of (pathological) α-syn in the ENS of Parkinson’s disease patients (Wakabayashi et al., 1988; Wakabayashi et al., 1990; Braak et al., 2006; Beach et al., 2010). However, it is yet not clear whether this enteric α-syn is directly responsible for the GI dysfunction seen in Parkinson’s disease patients, or whether it is an unrelated epiphenomenon. α-syn transgenic mice exhibit a GI phenotype (Kuo et al., 2010; Hallett et al., 2012; Wang et al., 2012; Farrell et al., 2014). However, because of the widespread α-syn brain and spinal cord pathology it is not possible to draw the conclusion that enteric α-syn pathology is the root cause of GI dysfunction in these mice. Herein, we describe, for the first time, that pathological forms of α-syn delivered directly to the ENS cause a GI phenotype in the form of reduced colonic motility. Although the DMN certainly is a crucial component of normal GI function (Pagani et al., 1985; Travagli and Anselmi, 2016) the sporadic α-syn pathology observed, and the lack of neuronal loss, argues against a CNS-mediated component to the GI phenotype that was observed in this study.
Data collected from Parkinson’s disease patients suggest that enteric α-syn pathology does not correlate with neuronal loss in the ENS (Annerino et al., 2012); accordingly, it is unlikely that neuronal loss is the root cause of GI dysfunction. Our long-term data collected from NHPs are in agreement with this observation as we did observe profound α-syn pathology (i.e. “Lewy-like” pathology in surviving neurons) without any overt enteric neuron loss. This suggests that the aggregates themselves ultimately serve to induce functional changes in enteric neurons. We have previously postulated that α-syn aggregation may act to sequester soluble (i.e. functional forms) of α-syn, depleting the cell of this protein (Benskey et al., 2016a). This phenomenon has been observed in a number of in vitro and in vivo models (Cali et al., 2012; Osterberg et al., 2015). Interestingly, our data suggest that aggregation itself does not correlate well with a loss of soluble α-syn, rather, we observed an apparent increase in α-syn immunoreactivity between 1 and 6 months following the injections. Moreover, S129+ synuclein was observed in a diffuse pattern in enteric neurons both with immunofluorescent and immunohistochemical imaging. It is possible that the pronounced aggregation of α-syn resulted in a compensatory increase in expression of endogenous α-syn, ultimately resulting in a functional phenotype.
4.2. Persistent α-syn pathology of the ENS does not result in sustained pathology of the CNS
A central tenet of the prion hypothesis of α-syn suggests that pathological forms of α-syn exists in a perpetual cycle of 1) neuronal uptake, 2) retrograde transport within the neuron, 3) seeding of endogenous α-syn to pathological forms within the recipient cell, and 4) subsequent propagation of the pathology to recipient cells (Luna and Luk, 2015). This theory is very powerful as it holds great explanatory power in regards to the temporal presentation of α-syn pathology and symptoms in Parkinson’s disease patients (Braak et al., 2003a). One aim with our study was to determine whether trans-synaptic spread readily takes place, or whether axonal transport is the chief mechanism whereby α-syn moves from the periphery to the brain. For the rodent studies we thus chose an area with minimal, but with some vagal innervation. This would allow us to track the expansion/progression of α-syn pathology over time. On the other hand, in the NHP cohort we also injected the subjects in to the stomach ENS, thus, providing for a much higher degree of exposure to vagal innervation. In our approach we observed uptake of recombinant α-syn protein into enteric neurons (rat and NHP), aggregate formation in enteric neurons (rat and NHP), and shortly following the injection we observed pathological α-syn in the rodent brainstem, suggestive of uptake and transport of the protein via the vagal nerve. This finding is in agreement with prior findings which have described transport of α-syn via the vagus nerve from its injection site in the stomach wall to the DMN (Holmqvist et al., 2014). These studies however, did not look at the later time points evaluated herein (6 days was the longest time point evaluated in the prior work). The difference in pathology seen between these 2 studies is thus likely the result of a temporal pattern of clearance of the retrogradely transported α-syn which originated from the single injection bolus and was taken up directly by vagal afferents within the colon. It is unlikely that the pathological α-syn observed in brainstem nuclei originated from enteric neurons and was subsequently propagated to CNS neurons (i.e. it is unlikely that α-syn was first endocytosed by enteric neurons and that pathological α-syn was thereafter transmitted to the vagus nerve from these neurons) since we observed no sustained brainstem pathology 12 months after the injections, despite the persistence of robust enteric α-syn pathology over time. Along those lines, because we observed no pathology in Parkinson’s disease -relevant structures, and because the limited α-syn pathology that was observed in the CNS diminished over time, our findings argue that the transported pathology is not sufficient to induce, nor sustain, a Parkinson’s disease -like pathology in the brain (e.g. induce propagation to the substantia nigra). Taken together, our findings thus suggest that α-syn pathology in enteric neurons is not the source of CNS pathology in disease.
Our findings pose several alternate possibilities to the ENS-CNS prion hypothesis of Parkinson’s disease. 1) A single bolus of pathological α-syn in the ENS is not sufficient to facilitate propagation. This is an unlikely scenario as overexpression (i.e. continuous production of pathological α-syn) also failed to elicit CNS pathology. Moreover, the concentration of PFFs injected in this study mirrored that in other studies performed in the PNS (Holmqvist et al., 2014) or the CNS (Luk et al., 2012a; Paumier et al., 2015). 2) Additional factors are required for the propagation of Parkinson’s disease -like α-syn pathology. Our studies outlined herein did not incorporate crucial factors such as aging (main risk factor for Parkinson’s disease (Collier et al., 2011)), a concurrent disease state, or systemic issues such as inflammation (Lema Tome et al., 2013; Kelly et al., 2014). It is possible that future studies incorporating additional factors such as these will be able to elicit propagation of α-syn from the ENS to the CNS. 3) ENS-CNS spread takes place, but over a much longer time span. It is thus important to note that it may be difficult to faithfully model the natural progression of human synucleinopathies in animal models. Although we utilized a long-time survival in this study, one year may not be representative of the time span required for α-syn to spread in Parkinson’s disease. Indeed, enteric dysfunction can be observed many years prior to CNS pathology (Ross et al., 2012), and it is possible that the spread and progression of pathogenic α-syn requires decades as opposed to years. Studies have found that a full truncal vagotomy is associated with a lower risk of developing Parkinson’s disease (Svensson et al., 2015). However, subsequent analyses of the same data set, as well as other studies failed to replicate this finding (Tysnes et al., 2015; Liu et al., 2017). It is important to note, however, that the later Tysnes study did not distinguish patients that were diagnosed immediately after the procedure from those that developed Parkinsonism much later (Tysnes et al., 2015). Thus, this analysis did not take in to account existing brain pathology at the time of vagotomy (see (Borghammer, 2018)). Regardless, the finding that the brainstem pathology that we observed early following the injections disappeared without spreading speaks against this point. 4) Propagation from the peripheral nervous system to the CNS does not take place; instead enteric and central α-syn pathology are two distinct phenomena which occur in Parkinson’s disease patients on different time lines. Perhaps they are related to a common intrinsic state of susceptible neurons in Parkinson’s disease but they cannot be directly connected. From this point of view, it is likely that Parkinson’s disease, and the synucleinopathy observed in Parkinson’s disease, represent a systemic disease state affecting many tissues throughout the body, and that any differential susceptibility of separate groups of neurons to α-syn pathology may represent differential functional reserves between neurons/circuits affected in Parkinson’s disease (Engelender and Isacson, 2017). This is supported by pathological reports showing that there is rarely a perfect progression of a-syn pathology from ENS to brain (or within the brain, pathology does not always progress in an ascending pattern) (Beach et al., 2009; Beach et al., 2010). Rather, more often than not, α-syn pathology is observed in many places concomitantly. 5) Propagation of α-syn pathology is a receptor-mediated event, and enteric neurons do not express these receptors. Recent advances in the fields has identified transmembrane proteins which are responsible for the endocytosis of PFF; lymphocyte-activation gene 3 (LAG3) and transmembrane protein 9 superfamily member 2 (TM9SF2) (Mao et al., 2016; Wadman, 2016). Little is known about TM9SF2, and although LAG3 is expressed in CNS neurons, it is unclear whether this protein is also expressed in the ENS or on distal vagal nerve endings. Indeed, recent findings from the Di Monte group indicated that α-syn pathology (via AAV-α-syn overexpression) can spread from midbrain nuclei to the DMN throughout the vagal innervation of the ENS, but no pathology was observed in enteric neurons despite high levels of ectopic α-syn in the vagus nerve (Ulusoy et al., 2017). Alternatively, it is possible that enteric neurons in the colon do not express high enough levels of endogenous α-syn to effectively seed aggregates. It is clear from studies in the CNS that PFF-mediated pathology is dependent on endogenous expression of α-syn, where PFF-induced pathology is not observed in neurons of α-syn knockout mice (Luk et al., 2012b; Volpicelli-Daley et al., 2014). However, α-syn expression is present throughout the gastrointestinal tract in both humans and rodents, including the colon (Phillips et al., 2013; Barrenschee et al., 2017), thus a lack of endogenous α-syn is likely not the cause of our findings. 6). α-syn propagation requires a high degree of vagal innervation. In the rodent study we targeted an area with minimal vagal innervation; the descending colon. It is conceivable that sustained propagation of α-syn pathology from the periphery to the brain originates form an area with a higher degree of innervation. Nevertheless, in this study NHPs were also injected in to the stomach, with the same lack of sustained brain pathology.
4.3. What is the role of enteric α-syn pathology in Parkinson’s disease?
One important outcome of this study is the finding that pathological α-syn, almost exclusively localized within enteric neurons, induced a GI phenotype in the absence of overt neurodegeneration. Although the reduction in colonic motility was transient in nature, this phenotype was likely an early response to the protein injections, and the recovery in colonic motility may be the result of the dwindling pathology that we observed. Indeed, in parallel studies in rodents we have found that continuous α-syn overexpression (via AAV) in enteric neurons results in long-term dysmotility (Benskey et al., 2015a). Our finding is not entirely surprising as there is no loss of enteric neurons observed in Par-kinson’s disease (Annerino et al., 2012). Importantly, the induction of α-syn pathology in non-dopaminergic neurons in the CNS can have profound effects on neuronal function and neurotransmission, without neurodegeneration (Caudal et al., 2015; Alvarsson et al., 2016). For instance, a chief role of α-syn seems to be that of a “brake” in vesicular neurotransmission (Benskey et al., 2016a). Thus, it is possible that perturbation in α-syn function (e.g. increased α-syn, pathological α-syn) can cause an imbalance between vesicular and nitrergic non-vesicular transmission, a phenomenon which could explain the GI dysmotility. Nevertheless, the precise role of (pathological) α-syn in GI function remains unknown and should be a focus of future research efforts.
Equally important to address is the genesis of α-syn pathology in the ENS in Parkinson’s disease. As mentioned above, the aging neurons of Parkinson’s disease patients may exhibit properties making them prone to α-syn aggregation regardless of anatomic location; but again, these events are not necessarily connected. For instance, increased levels of expression, or a shift in the subcellular localization of α-syn to the soma, will enhance cellular crowding and promote aggregation (Uversky et al., 2002). In addition, a significant focus has been placed on the microbiota and other extrinsic factors affecting the state of α-syn in enteric neurons. For instance, recent findings drew the correlation between specific GI microbiota and CNS disease progression in an α-syn transgenic mouse (Sampson et al., 2016). Although the underlying cause of this phenomenon is yet to be elucidated and likely multi-factorial; the authors demonstrated that the increased production of short-chain fatty acids mediated, at least in part, the CNS pathological process in these mice(Sampson et al., 2016). Importantly, short-chain fatty acids can cross the blood brain barrier. Thus, although there may not be a direct physical connection between ENS and CNS α-syn pathology in Parkinson’s disease, these pathologies may be linked via “endocrine-like” mechanisms.
5. Conclusion
In conclusion, our observations suggest that spread of α-syn pathology from enteric neurons to the brain is extremely limited in its scope, and not sufficient to sustain pathological spread of α-syn throughout the CNS on its own. Our findings give pause to the idea that the ENS is the original source of CNS pathology in Parkinson’s disease, or indicate that factors in addition to exposure to pathological α-syn may be required for efficient transmittal to the CNS. Nevertheless, our findings do not diminish the importance of the ENS in the Parkinson’s disease disease progress. Rather, we observed that enteric α-syn pathology is sufficient to elicit colonic dysmotility without the influence of central input. Given that GI symptoms are so prevalent in Parkinson’s disease, our findings thus emphasizes the importance of focusing on enteric neuron function as treatments for these devastating comorbidities are being therapeutically investigated.
Supplementary Material
Acknowledgments
We thank Dr. James J. Galligan for critical reading of this manuscript. We thank Dr. Caryl Sortwell for providing cryoprotected rat PFF treated brain tissue as a positive control for α-syn S129 staining. This work was supported by NIH R01DK10879801A1 & DOD W81XWH1610676 (FPM).
Funding sources
NIH R01DK10879801A1 & DOD W81XWH1610676 (FPM).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2018.01.008.
Author contributions
FPM and JHK conceived, and oversaw the execution, of these experiments. KCL provided recombinant protein and PFFs. JG performed rat injections and colonic motility assays. MJB performed the CNS histology of rats. AG and AOM performed enteric histology of rats. IMS assisted with vector preparation and in situ vector analysis. JRP performed the EM. NCK and RY performed all enteric analyses of NHP tissue.
Financial disclosure/conflict of interest
None.
References
- Abdelmotilib H, Maltbie T, Delic V, Liu Z, Hu X, Fraser KB, et al. alpha-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic neurodegeneration. Neurobiol Dis. 2017;105:84–98. doi: 10.1016/j.nbd.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104(2):502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- Alvarsson A, Caudal D, Bjorklund A, Svenningsson P. Emotional memory impairments induced by AAV-mediated overexpression of human alpha-synuclein in dopaminergic neurons of the ventral tegmental area. Behav Brain Res. 2016;296:129–133. doi: 10.1016/j.bbr.2015.08.034. [DOI] [PubMed] [Google Scholar]
- Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124(5):665–680. doi: 10.1007/s00401-012-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrenschee M, Zorenkov D, Bottner M, Lange C, Cossais F, Scharf AB, et al. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol Commun. 2017;5(1):1. doi: 10.1186/s40478-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Corbille AG, Letournel F, Kordower JH, Kremer T, Munoz DG, et al. Multicenter assessment of Immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J Park Dis. 2016;6(4):761–770. doi: 10.3233/JPD-160888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Manfredsson FP. Gene therapy of the peripheral nervous system: the enteric nervous system. Methods Mol Biol. 2016;1382:263–274. doi: 10.1007/978-1-4939-3271-9_19. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, Kuhn NC, Galligan JJ, Garcia J, Boye SE, Hauswirth WW, et al. AAV delivery of alpha synuclein to the enteric nervous system impairs colonic motility. Cell Transplant. 2015a;24(4):753. [Google Scholar]
- Benskey MJ, Kuhn NC, Galligan JJ, Garcia J, Boye SE, Hauswirth WW, et al. Targeted gene delivery to the enteric nervous system using AAV: a comparison across serotypes and capsid mutants. Mol Ther J Am Soc Gene Ther. 2015b;23(3):488–500. doi: 10.1038/mt.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Perez RG, Manfredsson FP. The contribution of alpha synuclein to neuronal survival and function implications for Parkinson’s Disease. J Neurochem. 2016a;137(3):331–359. doi: 10.1111/jnc.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Sandoval IM, Manfredsson FP. Continuous collection of adeno-associated virus from producer cell medium significantly increases total viral yield. Hum Gene Ther Methods. 2016b;27(1):32–45. doi: 10.1089/hgtb.2015.117. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Phys. 1991;260(1 Pt 2):R200–7. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33(1):48–57. doi: 10.1002/mds.27138. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003a;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 2003b;110(5):517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Brahic M, Bousset L, Bieri G, Melki R, Gitler AD. Axonal transport and secretion of fibrillar forms of alpha-synuclein, Abeta42 peptide and HTTExon 1. Acta Neuropathol. 2016;131(4):539–548. doi: 10.1007/s00401-016-1538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T, Ottolini D, Negro A, Brini M. alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem. 2012;287(22):17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudal D, Alvarsson A, Bjorklund A, Svenningsson P. Depressive-like phenotype induced by AAV-mediated overexpression of human alpha-synuclein in midbrain dopaminergic neurons. Exp Neurol. 2015;273:243–252. doi: 10.1016/j.expneurol.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao EJ, Zhang W, Lu Y, Liu R, Huang X, et al. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener. 2015;4(1):1. doi: 10.1186/2047-9158-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12(6):359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Selkoe D, Bartels T. New insights into cellular alpha-synuclein homeostasis in health and disease. Curr Opin Neurobiol. 2016;36:15–22. doi: 10.1016/j.conb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Devries MP, Vessalo M, Galligan JJ. Deletion of P2X2 and P2X3 receptor subunits does not alter motility of the mouse colon. Front Neurosci. 2010;4(22) doi: 10.3389/fnent.2010.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord. 1991;6(2):151–156. doi: 10.1002/mds.870060211. [DOI] [PubMed] [Google Scholar]
- Engelender S, Isacson O. The threshold theory for Parkinson’s disease. Trends Neurosci. 2017;40(1):4–14. doi: 10.1016/j.tins.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, et al. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain J Neurol. 2007;130(Pt 3):799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Farrell KF, Krishnamachari S, Villanueva E, Lou H, Alerte TN, Peet E, et al. Non-motor parkinsonian pathology in aging A53T alpha-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J Neurochem. 2014;128(4):536–546. doi: 10.1111/jnc.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried DE, Gulbransen BD. In situ Ca2+ imaging of the enteric nervous system. J Vis Exp. 2015;95 doi: 10.3791/52506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombash SE, Manfredsson FP, Kemp CJ, Kuhn NC, Fleming SM, Egan AE, et al. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS One. 2013;8(11):e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, McLean JR, Kartunen A, Langston JW, Isacson O. alpha-Synuclein overexpressing transgenic mice show internal organ pathology and autonomic deficits. Neurobiol Dis. 2012;47(2):258–267. doi: 10.1016/j.nbd.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, et al. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277(50):49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127(2):235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364(9440):1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, et al. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67(10):945–953. doi: 10.1097/NEN.0b013e318186de48. [DOI] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, et al. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14(2):467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Iwanaga K, Wakabayashi K, Yoshimoto M, Tomita I, Satoh H, Takashima H, et al. Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology. 1999;52(6):1269–1271. doi: 10.1212/wnl.52.6.1269. [DOI] [PubMed] [Google Scholar]
- Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, et al. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord. 2014;29(8):999–1009. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22(7):2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23(16):2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43(3):552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Goetz CG, Chu Y, Halliday GM, Nicholson DA, Musial TF, et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann Neurol. 2017;81(1):46–57. doi: 10.1002/ana.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19(9):1633–1650. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema Tome CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P. Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease–is there a link? Mol Neurobiol. 2013;47(2):561–574. doi: 10.1007/s12035-012-8267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, et al. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25(8):1091–1096. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- Li W, Englund E, Widner H, Mattsson B, van Westen D, Latt J, et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc Natl Acad Sci U S A. 2016;113(23):6544–6549. doi: 10.1073/pnas.1605245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88(21):1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338(6109):949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012b;209(5):975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Luk KC. Bent out of shape: alpha-Synuclein misfolding and the convergence of pathogenic pathways in Parkinson’s disease. FEBS Lett. 2015;589(24 Pt A):3749–3759. doi: 10.1016/j.febslet.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A. 2012;109(9):3213–3219. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307) doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WJ, Jr, Baumann TL, Bartus RT. Long-term safety of patients with Parkinson’s disease receiving rAAV2-neurturin (CERE-120) gene transfer. Hum Gene Ther. 2016;27(7):522–527. doi: 10.1089/hum.2015.134. [DOI] [PubMed] [Google Scholar]
- Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK. Progressive aggregation of alpha-synuclein and selective degeneration of Lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 2015;10(8):1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani FD, Norman WP, Kasbekar DK, Gillis RA. Localization of sites within dorsal motor nucleus of vagus that affect gastric motility. Am J Phys. 1985;249(1 Pt 1):G73–84. doi: 10.1152/ajpgi.1985.249.1.G73. [DOI] [PubMed] [Google Scholar]
- Park H, Lee JY, Shin CM, Kim JM, Kim TJ, Kim JW. Characterization of gastrointestinal disorders in patients with parkinsonian syndromes. Parkinsonism Relat Disord. 2015;21(5):455–460. doi: 10.1016/j.parkreldis.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(1):10–15. doi: 10.1016/j.parkreldis.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Martin FN, Billingsley CN, Powley TL. Alpha-synuclein expression patterns in the colonic submucosal plexus of the aging Fischer 344 rat: implications for biopsies in aging and neurodegenerative disorders? Neurogastroenterol Motil. 2013;25(9):e621–33. doi: 10.1111/nmo.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Mathiasen JR, Jacoby HI. Colonic bead expulsion time in normal and mu-opioid receptor deficient (CXBK) mice following central (ICV) administration of mu- and delta-opioid agonists. Life Sci. 1987;41(19):2229–2234. doi: 10.1016/0024-3205(87)90520-0. [DOI] [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18(Suppl. 1):S199–202. doi: 10.1016/S1353-8020(11)70062-1. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480 (e12). doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012a;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012b;27(6):709–715. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith K. Neurogastroenterology: improving 3D imaging of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2011;8(11):600. doi: 10.1038/nrgastro.2011.167. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79(6):940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- Sung HY, Park JW, Kim JS. The frequency and severity of gastrointestinal symptoms in patients with early Parkinson’s disease. J Mov Disord. 2014;7(1):7–12. doi: 10.14802/jmd.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- Tarutani A, Suzuki G, Shimozawa A, Nonaka T, Akiyama H, Hisanaga S, et al. The effect of fragmented pathogenic alpha-synuclein seeds on prion-like propagation. J Biol Chem. 2016;291(36):18675–18688. doi: 10.1074/jbc.M116.734707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13(7):389–401. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes OB, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, et al. Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol. 2015;78(6):1011–1012. doi: 10.1002/ana.24531. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, Rusconi R, Perez-Revuelta BI, Musgrove RE, Helwig M, Winzen-Reichert B, et al. Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol Med. 2013;5(7):1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, Phillips RJ, Helwig M, Klinkenberg M, Powley TL, Di Monte DA. Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 2017;133(3):381–393. doi: 10.1007/s00401-016-1661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, MC E, Bower KS, Li J, Fink AL. Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett. 2002;515(1–3):99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc. 2014;9(9):2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadman M. Rogue protein’s partners offer hope in Parkinson’s disease. Science. 2016;354(6315):956. doi: 10.1126/science.354.6315.956. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76(3):217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79(6):581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet MF, Tache Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport. 2008;19(8):873–876. doi: 10.1097/WNR.0b013e3282ffda5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Magen I, Yuan PQ, Subramaniam SR, Richter F, Chesselet MF, et al. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol Motil. 2012;24(9):e425–36. doi: 10.1111/j.1365-2982.2012.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008;67(5):402–416. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6(6):973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


