Abstract
It is widely known that there is a high prevalence of cigarette smoking in schizophrenia. One of the explanations is the self-medication hypothesis. Based on this hypothesis, it has been suggested that nicotine has procognitive effect or even neuroprotective effect in schizophrenia. However, cigarettes contain numerous neurotoxic substances, making the net effect of cigarette smoking on brain function and structure complex. Indeed, recent studies have called into question the self-medication hypothesis. We aimed to test whether there is an interaction between diagnosis and smoking status in gray matter volume, ie, whether smoking has specific effects on gray matter or whether main effects of these 2 variables additively affect common brain regions. Magnetic resonance imaging (MRI) images were obtained from 4 groups: (1) normal controls with no smoking history, (2) normal controls currently smoking and/or with a past history of smoking, (3) schizophrenia patients with no smoking history, and (4) schizophrenia patients currently smoking and/or with a past history of smoking. We used voxel-based morphometry to compare gray matter volumes among the 4 groups. We did not find any interaction between diagnosis and smoking, but we did find negative additive effects of schizophrenia diagnosis and smoking status in the left prefrontal cortex. The decrease in left prefrontal volume was associated with greater numbers of cigarette pack years and severe positive and negative symptoms. The current findings do not support the neuroprotective effect of smoking on gross brain structure in schizophrenia, emphasizing the necessity of longitudinal studies to test causal relationships among these variables.
Keywords: schizophrenia, smoking, voxel-based morphometry, gray matter, left prefrontal cortex
Introduction
According to a meta-analysis of 42 studies across 20 nations, on average, the rate of cigarette smoking in patients/persons with schizophrenia is 62%, which is much higher than the roughly 29% prevalence rate in the general population.1 A more recent survey in Japan reported that the rates of cigarette smoking in patients/persons with schizophrenia and the general population were 40.7% and 24.2%, respectively.2 One of the explanations for the high prevalence of cigarette smoking in schizophrenia is the self-medication hypothesis. Schizophrenia patients smoke in an attempt to self-medicate psychiatric and cognitive symptoms. This notion was supported by a body of preclinical studies demonstrating that nicotine has positive effects on various cognitive functions.3 Moreover, nicotine is reported to have a neuroprotective effect including an anti-inflammatory effect.4,5 However, cigarettes contain numerous harmful or neurotoxic substances besides nicotine, such as carbon monoxide. Therefore, the net effect of cigarette smoking on brain function and structure is complex. In fact, recent studies have called into question the self-medication hypothesis. A meta-analysis reported that cigarette smoking is associated with increased risk of psychosis, indicating the possibility of a causal link between smoking and psychosis.6 Boggs et al have shown that the effects of smoking abstinence and resumption on cognition in patients/persons with schizophrenia were minimal.7 Several cross-sectional studies have reported brain volume decreases in smokers without psychiatric disorders compared to nonsmokers.8–13 Although, because of the cross-sectional nature of the studies it is difficult to determine whether brain volume decreases are predisposing factors that lead to smoking or are the effects of chronic smoking, these studies and other circumstantial evidence suggest the possible negative effect of smoking on brain structure in smokers without psychiatric disorders.14 On the other hand, studies focusing on brain volume of smoking schizophrenia patients are limited and the results are mixed. A study has reported greater rather than smaller gray matter volumes in smoking schizophrenia patients compared to nonsmoking patients in temporal and lateral prefrontal cortices.15 The authors of this study suggested a neuroprotective effect of smoking in schizophrenia. However, a more recent study by Schneider et al16 reported smaller hippocampal and prefrontal volumes in smoking schizophrenia patients compared to nonsmoking patients.
Because neurobiological studies indicate that altered central nicotine receptors play a role in the pathophysiology of schizophrenia, the effect of smoking on the brain might be different between individuals with and without schizophrenia. As the self-medication hypothesis, or the study by Tregellas et al15 suggests, there could be antagonistic interaction between the diagnosis of schizophrenia and smoking status, ie, a positive or at least a less negative effect of smoking on brain structures could be observed in schizophrenia. On the other hand, synergistic interaction is also possible, namely, that a more pronounced negative effect of smoking could be observed in schizophrenia. To test whether there is an interaction or not, a 2 × 2 factorial design recruiting 4 groups (smokers with and without schizophrenia, nonsmokers with and without schizophrenia) is needed. To the best of our knowledge, there has been no study with 4 such groups to investigate gray matter volumes. One study investigating white matter integrity with 2 × 2 factorial design recruiting these 4 groups reported no interaction between diagnosis and smoking status.17 The study revealed that the main effects of schizophrenia diagnosis and smoking status were commonly associated with reduced white matter integrity in left anterior thalamic radiation. The authors of the study concluded that schizophrenia diagnosis and smoking status independently and additively affected white matter integrity in the region.
Here we examined gray matter volumes in 4 groups and tested whether there is interaction between diagnosis and smoking status, or whether the main effects of the 2 variables additively affect common brain regions.
Methods
Subjects
This study included 4 groups: (1) 20 normal controls with no smoking history, (2) 20 normal controls currently smoking and/or with a past history of smoking, (3) 30 schizophrenia patients with no smoking history, and (4) 30 schizophrenia patients currently smoking and/or with a past history of smoking. Hereafter, (1) normal controls with no smoking history are referred to as NC nonsmokers, (2) normal controls currently smoking and/or with a past history of smoking as NC smokers, (3) schizophrenia patients with no smoking history as SC nonsmokers, and (4) schizophrenia patients currently smoking and/or with a past history of smoking as SC smokers, in the text portion of this article, excluding quotes from previous studies. We asked all subjects about their age, gender, and handedness. Predicted IQ was measured by the Japanese Version of the National Adult Reading Test short form,18 which is considered to reflect the premorbid IQ of patients/persons with schizophrenia. Subjects with smoking history were asked how many cigarettes they smoked per day and how many years they smoked, and then we calculated the pack years (number of packs per day × number of years of smoking). Schizophrenia patients were referred to the Department of Psychiatry, Kyoto University Hospital. Each patient fulfilled the criteria for schizophrenia based on the Structural Clinical Interview for DSM-IV Axis I Disorders-Patients Edition version 2.0 (SCID-P). None of the patients were comorbid with neurological or other psychiatric disorders. We also asked them about medication and duration of illness. All patients were receiving antipsychotic medication. The medication dosage was converted to chlorpromazine equivalent according to the practice guidelines for the treatment of patients/persons with schizophrenia.19 Their symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS).20 Normal controls were also evaluated using SCID, and were found to have no history of neurological or psychiatric disorders.
This study was approved by the Committee on Medical Ethics of Kyoto University and was carried out in accordance with the Code of Ethics of the World Medical Association. After complete description of the study, written informed consent was obtained from all participants.
Magnetic Resonance Imaging Acquisition
All participants underwent magnetic resonance imaging (MRI) scans with a 3T whole body scanner equipped with an 8-channel phased-array head coil (Trio; Siemens). The scanning parameters of the T1-weighted 3-dimensional magnetization-prepared rapid gradient-echo (3D-MPRAGE) sequence were as follows: [TE] = 4.38 ms, [TR] = 2000 ms, [TI] = 990 ms, field of view = 225 × 240 mm2, 240 × 256 matrix, resolution = 0.9375 × 0.9375 × 1.0 mm3, slice number = 208.
Imaging Data Processing
T1-weighted images were analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Neuroscience) running on Matlab R2010b (MathWorks). We aimed to compare gray matter volumes between groups. We used an extension of SPM, and the VBM tools were written by Christian Gaser (VBM8; http://dbm.neuro.Uni-jena.de/vbm). The images were normalized and segmented into gray matter, white matter, and cerebrospinal fluid partitions in unified segmentation steps.21 The normalized and segmented images were resliced into 1 × 1 × 1 mm3 voxels and modulated by Jacobian determinates for nonlinear warping only. Then the modulated gray matter images were smoothed with a Gaussian kernel of 12 mm full-width at half-maximum, on which all the analyses were performed.
Demographic and Clinical Data
All data were analyzed using SPSS 23.0 (SPSS Inc.). We used 1-way ANOVA for age and the χ2 test for gender and handedness. Group differences of the mean scores of PANSS predicted by premorbid IQ, pack years, medication, and duration of illness were tested by Student t test, Mann-Whitney U test or Kruskal Wallis test as necessary. Differences were considered significant at P < .05 for all data.
Voxel-Based Morphometry Analysis
Using the full factorial model in the SPM menu, we conducted 2-way ANOVA to analyze the 2 factors of interest, smoking and schizophrenia. Age and gender were entered as nuisance covariates. The statistical significance level was set at P < .05, familywise error-corrected. First, the presence/absence of interaction was confirmed, and then the main effects of smoking and of schizophrenia were separately verified. To confirm the differences in gray matter volumes that were associated with main effect, smokers were compared to nonsmokers, and schizophrenia patients were compared to normal controls. The center of the region showing a volume decrease was expressed by MNI coordinates, and its range was expressed in voxels. The statistical significance level was set at P < .05, familywise error-corrected. MNI coordinates were transformed into Talairach coordinates using mni2tal.22 The Matlab script was written by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/).
Correlation Analyses
We set the region of interest (ROI) mask in the region where gray matter volume decrease was observed from the effect of both smoking and schizophrenia. This mask was derived using the WFU Pick Atlas (WFU Pickatlas v3.0; Wake Forest University School of Medicine). Using the volume of interest (VOI) function in SPM 8, we extracted the eigenvariate from the region. Correlation analyses were performed in SPSS 23.0 to investigate the association among eigenvariate, pack years, and PANSS.
Results
Demographic and Clinical Data
Demographic data, clinical measures and psychological tests are shown in table 1. Age, gender, handedness, and predicted premorbid IQ were matched between groups. There was a significant difference in pack years and a trend toward significant difference in PANSS Negative. We found no difference in medication, duration of illness, PANSS Positive and General.
Table 1.
Demographic and Clinical Data
| Normal Controls | Schizophrenia Patients | ||||
|---|---|---|---|---|---|
| Nonsmokers (n = 20) | Smokers (n = 20) | Nonsmokers (n = 30) | Smokers (n = 30) | P or χ2 Value | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 34.60 (8.90) | 35.50 (6.30) | 36.83 (9.24) | 39.23 (8.64) | .240a |
| Gender (male/female) | 12/8 | 17/3 | 17/13 | 21/9 | .144b |
| Handedness | 19/1 | 20/0 | 29/1 | 28/2 | .688b |
| Predicted premorbid IQ | 107.50 (6.77) | 106.90 (11.22) | 104.01 (9.35) | 102.10 (9.35) | .139c |
| Pack years | 9.25 (6.84) | 23.30 (19.54) | .012d | ||
| CPZ | 437.10 (293.58) | 624.70 (535.99) | .382d | ||
| Duration of illness | 11.15 (8.68) | 14.23 (7.81) | .100d | ||
| PANSS Positive | 14.27 (4.47) | 13.30 (4.60) | .412e | ||
| PANSS Negative | 17.20 (5.68) | 14.53 (5.22) | .063e | ||
| PANSS General | 31.47 (9.22) | 28.93 (9.13) | .321d | ||
Note: IQ, Intelligence Quotient; CPZ, chlorpromazine equivalent dose; PANSS, Positive and Negative Syndrome Scale.
a1-way ANOVA.
bChi-square test.
cKruskal Wallis test.
dMann-Whitney test.
eStudent t test.
Voxel-Based Morphometry
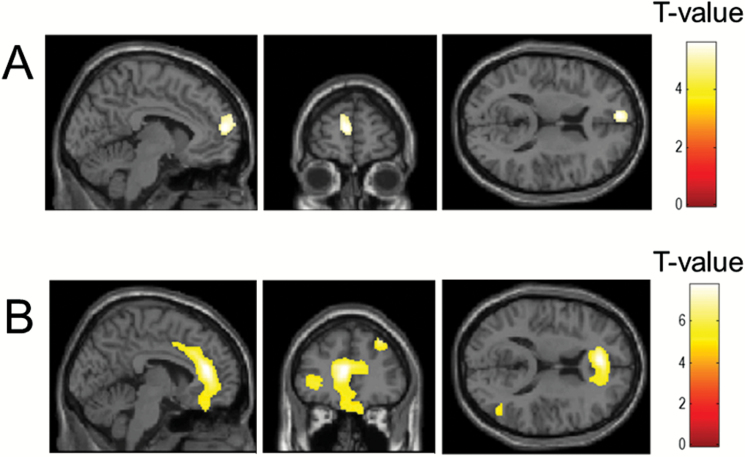
No interaction was found between smoking and schizophrenia. The main effect of smoking was found in the left prefrontal cortex (supplementary table 1). The main effect of schizophrenia was found in the left prefrontal cortices, left anterior cingulate cortex, left and right hippocampus and right insula (supplementary table 1). To verify the difference in volume of the regions showing the main effects, smoking subjects were compared to nonsmoking subjects, and schizophrenia patients were compared to normal controls. In smoking subjects, there were volume decreases in the left prefrontal cortex compared to nonsmoking subjects (figure 1A, supplementary table 2). In schizophrenia patients, the volumes of the left prefrontal cortices, left anterior cingulate cortex, left and right hippocampus, right insular and right temporal cortex were decreased compared to those in normal controls (figure 1B, supplementary table 2).
Fig. 1.
(A) Decreased gray matter volume in smokers (n = 50) compared to nonsmokers (n = 50). (B) Decreased gray matter volume in schizophrenia patients (n = 60) compared to normal controls (n = 40). Statistical significance level was set at P < .05, familywise error corrected. Both color bars indicated T value from 0 to 7.
Correlation Analysis
A volume decrease suggestive of the effects of smoking and schizophrenia was found to have manifested in the left prefrontal cortex. Since there was no interaction between these 2 factors, their effects were considered to be independent of each other, although additive effects of these factors might have been present. Therefore, to investigate effects on the gray matter of schizophrenia and smoking in the left prefrontal cortex, we first extracted the eigenvariate of the voxels within the cluster identified in left prefrontal cortex mask. Correlations among the volume of the left prefrontal cortex (eigenvariate), pack years, and PANSS were analyzed by Pearson’s correlation coefficient. Statistical values were regarded as significant at P < .05.
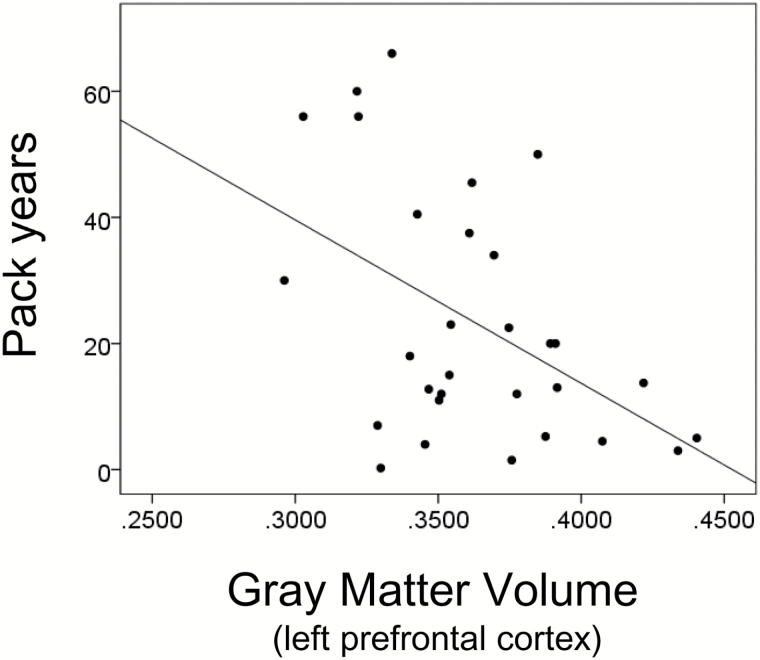
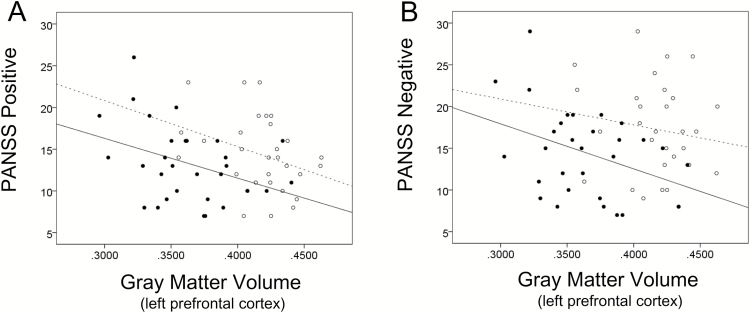
In SC smokers, pack years correlated positively with PANSS Positive (r = .596, P = .001), PANSS Negative (r = .364, P = .048), and PANSS General (r = .363, P = .049) Next, the left prefrontal cortex volume correlated negatively with pack years (r = −.473, P = .008; figure 2), PANSS Positive (r = −.371, P = .044; figure 3A) and PANSS Negative (r = −.368, P = .045; figure 3B) in SC smokers. There was no significant correlation between this cortex volume and PANSS General (r = −.163, P = .388). In SC nonsmokers, the positive correlations between the left prefrontal cortex volume and PANSS Positive (r = −.320, P = .085) were close to levels of statistical significance. There was no significant correlation between the left prefrontal cortex volume and PANSS Negative (r = −.179, P = .344) and PANSS General (r = −.219, P = .244). There was no correlation between the left prefrontal cortex volume and pack years in NC smokers (r = −.179, P = .450). Our results suggest that in SC smokers, the smaller the volume of the left prefrontal cortex is, the greater the lifetime amount of smoking and the more serious the scores of PANSS Negative and Positive symptoms are.
Fig. 2.
Correlation between volume of left prefrontal cortex and pack years in smokers with schizophrenia (n = 30). Left prefrontal cortex volume correlated negatively with pack years (r = −.473, P = .008).
Fig. 3.
Correlation between volume of left prefrontal cortex and PANSS Positive and Negative scores in schizophrenia patients. In smokers with schizophrenia, left prefrontal cortex volume was correlated negatively with PANSS Positive (r = −.371, P = .044) (A) and PANSS Negative (r = −.368, P = .045) (B), while positive correlations between left prefrontal cortex volume and PANSS Positive (r = −.320, P = .085) were close to levels of statistical significance (A). There was no significant correlation between left prefrontal cortex volume and PANSS Negative (r = −.179, P = .344) (B). ○ and ...... are nonsmokers with schizophrenia (n = 30); ● and ------ are smokers with schizophrenia (n = 30).
Discussion
This study demonstrated that there was no interaction between diagnosis and smoking status in relation to regional gray matter volume. Instead, the main effects of schizophrenia diagnosis and smoking status were commonly and additively associated with smaller volume in the left prefrontal cortex. The lifetime amount of smoking was also suggested to be greater in patients who showed a relatively pronounced volume decrease in the left prefrontal cortex, suggesting that smoking is associated with exacerbation of schizophrenia symptoms.
Previous studies have demonstrated that the left prefrontal cortex is a region characterized by a volume decrease in smokers without psychiatric disorders compared to nonsmokers,8–13 and in schizophrenia patients compared to normal controls.23,24 A study focusing on white matter found schizophrenia and smoking to be independently and additively involved in reduced integrity, with left anterior thalamic radiation and anterior limb of the internal capsule that connects the striatum and the prefrontal cortex.17 Therefore, the lack of interaction between diagnosis and smoking and the additive volume decrease observed in the left prefrontal cortex in SC smokers seem to be similar to the white matter findings. Negative correlation between the lifetime amount of smoking and left prefrontal cortex volume was demonstrated in several previous studies of NC smokers,8,9 and our present study obtained similar results in SC smokers. Previous studies reported a negative correlation between pack years and PANSS Negative in SC smokers,25 and a negative correlation between prefrontal cortex and PANSS in schizophrenia patients (regardless of with or without smoking history).26 Therefore, the results of the current study indicate that 3 factors—left prefrontal cortex volume decrease, severer psychiatric symptoms, smoking habit—are closely inter-related. Along with the absence of an interaction between diagnosis and smoking, and the additive negative effect on prefrontal regions, our finding suggests that a neuroprotective effect of smoking on the gross brain structure seems unlikely.
The strength of the current study is that we recruited 4 groups (smokers with and without schizophrenia, nonsmokers with and without schizophrenia), which enabled us to test whether there is an interaction between diagnosis and smoking status. Another unique feature is our sample of Japanese schizophrenia smokers. It is reported that schizophrenia patients are highly comorbid with substance use disorders, with most of them having overlapped use of cigarettes, cannabis, cocaine, and so on.27 However, cannabis in particular is strictly prohibited in Japan and the comorbidity rate between its use and schizophrenia is extremely low. Thus, we were able to recruit schizophrenia smokers free of any substance abuse.
The present study has some limitations. First, the scores of PANSS Negative between SC smokers and SC nonsmokers did not match, ie, they were lower (indicating less severe symptoms) in SC smokers than in SC nonsmokers. Still, the left prefrontal cortex volume was significantly smaller in SC smokers than in SC nonsmokers. Therefore, a self-medication effect of nicotine on psychiatric symptoms might be possible. Indeed, some studies have reported the PANSS score of SC smokers to be lower than that of SC nonsmokers,25 although others have reported the opposite.28 Moreover, it has recently been reported that smoking has no self-medication effect, and that chronic habitual smoking instead tends to worsen cognitive impairment29 or negative symptoms.30 Therefore, the possible self-medication effect of smoking on psychiatric symptoms remains elusive. Second, smoking behavior prior to the examination was not strictly controlled in SC smokers, data of blood concentration of nicotine (cotinine) were not collected, and carbon monoxide exhalation was not monitored. Finally, and most importantly, because this was a cross-sectional study, we cannot determine whether prefrontal gray matter decrease is a predisposing factor that leads to smoking, whether it is a result of chronic smoking, or their combination. A longitudinal study that includes a smoking cessation group is highly recommended.
Conclusion
Our results indicated that schizophrenia patients who currently smoked and/or had a past history of smoking showed pronounced abnormalities in the left prefrontal cortex, reflecting the effect of smoking and schizophrenia. These structural abnormalities were also associated with their amount of smoking and severity of schizophrenia symptoms. Current findings do not support the neuroprotective or self-medication effect of smoking at least on the gross brain structure in schizophrenia, and at the same time, emphasize the necessity of longitudinal studies to test causal relationships among these variables.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by: The Japan Society for the Promotion of Science (Young Scientists A 23680045, Scientific Research A 24243061, 15H01690, B 15H04893, C 26461767, and S 22220003) and Grant-in-Aid for challenging Exploratory Research (16K13106); The Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (on innovative areas 23118004, 23120009, 16H06572, 16H01504); The Uehara Memorial Foundation; The Smoking Research Foundation; The Takeda Science Foundation; Kobayashi Magobei Memorial Foundation; and Japan Foundation for Aging and Health. A part of this study is the result of Development of BMI Technologies for Clinical Application carried out under the Strategic Research Program for Brain Sciences by MEXT and “Research and development of technology for enhancing functional recovery of elderly and disabled people based on non-invasive brain imaging and robotic assistive devices”, the Commissioned Research of National Institute of Information and Communications Technology, Japan.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. [DOI] [PubMed] [Google Scholar]
- 2. Shinozaki Y, Nakao M, Takeuchi T, Yano E. Smoking rates among schizophrenia patients in Japan. Psychiatry Res. 2011;186:165–169. [DOI] [PubMed] [Google Scholar]
- 3. Mudo G, Belluardo N, Fuxe K. Nicotinic receptor agonists as neuroprotective/neurotrophic drugs. Progress in molecular mechanisms. J Neural Transm (Vienna). 2007;114:135–147. [DOI] [PubMed] [Google Scholar]
- 4. Fond G, Berna F, Andrianarisoa M et al. Chronic low-grade peripheral inflammation is associated with severe nicotine dependence in schizophrenia: results from the national multicentric FACE-SZ cohort [published online ahead of print February 25, 2017]. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-017-0771-4 [DOI] [PubMed] [Google Scholar]
- 5. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord. 2012;27:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. 2015;2:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boggs DL, Surti TS, Esterlis I et al. Minimal effects of prolonged smoking abstinence or resumption on cognitive performance challenge the “self-medication” hypothesis in schizophrenia [published online ahead of print April 6, 2017]. Schizophr Res. doi:10.1016/j.schres.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brody AL, Mandelkern MA, Jarvik ME et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. [DOI] [PubMed] [Google Scholar]
- 9. Gallinat J, Meisenzahl E, Jacobsen LK et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. [DOI] [PubMed] [Google Scholar]
- 10. Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17:977–980. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan P, Shi H, Zhong J et al. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34:813–817. [DOI] [PubMed] [Google Scholar]
- 13. Fritz HC, Wittfeld K, Schmidt CO et al. Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology. 2014;39:2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–273. [DOI] [PubMed] [Google Scholar]
- 15. Tregellas JR, Shatti S, Tanabe JL et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophr Res. 2007;97:242–249. [DOI] [PubMed] [Google Scholar]
- 16. Schneider CE, White T, Hass J et al. Smoking status as a potential confounder in the study of brain structure in schizophrenia. J Psychiatr Res. 2014;50:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Stein EA, Hong LE. Smoking and schizophrenia independently and additively reduce white matter integrity between striatum and frontal cortex. Biol Psychiatry. 2010;68:674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–339. [DOI] [PubMed] [Google Scholar]
- 19. Davis JM. Dose equivalence of the antipsychotic drugs. J Psychiatr Res. 1974;11:65–69. [DOI] [PubMed] [Google Scholar]
- 20. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 21. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 22. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY:Thieme Medical Publishers; 1988. [Google Scholar]
- 23. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. [DOI] [PubMed] [Google Scholar]
- 24. Lui S, Deng W, Huang X et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. [DOI] [PubMed] [Google Scholar]
- 25. Misiak B, Kiejna A, Frydecka D. Assessment of cigarette smoking status with respect to symptomatic manifestation in first-episode schizophrenia patients. Compr Psychiatry. 2015;58:146–151. [DOI] [PubMed] [Google Scholar]
- 26. Tang J, Liao Y, Zhou B et al. Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS One. 2012;7:e40247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volkow ND. Substance use disorders in schizophrenia–clinical implications of comorbidity. Schizophr Bull. 2009;35:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krishnadas R, Jauhar S, Telfer S, Shivashankar S, McCreadie RG. Nicotine dependence and illness severity in schizophrenia. Br J Psychiatry. 2012;201:306–312. [DOI] [PubMed] [Google Scholar]
- 29. Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iasevoli F, Balletta R, Gilardi V, Giordano S, de Bartolomeis A. Tobacco smoking in treatment-resistant schizophrenia patients is associated with impaired cognitive functioning, more severe negative symptoms, and poorer social adjustment. Neuropsychiatr Dis Treat. 2013;9:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.