Abstract
Background
The declining transition rate to psychotic disorder and the increasing rate of nonpsychotic poor outcomes among subjects at clinical high risk (CHR) for psychosis have increased the need for biomarkers to predict remission regardless of transition. This study investigated whether mismatch negativity (MMN) predicts the prognosis of CHR individuals during a 6-year follow-up period.
Methods
A total of 47 healthy control (HC) subjects and 48 subjects at CHR for psychosis participated in the MMN assessment. The clinical statuses of the CHR subjects were examined at baseline and regularly for up to 6 years. The CHR subjects were divided into remitter and nonremitter groups, and the baseline MMN amplitudes and latencies were compared across the remitter, nonremitter, and HC groups. Regression analyses were performed to identify the predictive factors of remission, the improvement of attenuated positive symptoms, and functional recovery.
Results
CHR nonremitters showed reduced MMN amplitudes at baseline compared to CHR remitters and HC subjects. A logistic regression analysis revealed that the baseline MMN amplitude at the frontal electrode site was the only significant predictor of remission. In a multiple regression analysis, the MMN amplitude, antipsychotic use, and years of education predicted an improvement in attenuated positive symptoms. The MMN amplitude at baseline predicted functional recovery.
Conclusions
These results suggest that MMN is a putative predictor of prognosis regardless of the transition to psychotic disorder in subjects at CHR. Early prognosis prediction and the provision of appropriate interventions based on the initial CHR status might be aided using MMN.
Keywords: clinical high risk for psychosis, event-related potential, mismatch negativity, remission, prognosis, schizophrenia
Introduction
Efforts aimed at early detection and intervention in patients with the psychotic disorder have led the establishment of “clinical high risk (CHR),” “ultra-high risk,” or “basic symptoms” criteria.1 The use of these approaches in identifying markers predictive of the transition to psychotic disorder has been a major focus of researchers, and sociodemographic, clinical, neuropsychological, neuroanatomical, and electrophysiological markers of this transition have been suggested.2–5 However, the initially reported high transition rate of 54% within 1 year later decreased to 22% within 1 year, 29% after 2 years, and 36% after 3 years.6,7 A declining transition rate in subjects at CHR has been consistently reported in different cohorts, and the dilution effect, early referral, source of referral, and comorbidity were suggested as causes of this phenomenon.8–11 This resulted in an increase in the proportion of CHR nonconverters who do not transition to psychotic disorder within a limited observational period. Longitudinal studies had reported that CHR nonconverters remained at a poor functional status even when they improved during the follow-up period.12,13 In addition, the high prevalence of nonpsychotic psychiatric disorders has been consistently reported, and comorbid mental disorders are associated with poor functional outcomes in CHR nonconverters.14–17 These findings suggest that attention should be paid not only to conversion status but also to general psychiatric conditions, including functional outcomes in subjects at CHR for psychosis.
Given the declining transition rate and increasing rate of nonpsychotic poor outcomes, predicting remission from initial CHR status might provide useful information, especially for clinical practice. The early detection of putative remitters might reduce the problems of unnecessary treatment and stigmatization. Furthermore, nonremitters, including converters, can receive more intensive care from the beginning of treatment to improve later outcomes. Although the predictors of or factors associated with remission from CHR status have not yet been sufficiently studied, the extant literature has shown that the factors associated with transition also show potential as markers for remission (ie, symptomatic, functional, or both types of improvement). Baseline sociodemographic characteristics and clinical symptoms do not differ between remitters and nonremitters,18 whereas remitters show better neurocognitive function than nonremitters at baseline.19 Egerton et al20 found that compared with remitters, the baseline thalamic glutamate level is lower in nonremitters and is associated with a change in attenuated positive symptom severity during the course of disease. Kim et al21 reported that baseline P300 amplitudes predict later improvement in the negative and general symptoms of subjects at CHR, although no baseline P300 difference was found between remitters and nonremitters. In addition, recent neuroimaging studies have attempted to predict functional improvements in individuals at CHR using a support vector regression of subcortical volumes.22 Therefore, other suggested biomarkers for schizophrenia pathophysiology or transition to psychosis might predict remission from CHR status.
Of the potential biomarkers for predicting remission in subjects at CHR, auditory mismatch negativity (MMN) is a promising candidate. MMN is an event-related potential (ERP) component that represents preattentive auditory processing and depends on the N-methyl-D-aspartate (NMDA) receptor-mediated glutamate system.23,24 Impaired MMN in patients with schizophrenia and its association with impaired functional status have been consistently reported.25–27 In subjects at CHR for psychosis, aberrant MMN activity and its relationship with positive prodromal symptom severity were found.28 Moreover, baseline MMN predicts the later transition to psychotic disorder and time to conversion.5,29,30 Because symptomatic and functional improvement should be considered simultaneously to better define remission from CHR status,12,13 MMN shows the additional possibility of being a potential biomarker for remission due to its representativeness of positive prodromal symptoms and general functional status.
Despite the clinical significance of predicting remission from CHR status and the potential use of MMN as a biomarker for remission, no study has attempted to predict remission in subjects at CHR using MMN. Therefore, we aimed to determine whether baseline MMN responses predict later remission and symptomatic or functional improvement during a maximal 6-year follow-up period. We hypothesized that individuals whose CHR statuses go into remission would show larger baseline MMN amplitudes, similar to healthy control (HC) subjects, than those whose statuses do not. We also hypothesized that the baseline MMN amplitude would predict later remission as well as symptomatic and functional improvement.
Methods
Participants
We recruited 140 subjects at CHR between January 2005 and January 2014 via the Seoul Youth Clinic (www.youthclinic.org), a center for the early detection of and intervention for people at high risk for psychosis.31 Among these subjects, 70 individuals at CHR participated in the baseline MMN measurement. CHR status was confirmed using the criteria of the Structured Interview for Prodromal Symptoms (SIPS).6 The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I Disorders (SCID-I) was used to determine the past and current psychiatric disorders. Prodromal symptoms were assessed using the validated Korean version of the SIPS,32 and the Global Assessment of Functioning (GAF) was used to define general functional status. The duration of untreated prodromal psychosis (DUPP) was obtained from medical records and interviews with the participants and their family members. Medication use was documented, and antipsychotic use was also recorded as the mean olanzapine equivalent dose.33 The exclusion criteria included a lifetime diagnosis of psychotic disorder, a history of antipsychotic use, substance abuse or dependence, neurological disease or significant head trauma, medical illness with cognitive sequelae, sensory impairments, and intellectual disability (intelligence quotient [IQ] < 70).
After baseline assessment, the subjects at CHR were followed up and assessed regularly for 1–6 years. A total of 48 subjects at CHR who participated in the baseline MMN assessment and were followed up at least once over 6 years were included in this study. Remission from CHR status was defined as an individual at CHR meeting a score of 2 or lower on the Scale of Prodromal Symptoms (SOPS) positive subscale and a score of 60 or more on the GAF at the last follow-up point.13,21 The remitter group (CHR-R) included 17 participants at CHR, and the nonremitter group (CHR-NR) included 31 subjects at CHR. Among the nonremitters, 7 CHR subjects made the transition to overt psychotic disorder and finished the last follow-up assessment as CHR at the time of transition. The demographic and MMN data of the 47 HC subjects, which were presented in a previously published study, were used for the group comparisons in the current study.34
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital. Written informed consent was obtained from all of the participants after a full explanation of the study procedure was provided.
EEG Recording
The electroencephalographic (EEG) recording protocol used in this study was identical to that of a prior study conducted in our lab.34 Participants were assigned to a passive auditory oddball task while their EEGs were recorded. While subjects concentrated on a “Where’s Waldo?” picture book, a pseudorandom series of 1000 Hz (80 dB, 10 ms rise/fall) auditory stimuli were binaurally presented using a STIM2 sound generator (Compumedics). The duration of the frequent standard stimuli (81.8%, 982/1200) was 50 ms, and the duration of the infrequent deviant stimuli (18.2%, 218/1200) was 100 ms. The intertrial interval was 600 ms.
Continuous EEG recordings were acquired using a Neuroscan 128 Channel SynAmps system equipped with a 128-channel Quick-Cap based on the modified 10–20 international system (Compumedics). The electrodes at the mastoid sites served as the reference electrodes. The EEG data were digitized at a 1000 Hz sampling rate with an online filter of 0.05–100 Hz. Eye movement artifacts were monitored by recording the vertical and horizontal electrooculogram using electrodes below and on the outer canthus of the left eye. The resistance at all electrode sites was below 5 kΩ.
ERP Analysis
The preprocessing of the ERP data and source reconstruction were performed using Curry version 7 software (Compumedics). Bad channels were replaced via the linear interpolation of the adjacent channels (up to 7% per participant). Eye movement artifacts were reduced using the artifact reduction algorithm implemented in Curry 7 software.35 EEG recordings were re-referenced to the common average reference data, bandpass filtered between 0.1 and 30 Hz, epoched to a 100 ms prestimulus and a 300 ms poststimulus, and baseline-corrected using the averaged prestimulus interval voltage. Epochs containing EEG amplitudes that exceeded ±75 μV were rejected automatically, and the number of remaining epochs exceeded 100 in all participants. MMN response activity was obtained by subtracting the ERPs elicited by the standard stimuli from those elicited by the deviant stimuli. A peak detection method was used to determine the peak MMN amplitude and latency, which was defined as the most negative deflection between 130 and 250 ms post-stimulus onset at the F3, Fz, F4, FC3, FCz, and FC4 electrode sites. We performed an exploratory source-level analysis of MMN using the data of 26 individuals at CHR who had both digitized channel locations and 3T MRI data. See the supplementary material for the detailed description of the image acquisition, data processing, and source reconstruction.
Statistical Analyses
The demographic and clinical characteristics of the subjects were compared across groups using analysis of variance (ANOVA). A χ2 test or Fisher’s exact test was used to analyze the categorical data. Group comparisons of MMN amplitudes and latency were performed using a repeated measures ANOVA with 6 frontocentral electrode sites (F3, Fz, F4, FC3, FCz, and FC4) as the within-subjects factor, group (HC vs CHR-R vs CHR-NR) as the between-subjects factor, and age as a covariate. A post hoc simple contrast test was used to reveal specific group differences. To identify the factors that predicted remission, a binary logistic regression with the backward selection method was used. A multiple regression analysis with the backward selection method was used to identify the factors that significantly predicted improvement in positive prodromal symptoms or general functional states during the follow-up period. The anticipated predictive factors included MMN peak amplitude at Fz assessed at baseline; demographic characteristics (ie, sex, handedness, age, IQ, and years of education); SOPS positive subscale score or GAF score measured at baseline; follow-up duration; medication use during the follow-up period (ie, mean olanzapine equivalent dose of antipsychotics, antidepressant use, mood stabilizer use, and anxiolytic use); and DUPP. IBM SPSS version 24 (IBM) was used for the statistical analyses. The threshold for statistical significance was set at P < .05.
Results
Subject Characteristics
All subjects at CHR were antipsychotic-naïve at the time of enrollment; 36 subjects were medication-naïve, 9 subjects were taking antidepressants, and 11 subjects were taking benzodiazepines. Table 1 summarizes the demographic and clinical characteristics at baseline and during the follow-up period. The HC subjects were older and more educated than both the CHR-R (age, P = .001; education years, P < .001) and CHR-NR (age, P < .001; education years, P < .001) subjects. IQ scores were not different between the HC and the CHR-R groups (P = .092); however, the CHR-NR group showed lower IQ scores than the HC group (P = .017). No differences were found in the demographic or clinical characteristics between the CHR-R and CHR-NR groups assessed at baseline. The CHR-R and CHR-NR groups did not differ with regard to follow-up duration, change in SOPS positive subscale scores or use of medication. However, the CHR-NR subjects were prescribed greater olanzapine equivalent doses of antipsychotics (t = −2.080, P = .043) and showed less functional improvement (t = 4.586, P < .001) during the follow-up period than the CHR-R subjects. A comparison of the 48 CHR subjects who participated in the follow-up assessment at least once and 22 CHR subjects who did not is provided in supplementary table 1.
Table 1.
Demographic Data of the Participants and the Clinical Characteristics at Baseline and Follow-Up of the Subjects at Clinical High Risk (CHR) for Psychosis
| HC | CHR-Ra | CHR-NRb | Statistical Analysisc | |||||
|---|---|---|---|---|---|---|---|---|
| (N = 47) | (N = 17) | (N = 31) | ||||||
| Mean | SD | Mean | SD | Mean | SD | χ2 or F or t | P | |
| Baseline characteristics | ||||||||
| Sex (male/female) | 29/18 | 11/6 | 24/7 | 2.166 | .339 | |||
| Handedness (right/left) | 46/1 | 17/0 | 28/3 | 3.550 | .169 | |||
| Age (years) | 24.6 | 5.3 | 19.8 | 3.6 | 19.5 | 3.4 | 14.696 | <.001** |
| IQ | 115.3 | 13.4 | 107.6 | 12.0 | 106.9 | 12.5 | 4.758 | .011* |
| Education (years) | 14.5 | 1.8 | 12.1 | 1.4 | 12.1 | 1.7 | 23.133 | <.001* |
| DUPP (months) | NA | 22.4 | 23.0 | 21.0 | 17.0 | 0.237 | .814 | |
| SOPS | ||||||||
| Positive symptoms | NA | 7.8 | 3.5 | 8.3 | 5.5 | −0.335 | .739 | |
| Negative symptoms | NA | 15.6 | 5.9 | 14.9 | 6.5 | 0.376 | .709 | |
| Disorganization | NA | 4.2 | 1.9 | 4.7 | 2.8 | −0.665 | .509 | |
| General symptoms | NA | 7.8 | 3.9 | 7.3 | 4.3 | 0.400 | .691 | |
| GAF | NA | 48.5 | 8.4 | 41.9 | 23.2 | 1.422 | .163 | |
| Follow-up characteristics | ||||||||
| Follow-up duration (days) | NA | 1141.7 | 612.5 | 1059.8 | 511.7 | 0.494 | .624 | |
| Antipsychotics dosed | NA | 1.9 | 2.3 | 3.6 | 3.4 | −2.080 | .043* | |
| Change in | ||||||||
| SOPS positive symptomse | NA | 4.7 | 3.3 | 4.1 | 8.3 | 0.378 | .707 | |
| GAFf | NA | 20.4 | 9.5 | 4.4 | 12.4 | 4.586 | <.001** | |
| Number of follow-up (months)g | ||||||||
| 12 months | NA | 14 (29.2) | 21 (43.8) | 1.187 | .276 | |||
| 18 months | NA | 12 (25.0) | 13 (27.1) | 3.612 | .057 | |||
| 24 months | NA | 10 (20.8) | 18 (37.5) | 0.003 | .959 | |||
| 36 months | NA | 7 (14.6) | 13 (27.1) | 0.003 | .959 | |||
| 48 months | NA | 5 (10.4) | 6 (12.5) | 0.629 | .428 | |||
| 60 months | NA | 4 (8.3) | 4 (8.3) | 0.893 | .345 | |||
| 72 months | NA | 2 (4.2) | 2 (4.2) | 0.406 | .524 | |||
| Use of medicationh | ||||||||
| Antipsychotics | NA | 14 (82.3) | 29 (93.5) | 1.475 | .225 | |||
| Antidepressants | NA | 12 (70.6) | 21 (67.7) | 0.041 | .839 | |||
| Mood stabilizers | NA | 11 (64.7) | 22 (71.0) | 0.200 | .654 | |||
| Anxiolytics | NA | 4 (23.5) | 13 (41.9) | 1.626 | .202 | |||
Note: HC, healthy control; SD, standard deviation; IQ, intelligence quotient; DUPP, duration of untreated prodromal psychosis; SOPS, Scale of Prodromal Symptoms; GAF, Global Assessment of Functioning; NA, not applicable.
aRemitted at last follow-up point.
bDid not remit at last follow-up point.
cAnalysis of variance, independent t-test or Welch’s t-test if the variances were not equal; χ2 analysis or Fisher’s exact test for categorical data.
dMean daily olanzapine equivalent dose prescribed during the follow-up period.
eCalculated by subtracting scores at last follow-up point from scores at baseline.
fCalculated by subtracting scores at baseline from scores at last follow-up point.
gNumber (percentage) of subjects who were followed-up at that time point.
hNumber (percentage) of subjects who were prescribed each medication during the follow-up period.
*The mean difference is significant at the .05 level.
**The mean difference is significant at the .005 level.
MMN Amplitude at Baseline Predicts Remission and Symptomatic or Functional Improvement
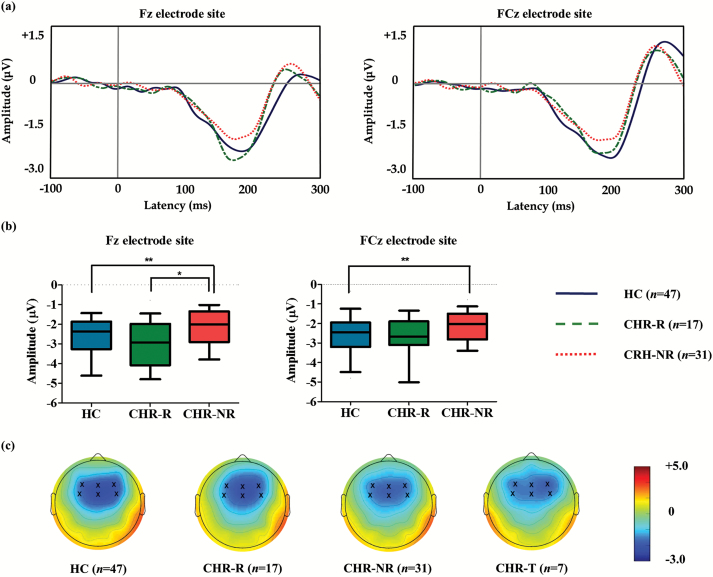
Figure 1a displays the grand-average MMN waveforms, and Figure 1b shows the MMN peak amplitudes across the 3 groups. Figure 1c displays 2-dimensional topographic maps of the MMN amplitudes for the HC, CHR-R, CHR-NR, and CHR subjects who transitioned to overt psychotic disorder (CHR-T). Table 2 summarizes the group comparison results for the baseline MMN amplitudes and latencies. A repeated measures ANOVA revealed a significant main effect of group (F2,91 = 4.876, P = .010), electrode site (F5,87 = 4.758, P = .001), and age (F1,91 = 8.929, P = .004) on the MMN amplitude at baseline. The group by electrode interaction was not significant (F10,176 = 1.670, P = .091). The post hoc analysis revealed that the MMN amplitude at baseline was smaller in the CHR-NR group than in both the HC (P = .004) and CHR-R (P = .042) groups. Furthermore, the MMN amplitude at baseline was not different between the HCs and CHR-R subjects (P = .608). Regarding the MMN latency at baseline, there was no significant effect of group (F2,91 = 2.436, P = .093), electrode site (F5,87 = 1.705, P = .142), and age (F1,91 = 0.781, P = .379), as well as no significant group by electrode interaction (F10,176 = 0.817, P = .613).
Fig. 1.
(a) Grand-averaged mismatch negativity (MMN) waveforms across the healthy controls (HC) and subjects at clinical high risk (CHR) for psychosis who remitted (CHR-R) or did not remit (CHR-NR). (b) The MMN amplitudes at the Fz and FCz electrode sites across the groups. The horizontal lines in the group indicate the means, and the vertical lines in the group indicate the10 to 90 percentile. * indicates that the mean difference is significant at the .05 level; ** indicates that the mean difference is significant at the .005 level. (c) Two-dimensional topographic maps of MMN in the HC, CHR-R, CHR-NR, and CHR-T subjects. The 6 frontocentral electrodes are indicated by an x in the topographic maps. The colored bar with numbers indicates the amplitude of MMN (μV).
Table 2.
Means and Standard Deviations (SD) of the Mismatch Negativity (MMN) Peak Amplitudes and Latencies at the Surface Electrodes Across the Groups
| HC | CHR-Ra | CHR-NRb | Statistical Analysisc | Post Hoc Analysisd | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 47) | (N = 17) | (N = 31) | |||||||||
| Mean | SD | Mean | SD | Mean | SD | F | P | A vs B | A vs C | B vs C | |
| Amplitude (μV) | |||||||||||
| F3 | −2.2 | 1.1 | −2.5 | 1.0 | −1.8 | 0.8 | 4.627 | .012* | .955 | .008* | .019* |
| Fz | −2.7 | 1.2 | −3.0 | 1.2 | −2.2 | 1.0 | 5.875 | .004** | .814 | .002** | .014* |
| F4 | −2.7 | 1.2 | −3.0 | 1.1 | −2.4 | 0.9 | 3.614 | .031* | .793 | .013* | .060 |
| FC3 | −1.9 | 1.0 | −2.0 | 0.8 | −1.7 | 0.7 | 2.009 | .140 | .745 | .058 | .189 |
| FCz | −2.7 | 1.7 | −2.7 | 1.2 | −2.2 | 0.8 | 6.491 | .002** | .225 | .001** | .067 |
| FC4 | −2.6 | 1.2 | −2.5 | 0.9 | −2.3 | 0.9 | 1.200 | .306 | .519 | .125 | .498 |
| Latency (ms) | |||||||||||
| F3 | 181.9 | 25.9 | 173.0 | 25.0 | 170.7 | 24.3 | 1.464 | .237 | .269 | .101 | .764 |
| Fz | 182.5 | 25.1 | 177.3 | 23.6 | 172.9 | 21.9 | 1.904 | .155 | .304 | .055 | .533 |
| F4 | 182.4 | 25.5 | 174.8 | 24.2 | 170.9 | 21.3 | 3.694 | .029* | .093 | .009** | .571 |
| FC3 | 177.5 | 29.2 | 176.8 | 26.4 | 173.6 | 26.8 | 0.484 | .618 | .656 | .328 | .693 |
| FCz | 183.3 | 22.5 | 178.2 | 17.9 | 174.7 | 22.4 | 1.154 | .320 | .450 | .133 | .591 |
| FC4 | 175.5 | 23.4 | 175.4 | 24.2 | 162.3 | 15.8 | 4.351 | .016* | .727 | .006** | .044* |
Note: HC, healthy control; CHR, clinical high risk; A, HC; B, CHR-R; C, CHR-NR.
aRemitted at last follow-up point.
bDid not remit at last follow-up point.
cAnalysis of variance with age as covariate.
d P value of post hoc analysis using simple contrast test.
*The mean difference is significant at the .05 level.
**The mean difference is significant at the .005 level.
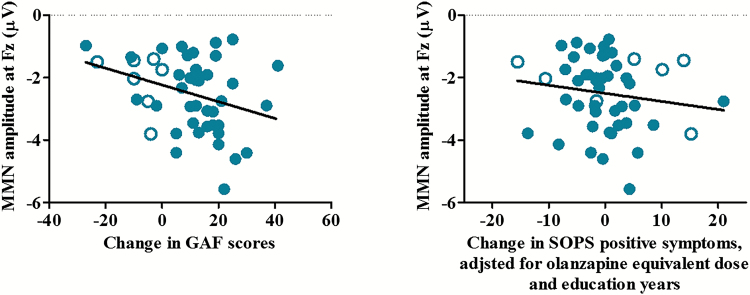
According to the binary logistic regression analysis, the baseline MMN amplitude at Fz was the only significant predictor of remission (Exp [β] = 0.472, 95% confidence interval [95% CI] = 0.254 to 0.877, P = .018). According to the multiple regression analysis, improvement in SOPS positive symptoms was significantly predicted by the baseline MMN amplitude at Fz, the dose of antipsychotics used, and years of education. The only significant predictor of GAF improvement was the baseline MMN amplitude at Fz (table 3, figure 2). The results of the exploratory MMN source analysis are presented in the supplementary material (supplementary tables 2 and 3; supplementary figure 1).
Table 3.
Significant Predictors of Remission, Improvement of Attenuated Positive Symptoms and General Functioning
| Outcome Variables | Significant Predictors | R 2 or Partial R2 | Exp (B) or Beta (SB) | P | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Remissiona | MMN amplitude at Fz | 0.244 | 0.472 | .018* | 0.254 | 0.877 |
| Improvement of SOPS positive symptomsb | MMN amplitude at Fz | −0.289 | −2.028 (−2.205) | .033* | −3.888 | −0.169 |
| Antipsychotics dosec | 0.389 | 1.024 (2.967) | .005* | 0.326 | 1.721 | |
| Education years | 0.350 | 2.613 (2.669) | .011* | 0.634 | 4.592 | |
| Improvement of GAFb | MMN amplitude at Fz | 0.168 | −3.696 (−2.265) | .028* | −0.692 | −0.410 |
Note: SB, standardized beta; MMN, mismatch negativity; SOPS, Scale of Prodromal Symptoms; GAF, Global Assessment of Functioning; CI, confidence interval.
aBinary logistic regression with backward method.
bMultiple regression with backward method.
cMean olanzapine equivalent dose.
*The mean difference is significant at the .05 level.
Fig. 2.
The correlation between the change in the Global Assessment of Functioning (GAF) scores and the MMN amplitude at baseline (left). The partial correlation between the baseline MMN amplitude at Fz and the change in the Scale of Prodromal Symptoms (SOPS) positive symptom scores adjusted for the olanzapine equivalent antipsychotic dose and education years (right). The subjects at CHR who transitioned to psychosis are indicated by the open circles.
Discussion
This study investigated MMN as a predictor of prognosis after a 6-year follow-up period among subjects at CHR for psychosis. As expected, the baseline MMN amplitudes at the frontal electrode sites were reduced in CHR-NR subjects compared with CHR-R subjects and HC subjects, and a larger baseline MMN amplitude was the only significant predictor of remission. The MMN amplitude obtained at baseline predicted improvement in general functional status during the follow-up period in the whole CHR group. The significant predictors of reduction in attenuated positive symptoms were baseline MMN amplitude, antipsychotic dosage, and years of education.
Because nontransition or the amelioration of attenuated positive symptoms does not ensure a positive prognosis, especially in terms of functional outcomes,12,13,36 to be clinically relevant, the concept of remission from CHR status should include both symptomatic and functional improvement. The results of the current study show that the baseline MMN amplitude predicts later remission, which was defined using both the SOPS positive subscale score and the GAF score; these findings suggested that MMN can be used as a putative biomarker for the early detection of clinically relevant remission in subjects at CHR. Furthermore, we found that the baseline MMN amplitude separately predicted the improvement of attenuated positive symptoms and general functional status in the CHR group as a whole. These results are consistent with the previous literature, which reports a relationship between MMN and positive symptom severity or general functional status in patients with schizophrenia and those at CHR for psychosis.26,28,37–39 In addition, Thomas et al40 showed that early auditory processing significantly predicted functional outcomes in patients with schizophrenia, which further supports the results of the current study.
To date, all other CHR studies using MMN as a biomarker have attempted to reveal its potential utility to predict the transition to psychotic disorder.30 In particular, Perez et al5 showed that MMN was compromised prior to and a significant predictor of time to psychosis onset among subjects at CHR. However, the transition rate has declined from an initial 54% within 1 year to 10–15% within 2–3 years,6,9,41 and a large proportion of subjects at CHR have shown poor prognoses, although they did not transition to psychotic disorder.14,16 This phenomenon has raised questions about the clinical relevance of predictions limited to transition in CHR prognosis; in turn, the prediction of remission from an initial CHR status has gained as much clinical importance as the prediction of transition.20,21,36 The early classification of remitters and nonremitters among individuals with initial CHR statuses would be helpful for earlier clinical decisions regarding intervention factors such as timing and intensity. In line with the trend toward at-risk mental state research, the current study provides the first suggestion that MMN serves as a biomarker in predicting the prognosis of subjects at CHR, regardless of psychotic conversion.
This study has several limitations. First, the follow-up period varied among subjects at CHR, although the observational period of 1–6 years was relatively long. Although the follow-up duration of the CHR-R and CHR-NR groups did not differ and the prognostic changes were not explained by the length of the follow-up period according to the multiple regression analysis, a potential bias caused by the varying lengths of follow-up duration warrants caution when interpreting the results. Second, symptoms and functional status at a single last follow-up point was used to declare remission which was merely a snapshot of a status, thus caution would need to interpret the result of our study. Third, higher baseline GAF scores shown in CHR subjects who did not participate in follow-up assessment may lead potential bias to the result that MMN amplitude at baseline predicted improvement in general functional status. Fourth, the range of change in the clinical characteristics examined in the present study is limited to the scores derived from the SOPS positive subscale and the GAF scale following the definition of remission. Other important clinical variables, including negative, disorganization, and general symptoms, as well as neurocognition, are beyond the scope of the present study and would further augment the meaning of our findings.
The present study is the first to examine the possibility that baseline MMN predicts later functional and symptomatic prognoses in subjects at CHR for psychosis. We observed that the baseline MMN amplitude was associated with later remission as well as improvements in attenuated positive symptoms and general functional status. Our results not only suggest that MMN is a putative biomarker of remission in subjects at CHR but also provide the biological background for previous studies that argued for the importance of nonpsychotic outcomes and clinically relevant remission criteria, including functional improvement.12,13,15,21,42 Although challenges remain in translating electrophysiological findings into clinically feasible prognostic tests, the early prediction of prognosis and the provision of appropriate interventions for individuals at CHR for psychosis might be aided using MMN.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning, supported this research (grant no. 2016R1E1A1A02921618).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fusar-Poli P, Borgwardt S, Bechdolf A et al. . The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fusar-Poli P, Borgwardt S, Crescini A et al. . Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 3. Nieman DH, Ruhrmann S, Dragt S et al. . Psychosis prediction: stratification of risk estimation with information-processing and premorbid functioning variables. Schizophr Bull. 2014;40:1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Addington J, Liu L, Perkins DO, Carrion RE, Keefe RS, Woods SW. The role of cognition and social functioning as predictors in the transition to psychosis for youth with attenuated psychotic symptoms. Schizophr Bull. 2017;43:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez VB, Woods SW, Roach BJ et al. . Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller TJ, McGlashan TH, Rosen JL et al. . Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Bonoldi I, Yung AR et al. . Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 8. Yung AR, Yuen HP, Berger G et al. . Declining transition rate in ultra high risk (prodromal) services: dilution or reduction of risk?Schizophr Bull. 2007;33:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim KO, Lee TY, Kim M et al. . Early referral and comorbidity as possible causes of the declining transition rate in subjects at clinical high risk for psychosis. Early Interv Psychiatry. 2016. doi: 10.1111/eip.12363. [DOI] [PubMed] [Google Scholar]
- 10. Hartmann JA, Yuen HP, McGorry PD et al. . Declining transition rates to psychotic disorder in “ultra-high risk” clients: investigation of a dilution effect. Schizophr Res. 2016;170:130–136. [DOI] [PubMed] [Google Scholar]
- 11. Fusar-Poli P, Rutigliano G, Stahl D et al. . Deconstructing pretest risk enrichment to optimize prediction of psychosis in individuals at clinical high risk. JAMA Psychiatry. 2016;73:1260–1267. [DOI] [PubMed] [Google Scholar]
- 12. Addington J, Cornblatt BA, Cadenhead KS et al. . At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee TY, Kim SN, Correll CU et al. . Symptomatic and functional remission of subjects at clinical high risk for psychosis: a 2-year naturalistic observational study. Schizophr Res. 2014;156:266–271. [DOI] [PubMed] [Google Scholar]
- 14. Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172:249–258. [DOI] [PubMed] [Google Scholar]
- 15. Schlosser DA, Jacobson S, Chen Q et al. . Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr Bull. 2012;38:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutigliano G, Valmaggia L, Landi P et al. . Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord. 2016;203:101–110. [DOI] [PubMed] [Google Scholar]
- 17. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Wit S, Schothorst PF, Oranje B, Ziermans TB, Durston S, Kahn RS. Adolescents at ultra-high risk for psychosis: long-term outcome of individuals who recover from their at-risk state. Eur Neuropsychopharmacol. 2014;24:865–873. [DOI] [PubMed] [Google Scholar]
- 19. Lee TY, Shin YS, Shin NY et al. . Neurocognitive function as a possible marker for remission from clinical high risk for psychosis. Schizophr Res. 2014;153:48–53. [DOI] [PubMed] [Google Scholar]
- 20. Egerton A, Stone JM, Chaddock CA et al. . Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology. 2014;39:2891–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim M, Lee TY, Lee S, Kim SN, Kwon JS. Auditory P300 as a predictor of short-term prognosis in subjects at clinical high risk for psychosis. Schizophr Res. 2015;165:138–144. [DOI] [PubMed] [Google Scholar]
- 22. de Wit S, Ziermans TB, Nieuwenhuis M et al. . Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: applying machine learning techniques to brain imaging data. Hum Brain Mapp. 2017;38:704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends Neurosci. 2001;24:283–288. [DOI] [PubMed] [Google Scholar]
- 24. Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull. 2012;38:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 26. Kim M, Kim SN, Lee S et al. . Impaired mismatch negativity is associated with current functional status rather than genetic vulnerability to schizophrenia. Psychiatry Res. 2014;222:100–106. [DOI] [PubMed] [Google Scholar]
- 27. Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin KS, Kim JS, Kang DH et al. . Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009;65:1071–1078. [DOI] [PubMed] [Google Scholar]
- 29. Bodatsch M, Ruhrmann S, Wagner M et al. . Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. [DOI] [PubMed] [Google Scholar]
- 30. Bodatsch M, Brockhaus-Dumke A, Klosterkötter J, Ruhrmann S. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol Psychiatry. 2015;77:951–958. [DOI] [PubMed] [Google Scholar]
- 31. Kwon JS, Byun MS, Lee TY, An SK. Early intervention in psychosis: insights from Korea. Asian J Psychiatr. 2012;5:98–105. [DOI] [PubMed] [Google Scholar]
- 32. Jung MH, Jang JH, Kang DH et al. . The reliability and validity of the Korean version of the structured interview for prodromal syndrome. Psychiatry Investig. 2010;7:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 34. Kim M, Cho KI, Yoon YB, Lee TY, Kwon JS. Aberrant temporal behavior of mismatch negativity generators in schizophrenia patients and subjects at clinical high risk for psychosis. Clin Neurophysiol. 2017;128:331–339. [DOI] [PubMed] [Google Scholar]
- 35. Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. [DOI] [PubMed] [Google Scholar]
- 36. Yung AR, Nelson B, Thompson A, Wood SJ. The psychosis threshold in ultra high risk (prodromal) research: is it valid?Schizophr Res. 2010;120:1–6. [DOI] [PubMed] [Google Scholar]
- 37. Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW. Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophr Bull. 2017;43:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrión RE, Cornblatt BA, McLaughlin D et al. . Contributions of early cortical processing and reading ability to functional status in individuals at clinical high risk for psychosis. Schizophr Res. 2015;164:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified ‘optimal’ multi-feature paradigm. Int J Psychophysiol. 2011;81:245–251. [DOI] [PubMed] [Google Scholar]
- 40. Thomas ML, Green MF, Hellemann G et al. . Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry. 2017;74:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ziermans TB, Schothorst PF, Sprong M, van Engeland H. Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res. 2011;126:58–64. [DOI] [PubMed] [Google Scholar]
- 42. Nelson B, Yuen K, Yung AR. Ultra high risk (UHR) for psychosis criteria: are there different levels of risk for transition to psychosis?Schizophr Res. 2011;125:62–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.