Abstract
Background
Though olfactory deficits are well-documented in schizophrenia, fewer studies have examined olfactory performance profiles across the psychosis spectrum. The current study examined odor identification, discrimination, and detection threshold performance in first-episode psychosis (FEP) patients diagnosed with schizophrenia, schizoaffective disorder, bipolar disorder with psychotic features, major depression with psychotic features, and other psychotic conditions.
Method
FEP patients (n = 97) and healthy adults (n = 98) completed birhinal assessments of odor identification, discrimination, and detection threshold sensitivity for lyral and citralva. Participants also completed measures of anticipatory pleasure, anhedonia, and empathy. Differences in olfactory performances were assessed between FEP patients and controls and within FEP subgroups. Sex-stratified post hoc analyses were employed for a complete analysis of sex differences. Relationships between self-report measures and olfactory scores were also examined.
Results
Individuals with psychosis had poorer scores across all olfactory measures when compared to the control group. Within the psychosis cohort, patients with schizophrenia-associated psychosis had poorer odor identification, discrimination, and citralva detection threshold scores relative to controls. In schizophrenia patients, greater olfactory disturbance was associated with increased negative symptomatology, greater self-reported anhedonia, and lower self-reported anticipatory pleasure. Patients with mood-associated psychosis performed comparable to controls though men and women in this cohort showed differential olfactory profiles.
Conclusions
These findings indicate that olfactory deficits extend beyond measures of odor identification in FEP with greater deficits observed in schizophrenia-related subgroups of psychosis. Studies examining whether greater olfactory dysfunction confers greater risk for developing schizophrenia relative to other forms of psychosis are warranted.
Keywords: smell, olfaction, anhedonia, negative symptoms, early psychosis, mood disorders
Introduction
The central location of the olfactory system within the medial forebrain makes it especially vulnerable to disruption during early forebrain development. Developmental abnormalities in schizophrenia during a critical period of embryonic risk are thought to be mirrored in both disturbed functional output and compromised structural integrity of the central olfactory system.1–5 Olfactory epithelium-derived cells in patients with schizophrenia also demonstrate molecular and cellular changes relative to control cells,6–12 indicating that abnormalities extend to the most peripheral aspects of this system. Mounting evidence has pointed to the utility of olfactory measures as informative surrogate biological markers of disease susceptibility tied to this fetal risk period.13
Studies of olfactory functioning have received the greatest attention in schizophrenia with the largest deficits observed on measures of odor identification ability.14 These findings persist after controlling for the influence of sex,15 smoking burden,16 and task complexity.17 Odor identification deficits are associated with increased negative symptoms, anhedonia, and poor personal hygiene.18–20 Olfactory impairment is also present irrespective of medication status as unmedicated patients show robust deficits on measures of odor identification.21,22 Early investigations by Rupp et al23,24 found poor odor identification and discrimination ability in adult men with schizophrenia, a finding that was replicated in a larger cohort.25 Schizophrenia patients also have variably reduced odor detection threshold sensitivity. In particular, a selective odor detection threshold deficit for lyral, an odorant that has a low intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) response, but not citralva, an odorant with a high adenylyl cyclase response, has been reported in schizophrenia.26 This finding is mechanistically insightful as disrupted cAMP signaling has been implicated in the pathophysiology of schizophrenia.27
Olfactory deficits have also been documented in unaffected first-degree relatives of schizophrenia patients,28,29 adolescents and young adults with prodromal symptoms of psychosis and patients newly diagnosed with a psychotic condition.25,30,31 Though considerably fewer studies have examined odor discrimination performance in prodromal and first-episode psychosis (FEP) populations, at least 1 study found that prodromal youth, but not first-degree relatives, have impaired odor discrimination ability.25 In neuroleptic-naïve FEP subjects, the severity of olfactory dysfunction did not improve with medication stabilization21 and was found to predict poor outcome when assessed longitudinally.32
Findings of olfactory deficits in schizophrenia have propelled interest in other neuropsychiatric conditions characterized by anhedonia, emotional disturbance, and social impairment, particularly given the observation that increased anhedonia during the schizophrenia prodrome predicts conversion to psychosis.33 Studies in bipolar disorder have generally found intact odor identification and discrimination performances.34,35 In major depression, olfactory findings are generally reported as intact,35,38–40 though Clepce et al36 found reduced odor identification ability in patients during a depressive episode compared to their performances in the remitted state. Reduced odor detection thresholds have been reported in major depression,37–39 though see Swiecicki et al,40 with normalized olfactory scores following treatment.41 Though multiple studies have found that olfactory disturbance is associated with anhedonia and flat affect,42,43 these investigations have not yet extended beyond schizophrenia cohorts.
In the current study, we examined deficits in odor identification, discrimination, and detection threshold in newly diagnosed individuals across the psychosis spectrum and healthy controls. We then examined the extent to which FEP patients with affective psychosis and FEP patients with a core psychotic condition of schizophrenia or schizoaffective disorder differed from controls. The relationships between clinician-administered and self-report assessments of anhedonia and olfactory performance were then explored within the FEP cohort and these subgroups of interest.
Methods
Recruitment and Participants
This study was approved and conducted using guidelines established by the Johns Hopkins School of Medicine Institutional Review Board and in accordance with The Code of Ethics of the World Medical Association (1964 Declaration of Helsinki). Each participant received a full explanation of the study procedures. Written informed consent was obtained for all participants 18 years and older. Parental consent and assent was obtained for all participants below age 18.
Individuals with FEP (n = 97) and neurologically and psychiatrically healthy participants (n = 98) were recruited to the Johns Hopkins Schizophrenia Center. Recruitment was limited to individuals between 13 and 35 years of age with the onset of psychosis within 24 months of the study. Diagnoses were established using the Diagnostic Interview for Genetic Studies (DIGS)44 or the Structured Clinical Interview for DSM-IV—Patient Edition (SCID)45 and available information from the patient’s care providers and medical record. Individuals with a history of head trauma, nasal trauma, nasal surgery, neurologic disorder, cancer, viral infection, and reported history of intellectual disability were excluded. Furthermore, participants with an estimated intellect below 70 on the Hopkins Adult Reading Test46 were excluded from this study. Individuals who reported active substance abuse or produced a urine drug screen positive for illicit substance use, except marijuana, were excluded from participation. Individuals were also excluded if they were pregnant or taking anti-inflammatory agents. Healthy comparison subjects were additionally screened and excluded for a family history of schizophrenia or schizophrenia-spectrum disorder. All participants were instructed not to wear fragrances, smoke, eat or drink anything 2 hours prior to olfactory testing. Individuals were rescheduled if they had serious allergies or a sinus cold on the day of testing.
The FEP cohort was comprised of individuals with schizophrenia (n = 52), schizoaffective disorder (n = 12), bipolar disorder with psychotic features (n = 19), major depressive disorder with psychotic features (n = 6), psychotic disorder not otherwise specified (n = 3), schizophreniform disorder (n = 1), brief psychotic episode (n = 1) and drug-induced psychosis (n = 3). Within the FEP group, individuals were further categorized as having schizophrenia-associated psychosis (SAP) or MAP. As 2 recent meta-analyses47,48 found schizoaffective patients to have illness characteristics more similar to schizophrenia patients than that of individuals with bipolar psychosis and major depression, we categorized individuals with diagnoses of schizophrenia or schizoaffective disorder into the schizophrenia-associated psychosis (SAP) group (n = 64). Individuals with bipolar psychosis and major depression with psychosis comprised the mood-associated psychosis (MAP) group (n = 25). Individuals with psychotic disorder not otherwise specified, drug-induced psychosis, schizophreniform disorder, and brief psychosis were not categorized into the SAP and MAP subgroups.
In the FEP group, 11 patients were unmedicated at the time of the study visit, of which 8 had been medicated with a first or second generation antipsychotic medication in the past. Four patients were taking a first-generation antipsychotic medication, 76 were taking second-generation antipsychotic medication, and 6 were taking a combination of both first- and second-generation antipsychotic medications. Available antipsychotic medication dosages were converted to chlorpromazine equivalents using published reference tables.49 Medication dosage information was unavailable for 4 patients.
Demographic Analysis
Means, SDs, and frequencies for clinical and demographic variables are presented in table 1. The FEP group and controls did not differ with respect to age (F1,193 = 1.56, P = .21) and race (χ2 = 6.72, df = 4, P = .15). Groups differed with respect to sex composition (χ2 = 18.56, df = 1, P < .001), as the FEP group was comprised of a significantly greater proportion of men (74.23%) than the comparison group (43.88%). The control participants had more years of education (F1,193 = 19.37, P < .001). However, groups did not differ with regard to parental education (Wilks F2,137 = 0.05, P = .95), an estimate of potential that minimizes the confound of illness.50,51 Overall group differences in smoking, as measured by current packs per day, were statistically significant as the FEP group smoked more than control subjects (F1,193 = 13.00, P < .001).
Table 1.
Demographic and Clinical Characteristics of the Overall Sample
| FEP (n = 97) | SAP (n = 64) | MAP (n = 25) | HC (n = 98) | |
|---|---|---|---|---|
| Mean (SD)/ n | Mean (SD)/ n | Mean (SD)/ n | Mean (SD)/ n | |
| Age (y) | 22.45 (4.25) | 22.41 (4.15) | 23.52 (4.63) | 23.17 (3.78) |
| Sex (men:women) | 72:25 | 49:15 | 16:9 | 43:55 |
| Education level (y) | 13.25 (2.44) | 13.11 (2.34) | 14.00 (2.78) | 14.66 (2.04) |
| Pack-days | 0.08 (0.19) | 0.10 (0.22) | 0.04 (0.14) | 0.01 (0.05) |
| Illness duration (mo) | 14.02 (11.04) | 15.73 (11.40) | 11.88 (9.74) | — |
| Age of onset (y) | 21.30 (4.32) | 21.08 (4.28) | 22.56 (4.63) | — |
| Chlorpromazine equivalents | 272.52 (272.65) | 314.37 (290.51) | 179.93 (212.58) | — |
| MMSEa total score | 27.67 (2.19) | 27.48 (2.32) | 28.21 (1.79) | — |
| SANSb total score | 28.21 (20.38) | 32.77 (20.44) | 18.63 (17.60) | — |
| SAPSc total score | 14.93 (18.19) | 17.84 (17.95) | 9.67 (18.90) | — |
Note: FEP, First-Episode Psychosis; SAP, Schizophrenia-associated pyschosis; MAP, Mood-associated psychosis; HC, Healthy controls.
aMMSE, Mini-Mental State Examination.55
bSANS, Scale for the Assessment of Negative Symptoms.56
cSAPS, Scale for the Assessment of Positive Symptoms.57
Demographics variables were also compared between the control group and the aforementioned SAP and MAP subgroups. The 3 groups did not differ with respect to age, race, or parental education but showed significant overall differences with respect to sex composition (χ2 = 17.41, df = 2, P < .001), education (F2,184 = 9.21, P < .01), and current smoking (F2,184 = 8.34, P < .01). Healthy control subjects and MAP subjects did not differ with respect to education (F1,121 = 1.80, P = .18) but MAP subjects had higher smoking levels (F1,121 = 4.01, P = .05). Compared to controls, SAP subjects had lower educational attainment (F1,160 = 19.99, P < .01) and higher smoking levels (F1,160 = 16.57, P < .01). The MAP (χ2 = 3.23, df = 1, P = .07) and SAP (χ2 = 16.86, df = 1, P < .001) subgroups were comprised of a greater proportion of men than the comparison group. The SAP and MAP subgroups did not differ with respect to sex composition, education, mental status, packs per day, age of onset, or duration of illness (all Ps > .13). However, medication dosages, as measured by chlorpromazine equivalents, were greater in the SAP group (F1,83 = 4.23, P = .04).
Olfactory Assessment
Odor Identification and Discrimination.
Participants were first administered the Sniffin’ Sticks Odor Identification and Discrimination test.52,53 Each individual was presented with a sequence of 16 scented pens birhinally. Following the presentation of each odorant, individuals were asked to identify the correct odor from 4 choices. During the 16-trial odor discrimination test, 3 scented pens were placed under the subject’s nares in succession. Subjects were asked to identify which odorant differed from the other 2. Overall accuracy scores were calculated by tallying the number of odors correctly identified and discriminated.
Odor Detection Threshold.
Participants were then administered 2 odor detection threshold tasks utilizing lyral and citralva as the active odorants in a counterbalanced order. The task followed a single reversing staircase, forced-choice format in which individuals were presented with 2 vials, one with mineral oil and one containing the active odorant diluted in mineral oil. Individuals were then asked to identify which vial “smelled stronger.” The concentration of the active odorant, citralva or lyral, to mineral oil ranged from 10−10 molar (weakest) to 10−1 molar (strongest); the test began at the 10−5 molar step. The concentration was increased in full-molar increments until the participant correctly detected the odor on 5 consecutive trials. Once this baseline was established, the odor concentration was either increased following an incorrect trial or decreased after 2 correct trials in half-molar increments. The task was stopped once 7 reversal points were reached. As described in prior work,26,54 the average of the last 4 threshold reversal points out of 7 total reversals was calculated for each task in order to generate a total score. The total detection threshold score reflected the weakest odor concentration reliably identified as stronger than mineral oil. The odor detection task was initiated later in study enrollment resulting in smaller analytic sample sizes. Fifty-five FEP patients and 66 control subjects had valid odor detection results for inclusion.
Clinician-Administered and Self-Report Measures
The Mini-Mental State Examination (MMSE)55 and Scales for the Assessment of Negative Symptoms (SANS)56 and Positive Symptoms (SAPS)57 were administered by an experienced clinician to characterize mental status and positive and negative symptom severity in each patient. Participants completed inventories to assess physical anhedonia, hedonic capacity, and empathic concern. The 61-item Physical Anhedonia Scale (PAS)58,59 is a self-report scale that assesses the diminished ability to experience pleasure. The Temporal Experience of Pleasure Scale (TEPS)60 is 18-item self-report inventory designed to capture anticipatory and consummatory facets of hedonic pleasure. Individuals also completed two 7-item subscales of the Interpersonal Reactivity Index (IRI)61 to assess empathic concern and perspective-taking abilities.
Statistical Analyses
Raw olfactory scores were not normally distributed. However, Kolmogorov-Smirnov tests conducted on model residuals were not statistically significant and normal probability plots of the residuals did not show significant deviation from normality. As such, multivariate analysis of covariance was conducted to examine overall differences in odor identification and discrimination performance between FEP patients and controls. Group (FEP patient and control) and sex were entered as between-group factors with scores on the odor identification and odor discrimination measures entered as within-subject factors. Given the significant group differences in smoking, these analyses were repeated with pack-days entered as a covariate. We then compared olfactory performances between controls and the SAP and MAP subgroups. Group (control, SAP and MAP) and sex were entered as between-group factors with scores on the odor identification and odor discrimination measures entered as within-subject factors. Given the significant group differences in medication dosage between the MAP and SAP subgroups, all post-hoc analyses between these subgroups were repeated with chlorpromazine equivalents entered as a covariate. Sex-stratified post hoc analyses were employed as sex differences in olfactory performance have been noted.14,62,63 Means, SDs, and frequencies for olfactory scores stratified by sex are presented in supplementary table 1. To examine group differences in odor detection threshold task performances, the above analyses were repeated with performance on the lyral and citralva trials entered as the within-subject factors.
Relationships between olfactory performance indices and clinical symptoms (negative symptoms, positive symptoms), self-report measures, and medication dosage were assessed with Pearson correlations in the FEP group and MAP/SAP subgroups.
Finally, primary analyses were repeated using non-parametric methods (ie, Kruskal Wallis ANOVA and Spearman correlations) with the same pattern of findings noted below.
Results
Odor Identification and Discrimination Performance
We first examined differences in odor identification and discrimination performance between FEP patients and healthy controls. The FEP group had greater difficulty on measures of odor discrimination and odor identification ability compared to healthy controls (F1,191 = 10.26, P < .001). The main effect of sex (F1,191 = 1.94, P = .16), group by sex interaction (F1,191 < 0.01, P = .98), and group by task interaction (F1,191 = 1.13, P = .29) was not statistically significant. Overall group differences in olfactory task performance persisted after including current smoking as a covariate (F1,190 = 10.02, P < .001) and when men and women were examined separately. Means and SDs of olfactory scores appear in table 2.
Table 2.
Means (± SDs) of Olfactory Performance Scores
| FEP (n = 97) | SAP (n = 64) | MAP (n = 25) | HC (n = 98) | |
|---|---|---|---|---|
| Olfactory performance scores | ||||
| Odor identification (16 items) | 11.31 (2.46) | 11.09 (2.43) | 12.28 (2.28) | 12.03 (1.89) |
| Odor discrimination (16 items) | 9.72 (2.43) | 9.50 (2.44) | 10.32 (2.29) | 10.96 (1.99) |
| (n = 55) | (n = 35) | (n = 18) | (n = 66) | |
| Odor detection threshold scoresa | ||||
| Lyral detection threshold | −4.22 (0.77) | −4.18 (0.84) | −4.29 (0.68) | −4.49 (0.93) |
| Citralva detection threshold | −4.52 (0.93) | −4.26 (0.72) | −4.99 (1.15) | −4.96 (0.92) |
Note: FEP, First-Episode Psychosis; SAP, Schizophrenia and schizoaffective disorder; MAP, Mood associated psychosis; HC, Healthy controls.
aLower scores reflect better detection threshold performance.
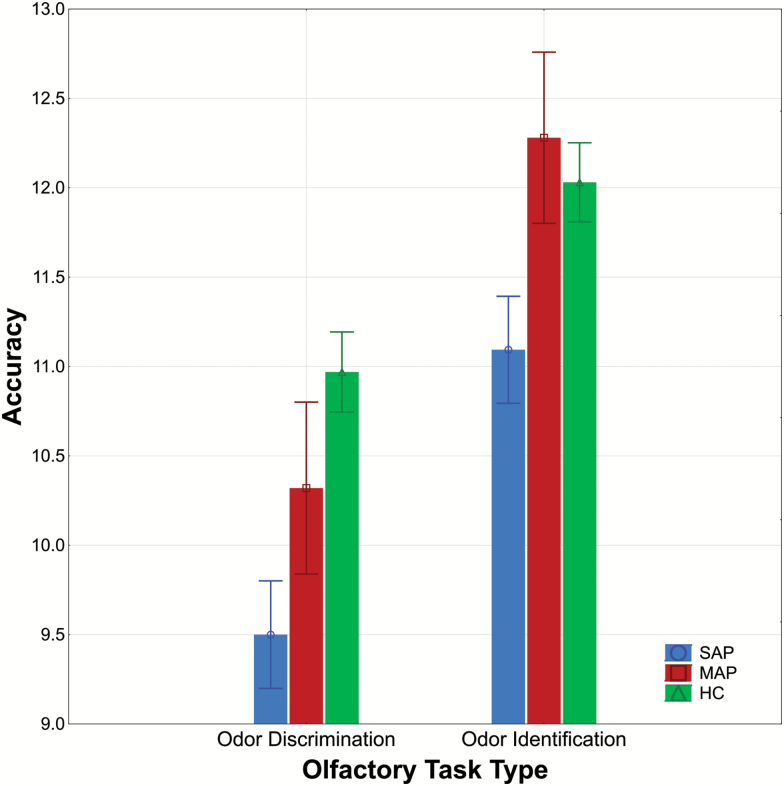
We then examined odor identification and discrimination performance in the controls and SAP and MAP subgroups. The main effect of group was statistically significant (F2,181 = 7.96, P < .001; see figure 1). Individuals in the MAP group did not differ significantly from controls (F1,119 = 0.04, P = .84). In contrast, the SAP group had significantly reduced odor identification and discrimination performances relative to controls (F1,158 = 15.59, P < .001) and the MAP subgroup (F1,85 = 5.23, P = .02). These latter differences between the SAP and MAP subgroups persisted after including medication dosage as a covariate (F1,80 = 4.82, P = .03). There was no statistically significant group by task interaction (F2,181 = 1.02, P = .36).
Fig. 1.
Olfactory task performance (±SE) in first-episode psychosis (FEP) patients with schizophrenia-associated psychosis (SAP) and mood-associated psychosis (MAP) relative to healthy comparison (HC) subjects. Note: Individuals in the SAP group performed significantly worse across both tasks compared to the control group (P < .001) whereas the MAP group did not differ significantly from controls.
Though the main effect of sex (F1,181 = 1.21, P = .27) and group by sex interaction (F2,181 = 0.50, P = .60) were not statistically significant, we employed exploratory post hoc analyses to examine differences separately between men and women. Men and women in the MAP group did not differ from healthy men and women (Ps > .62). Women in the SAP group produced poorer odor identification and discrimination scores compared to women in the MAP group (F1,22 = 4.72, P = .04) whereas differences between men in the SAP and MAP subgroups were not statistically significant (F1,63 = 1.52, P = .22). This pattern of findings remained after including current smoking burden as a covariate in the overall analyses and medication dosage as a covariate in contrasts between the FEP subgroups.
Relationship of Odor Identification and Discrimination Performance to Illness Characteristics and Self-Report Measures
We examined relationships between odor identification and discrimination performance and clinical and illness characteristics in the FEP group. In the FEP group, poorer odor discrimination ability was associated with increased clinician-rated negative symptomatology (r = –.27, P < .01) and lower self-reported empathic concern (r = .23, P = .03). Furthermore, poorer odor identification accuracy was associated with increased self-reported physical anhedonia (r = –.23, P = .03) and higher clinician-rated negative symptomatology (r = –.27, P < .01) in the FEP group. Chlorpromazine equivalents were not significantly related to odor identification or discrimination scores in FEP patients (all Ps > .39).
In the SAP subgroup, increased self-reported physical anhedonia (r = –.37, P < .01) and clinician-rated negative symptomatology (r = –.28, P = .03) were associated with reduced odor identification ability; these associations were not observed in the MAP subgroup (all Ps > .16). Better self-reported perspective-taking (r = .31, P = .01) and consummatory pleasure (r = .31, P = .01) was associated with increased odor identification ability in the SAP group, but not in the MAP group (all Ps > .16). These associations were not robust to correction for multiple comparisons.
Odor Detection Threshold for Lyral and Citralva
Odor detection thresholds for lyral and citralva were then examined in the FEP group relative to healthy subjects. FEP patients had poorer odor detection thresholds compared to the control group (F1,117 = 8.44, P < .01). The main effect of sex (F1,117 = 0.16, P = .69) and sex by group interaction (F1,117 = 0.63, P = .43) was not statistically significant. Similarly, the interaction of group and odorant type (lyral and citralva) was not statistically significant (F1,117 = 0.11, P = .74). After including smoking as a covariate, the main effect of group persisted (F1,116 = 7.77, P < .01) and all other interactions remained nonsignificant (all Ps > .18). This pattern of findings persisted when men and women were examined separately. Means and SDs for odor detection thresholds are presented in table 2.
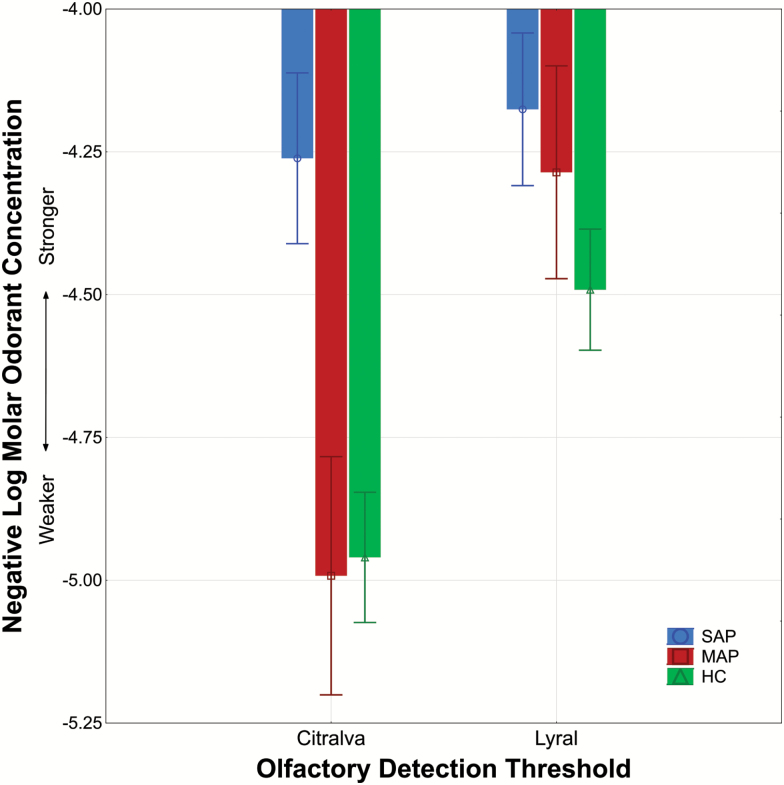
We then compared odor detection threshold performances between controls and SAP and MAP subgroups. The main effect of group was statistically significant (F2,113 = 9.04, P < .01; see figure 2) with individuals in the SAP, but not MAP group, showing overall differences from controls (F1,97 = 16.87, P < .01). Furthermore, the task (lyral and citralva) by sex by group interaction approached statistical significance (F2,113 = 2.70, P = .07). Women in the MAP group tended to show better odor detection thresholds for citralva but no differences for lyral compared to healthy women and women in the SAP group. Women in the SAP group produced poorer odor detection thresholds for citralva (F1,42 = 9.01, P < .01) but not lyral compared to healthy women (F1,42 = 2.88, P = .10). In contrast, men in the MAP group did not differ from healthy men or men in the SAP group on either odor detection threshold task (all Ps > .28). Men in the SAP group produced poorer odor detection thresholds for citralva (F1,55 = 6.97, P = .01) but not lyral (F1,55 = 0.18, P = .67) compared to healthy men. This pattern of findings remained after including current smoking burden as a covariate in the overall analyses and medication dosage as a covariate in contrasts between the FEP subgroups.
Fig. 2.
Odor threshold detection sensitivity (±SE) to lyral and citralva in first-episode psychosis (FEP) patients with schizophrenia-associated psychosis (SAP) and mood-associated psychosis (MAP) relative to healthy comparison (HC) subjects. Note: The SAP subgroup had poorer odor detection thresholds for citralva (P = .01) but not lyral compared to the control group. The MAP group did not differ significantly from controls. Y-axis values closer to −4.00 indicate poorer odor detection threshold sensitivity.
Relationship of Odor Detection Threshold Performance to Illness Characteristics
In the FEP group, better odor detection threshold for citralva was associated with decreased clinician-rated positive symptomatology (r = .31, P = .02) whereas better odor detection threshold for lyral was associated with increased self-reported physical anhedonia (r = –.31, P = .02). No significant associations were observed between lyral and citralva detection thresholds and chlorpromazine equivalents (all Ps > .29). In the SAP subgroup, better odor detection threshold for lyral was associated with increased self-reported physical anhedonia (r = –.39, P = .02), which was not demonstrated in the MAP cohort. These associations were not robust to correction for multiple comparisons.
Discussion
In the present study, we found that individuals newly diagnosed with a psychotic disorder demonstrated reduced odor identification, odor discrimination, and odor detection threshold performances when compared to healthy controls. These results could not be explained by the influence of sex or current smoking burden. Further investigation within the FEP group revealed that schizophrenia and schizoaffective patients had reduced olfactory functioning relative to controls, whereas olfactory profiles in individuals with bipolar psychosis and psychotic depression were similar to healthy controls. These findings indicate that olfactory deficits extend beyond measures of odor identification in FEP with greater olfactory dysfunction observed in schizophrenia-associated subgroups of psychosis.
Though it is well-established that the diagnosis of schizophrenia and schizoaffective disorder is associated with olfactory dysfunction,14 considerably fewer studies have examined the degree of olfactory impairment in other forms of psychosis. In one longitudinal study of individuals at ultra-high risk for psychosis, greater odor identification deficits at baseline assessment were observed in subjects that ultimately converted to schizophrenia when compared with individuals who transitioned to another mental illness, such as bipolar psychosis.31 Prior work has also indicated that youths with prodromal symptoms of psychosis show similar profiles of odor identification and discrimination impairment as those observed in adults with schizophrenia.25 Collectively, our findings indicate that olfactory measures may be informative surrogate biological markers of disease susceptibility given the widespread olfactory dysfunction observed in schizophrenia and schizoaffective disorder. In contrast, individuals with psychotic depression and bipolar psychosis showed mild reduction in olfactory functioning that was not statistically different from healthy controls. Though several studies in bipolar disorder have indicated intact odor identification and discrimination scores,34,35,64 2 investigations observed abnormal odor hedonic processing in bipolar disorder40,65 and potential relationships between odor identification accuracy and facial emotion recognition.66 Thus, further investigation on the utility of odor hedonic measures in mood disorders is warranted.
We examined birhinal odor detection thresholds for a strong cAMP activator, citralva, and a weak cAMP activator, lyral, across the psychosis spectrum. Previous work by Turetsky and colleagues26,54 found that schizophrenia patients, their adult first-degree relatives, and individuals with prodromal psychosis showed reduced ability to detect lyral despite normal perception of citralva. These differential odor detection threshold findings based on adenylyl cyclase activation were thought to be associated with altered intracellular cAMP signaling in schizophrenia. Contrary to expectation, we found the opposite effect, in that first-episode patients with schizophrenia-associated psychosis displayed reduced detection threshold deficits for citralva but not lyral. In contrast, detection threshold performances within the MAP group varied as a function of sex. It remains unclear if differences in the clinical characteristics of our population (positive symptomatology, medication effects, sex distribution, estrogen levels), method of assessment (birhinal vs unirhinal) and instrumentation accounted for the inability to replicate prior work. It is also possible that these differences reflect the heterogeneity of schizophrenia populations assessed.
Though the small sample sizes within the MAP cohort limited our ability to make inferences regarding the sex differences observed, our preliminary findings indicate possible differences in olfactory profiles between men and women with bipolar psychosis and psychotic depression. The extant literature in schizophrenia14,62,63 and healthy people67 has indicated that sex influences olfactory functioning; however, the largest study of early psychosis patients to date found no diagnosis-specific sex differences in odor identification performance.15 Even less is known about how sex moderates olfactory performance in MAP though investigations thus far have found no influence of sex in bipolar disorder.65,68 As multiple studies have described differences in illness characteristics, social functioning, disability level, and coping strategies between men and women with psychosis (for review, see: Hanlon69), future studies examining these factors in relation to olfactory performance would be an important step in furthering our understanding of sex effects and olfactory functioning across the psychosis spectrum.
Relationships between olfactory deficits and increased self-reported and clinician-rated negative symptoms were examined in the FEP group and in the SAP and MAP subgroups. Across all FEP patients, greater olfactory disturbance was associated with increased clinician-rated negative symptomatology, greater self-reported physical anhedonia, and lower self-reported anticipatory pleasure. These findings persisted in the schizophrenia and schizoaffective subgroup but were absent in the MAP subgroup. Though these associations were not robust to correction for multiple comparisons, our results are highly consistent with prior examinations noting a strong association between olfactory dysfunction and negative symptoms of schizophrenia.19,21,25,42,70–72 It is possible that abnormalities in orbitofrontal-limbic pathways contribute to the olfactory dysfunction and negative symptoms observed in schizophrenia. Prior work has demonstrated that anhedonia is a significant predictor of conversion to psychosis33 and functional outcome in schizophrenia.73 Therefore, olfactory assessment may be useful in identifying FEP patients at greatest need for psychosocial intervention and as a translational correlate of negative symptoms in treatment studies.
Collectively, the current study indicates that olfactory deficits extend beyond measures of odor identification in FEP and further indicates that olfactory processing abnormalities dissociate between primary mood and schizophrenia-related subgroups. Future studies will allow us to examine the relationship between olfactory measures and neurocognitive performance, structural and functional neuroimaging indices, and molecular signatures ascertained from olfactory neuronal cells collected via nasal biopsy. Through these multilayered analyses, we previously found that a molecular signature of the downregulated SMAD pathway in olfactory neural epithelium was associated with cognitive deficits in an independent chronic schizophrenia cohort.74 The longitudinal component of the current study will also allow us to examine whether widespread olfactory deficits at the onset of illness can identify and properly stratify those patients at increased risk for poor outcome.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by the National Institutes of Health (MH092443, MH094268, and DA040127 to A.S. and MH105660 to K.I. and A.S.). A portion of participant recruitment costs were supported by the Mitsubishi Tanabe Pharma Corporation. V.K. is supported through the Johns Hopkins Clinical Research Scholars Program (KL2TR001077). The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of the sponsoring organizations, agencies, or US Government.
Supplementary Material
Acknowledgments
The authors wish to extend their gratitude to the participants in this study. We also wish to thank Thomas Sedlak, MD, PhD, Jennifer Coughlin, MD, Crystal Watkins, MD, PhD, Frederick Nucifora, DO, PhD, Carolyn Howell, MD, Kinya Okada, PhD, Yukiko Lema, Samantha DuBois, Danielle Sullivan, Aditi Trivedi, Candace Ford, Pearl Kim, Carrie Andrews, Stephanie Lechich, Jeffrey Crawford, Ashley Lloyd, Bernard Sarmiento, Lindsay Shaffer, Elizabeth Cifuentes, Ivana Su, Jamie Edwards, and Nao Gamo, PhD of the Johns Hopkins Schizophrenia Center for assistance with recruitment, assessment, data management and critical readings of the manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Lavoie J, Sawa A, Ishizuka K. Application of olfactory tissue and its neural progenitors to schizophrenia and psychiatric research. Curr Opin Psychiatry 2017;30:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen AD, Pelavin PE, Shenton ME et al. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 2011;5:252–261. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi T, Nakamura Y, Nakamura K et al. Altered depth of the olfactory sulcus in first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:167–172. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi T, Wood SJ, Yung AR et al. Altered depth of the olfactory sulcus in ultra high-risk individuals and patients with psychotic disorders. Schizophr Res. 2014;153:18–24. [DOI] [PubMed] [Google Scholar]
- 6. Arnold SE, Han LY, Moberg PJ et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. [DOI] [PubMed] [Google Scholar]
- 7. Borgmann-Winter K, Willard SL, Sinclair D et al. Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl Psychiatry. 2015;5:e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. English JA, Fan Y, Föcking M et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl Psychiatry. 2015;5:e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horiuchi Y, Kano S, Ishizuka K et al. Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res. 2013;77:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kano S, Colantuoni C, Han F et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry. 2013;18:740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackay-Sim A. Concise review: patient-derived olfactory stem cells: new models for brain diseases. Stem Cells. 2012;30:2361–2365. [DOI] [PubMed] [Google Scholar]
- 12. Mor E, Kano S, Colantuoni C, Sawa A, Navon R, Shomron N. MicroRNA-382 expression is elevated in the olfactory neuroepithelium of schizophrenia patients. Neurobiol Dis. 2013;55:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turetsky BI, Hahn CG, Borgmann-Winter K, Moberg PJ. Scents and nonsense: olfactory dysfunction in schizophrenia. Schizophr Bull. 2009;35:1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moberg PJ, Kamath V, Marchetto DM et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014;40:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Good KP, Leslie RA, McGlone J, Milliken HI, Kopala LC. Sex differences in olfactory function in young patients with psychotic disorders. Schizophr Res. 2007;97:97–102. [DOI] [PubMed] [Google Scholar]
- 16. Stedman TJ, Clair AL. Neuropsychological, neurological and symptom correlates of impaired olfactory identification in schizophrenia. Schizophr Res. 1998;32:23–30. [DOI] [PubMed] [Google Scholar]
- 17. Kopala L, Good K, Martzke J, Hurwitz T. Olfactory deficits in schizophrenia are not a function of task complexity. Schizophr Res. 1995;17:195–199. [DOI] [PubMed] [Google Scholar]
- 18. Brewer WJ, Edwards J, Anderson V, Robinson T, Pantelis C. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021–1031. [DOI] [PubMed] [Google Scholar]
- 19. Ishizuka K, Tajinda K, Colantuoni C et al. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neurosci Res. 2010;66:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cieslak K, Walsh-Messinger J, Stanford A et al. Olfactory performance segregates effects of anhedonia and anxiety on social function in patients with schizophrenia. J Psychiatry Neurosci. 2015;40:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brewer WJ, Pantelis C, Anderson V et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. [DOI] [PubMed] [Google Scholar]
- 22. Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1993;8:245–250. [DOI] [PubMed] [Google Scholar]
- 23. Rupp CI, Fleischhacker WW, Kemmler G et al. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull. 2005;31:155–165. [DOI] [PubMed] [Google Scholar]
- 24. Rupp CI, Fleischhacker WW, Kemmler G et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74:149–161. [DOI] [PubMed] [Google Scholar]
- 25. Kamath V, Turetsky BI, Calkins ME et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J Biol Psychiatry. 2014;15:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sawa A, Cascella NG. Peripheral olfactory system for clinical and basic psychiatry: a promising entry point to the mystery of brain mechanism and biomarker identification in schizophrenia. Am J Psychiatry. 2009;166:137–139. [DOI] [PubMed] [Google Scholar]
- 28. Kopala LC, Good KP, Torrey EF, Honer WG. Olfactory function in monozygotic twins discordant for schizophrenia. Am J Psychiatry. 1998;155:134–136. [DOI] [PubMed] [Google Scholar]
- 29. Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158:1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res. 2010;123:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brewer WJ, Wood SJ, McGorry PD et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. [DOI] [PubMed] [Google Scholar]
- 32. Good KP, Tibbo P, Milliken H et al. An investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosis. Schizophr Res. 2010;124:60–65. [DOI] [PubMed] [Google Scholar]
- 33. Velthorst E, Nieman DH, Becker HE et al. Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109:60–65. [DOI] [PubMed] [Google Scholar]
- 34. Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol Psychiatry. 1988;23:123–128. [DOI] [PubMed] [Google Scholar]
- 35. Krüger S, Frasnelli J, Bräunig P, Hummel T. Increased olfactory sensitivity in euthymic patients with bipolar disorder with event-related episodes compared with patients with bipolar disorder without such episodes. J Psychiatry Neurosci. 2006;31:263–270. [PMC free article] [PubMed] [Google Scholar]
- 36. Clepce M, Gossler A, Reich K, Kornhuber J, Thuerauf N. The relation between depression, anhedonia and olfactory hedonic estimates–a pilot study in major depression. Neurosci Lett. 2010;471:139–143. [DOI] [PubMed] [Google Scholar]
- 37. Pause BM, Miranda A, Göder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. [DOI] [PubMed] [Google Scholar]
- 38. Negoias S, Croy I, Gerber J et al. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience. 2010;169:415–421. [DOI] [PubMed] [Google Scholar]
- 39. Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL. Odor perception in patients with mood disorders. J Affect Disord. 2006;90:187–191. [DOI] [PubMed] [Google Scholar]
- 40. Swiecicki L, Zatorski P, Bzinkowska D, Sienkiewicz-Jarosz H, Szyndler J, Scinska A. Gustatory and olfactory function in patients with unipolar and bipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:827–834. [DOI] [PubMed] [Google Scholar]
- 41. Pause BM, Raack N, Sojka B, Göder R, Aldenhoff JB, Ferstl R. Convergent and divergent effects of odors and emotions in depression. Psychophysiology. 2003;40:209–225. [DOI] [PubMed] [Google Scholar]
- 42. Good KP, Whitehorn D, Rui Q, Milliken H, Kopala LC. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am J Psychiatry. 2006;163:932–933. [DOI] [PubMed] [Google Scholar]
- 43. Lin A, Wood SJ, Nelson B et al. Neurocognitive predictors of functional outcome two to 13 years after identification as ultra-high risk for psychosis. Schizophr Res. 2011;132:1–7. [DOI] [PubMed] [Google Scholar]
- 44. Nurnberger JI Jr, Blehar MC, Kaufmann CA et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859; discussion 863. [DOI] [PubMed] [Google Scholar]
- 45. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV - Patient Edition (SCID-P, Version 2.0).New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- 46. Schretlen DJ, Winicki JM, Meyer SM, Testa SM, Pearlson GD, Gordon B. Development, psychometric properties, and validity of the hopkins adult reading test (HART). Clin Neuropsychol. 2009;23:926–943. [DOI] [PubMed] [Google Scholar]
- 47. Rink L, Pagel T, Franklin J, Baethge C. Characteristics and heterogeneity of schizoaffective disorder compared with unipolar depression and schizophrenia - a systematic literature review and meta-analysis. J Affect Disord. 2016;191:8–14. [DOI] [PubMed] [Google Scholar]
- 48. Pagel T, Baldessarini RJ, Franklin J, Baethge C. Characteristics of patients diagnosed with schizoaffective disorder compared with schizophrenia and bipolar disorder. Bipolar Disord. 2013;15:229–239. [DOI] [PubMed] [Google Scholar]
- 49. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed]
- 50. Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990;47:1066–1072. [DOI] [PubMed] [Google Scholar]
- 51. Resnick SM. Matching for education in studies of schizophrenia. Arch Gen Psychiatry. 1992;49:246. [DOI] [PubMed] [Google Scholar]
- 52. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. [DOI] [PubMed] [Google Scholar]
- 53. Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. “Sniffin’ sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 54. Kamath V, Moberg PJ, Calkins ME et al. An odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosis. Schizophr Res. 2012;138:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 56. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 57. Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS).Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 58. Chapman LJ, Edell WS, Chapman JP. Physical anhedonia, perceptual aberration, and psychosis proneness. Schizophr Bull. 1980;6:639–653. [DOI] [PubMed] [Google Scholar]
- 59. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–382. [DOI] [PubMed] [Google Scholar]
- 60. Chan RC, Shi YF, Lai MK, Wang YN, Wang Y, Kring AM. The Temporal Experience of Pleasure Scale (TEPS): exploration and confirmation of factor structure in a healthy Chinese sample. PLoS One. 2012;7:e35352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85–100. [Google Scholar]
- 62. Malaspina D, Keller A, Antonius D et al. Olfaction and cognition in schizophrenia: sex matters. J Neuropsychiatry Clin Neurosci. 2012;24:165–175. [DOI] [PubMed] [Google Scholar]
- 63. Seidman LJ, Goldstein JM, Goodman JM et al. Sex differences in olfactory identification and Wisconsin Card Sorting performance in schizophrenia: relationship to attention and verbal ability. Biol Psychiatry. 1997;42:104–115. [DOI] [PubMed] [Google Scholar]
- 64. Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485. [DOI] [PubMed] [Google Scholar]
- 65. Cumming AG, Matthews NL, Park S. Olfactory identification and preference in bipolar disorder and schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2011;261:251–259. [DOI] [PubMed] [Google Scholar]
- 66. Lahera G, Ruiz-Murugarren S, Fernández-Liria A, Saiz-Ruiz J, Buck BE, Penn DL. Relationship between olfactory function and social cognition in euthymic bipolar patients. CNS Spectr. 2016;21:53–59. [DOI] [PubMed] [Google Scholar]
- 67. Good K, Kopala L. Sex differences in olfactory function. In: Brewer W, Castle D, Pantelis C, eds. Smell and the Brain: Window to the Mind. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 68. Hardy C, Rosedale M, Messinger JW et al. Olfactory acuity is associated with mood and function in a pilot study of stable bipolar disorder patients. Bipolar Disord. 2012;14:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hanlon MC, Campbell LE, Single N et al. Men and women with psychosis and the impact of illness-duration on sex-differences: the second Australian national survey of psychosis. Psychiatry Res. 2017;256:130–143. [DOI] [PubMed] [Google Scholar]
- 70. Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophr Bull. 2010;36:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. [DOI] [PubMed] [Google Scholar]
- 72. Moberg PJ, Arnold SE, Doty RL et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. [DOI] [PubMed] [Google Scholar]
- 73. Kiwanuka JN, Strauss GP, McMahon RP, Gold JM. Psychological predictors of functional outcome in people with schizophrenia. Schizophr Res. 2014;157:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Horiuchi Y, Kondo MA, Okada K et al. Molecular signatures associated with cognitive deficits in schizophrenia: a study of biopsied olfactory neural epithelium. Transl Psychiatry. 2016;6:e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.