Abstract
Compared with oral antipsychotics (OAPs), long-acting injectable antipsychotics (LAIs) should improve medication adherence and reduce relapses in schizophrenia. However, meta-analyses of randomized trials and mirror-image studies yielded inconsistent results. Nonrandomized cohort studies with parallel comparisons of LAIs and OAPs offer a third design to examine this issue. We meta-analyzed cohort studies with ≥24 weeks duration and hospitalization data. Primary outcome was hospitalization rate, ie, number of hospitalizations per person-year. Secondary outcomes included hospitalization risk, ie, proportion of patients experiencing ≥1 hospitalizations, all-cause discontinuation, and total hospitalization days. Patient severity and/or chronicity at baseline was also meta-analyzed and explored as a potential effect size moderator. Altogether, 42 studies (n = 101 624; follow-up = 18.6 ± 10.0 mo) were meta-analyzed. LAIs were superior to OAPs regarding hospitalization rate (studies = 15, person-years = 68 009, rate ratio = 0.85, 95% CI = 0.78–0.93, P < .001) and all-cause discontinuations (studies = 10, n = 37 293, risk ratio = 0.78, 95% CI = 0.67–0.91, P = .001), but not regarding hospitalization risk (studies = 33, n = 51 733, risk ratio = 0.92, 95% CI = 0.84–1.00, P = .06), and hospitalization days (studies = 11, n = 21 328, Hedges’ g = −0.05, 95% CI = −0.16 to 0.06, P = .39). Illness severity/chronicity was significantly greater in patients prescribed LAIs vs OAPs when all available information was pooled together (studies = 23, n = 61 806, Hedges’ g = 0.15, 95% CI = 0.03–0.26, P = .01), but not when examined separately. In summary, this meta-analysis of cohort studies, which included patients that are broadly representative of clinical practice, indicates that LAIs are superior to OAPs. The lack of significant superiority of LAIs for hospitalization risk and hospital days needs to be interpreted in the context of naturalistic treatment selection with subsequently greater illness severity/chronicity in LAI-treated patients.
Keywords: maintenance therapy, hospitalization, depot, psychosis, treatment discontinuation, moderators
Introduction
Because psychopathology and social functioning can worsen with repeated psychotic episodes in patients with schizophrenia,1,2 relapse prevention is a critical goal. There is strong evidence of antipsychotic efficacy for relapse prevention in chronic and first-episode patients.3,4 Relapse risk is 2–6 times higher without antipsychotics.3–6 However, because non-adherence, which occurs in up to 50% of patients, limits the clinical efficacy of pharmacotherapy,7,8 long-acting injectable antipsychotics (LAIs) are an important option.9 LAIs are often recommended for individuals with known or expected non-adherence to oral antipsychotics (OAPs).
There are inconsistencies in the literature comparing the effectiveness of LAIs and OAPs for schizophrenia. Several large randomized controlled trials (RCTs) have not shown significant superiority of LAIs over OAPs.10–13 However, significant advantages were identified in a few studies that targeted populations with early stage illness14,15 or a special population that required not only relapse prevention, but also reduction in risk of criminal justice involvement.16 A recent meta-analysis of RCTs did not find a significant difference between LAIs and OAPs in preventing relapse or hospitalization or in secondary outcomes related to relapse.17 However, RCTs might enroll a disproportionate number of patients with better treatment adherence and lower illness severity.18 In addition, participation in clinical trials can alter the ecology of treatment delivery and experience. For example, patients may receive appointment reminders, reimbursements, free medication, and adherence assessments.18 Therefore, the standard RCT might not be the best strategy to examine the effectiveness of LAIs compared to OAPs.19 Conversely, a subsequent meta-analysis of mirror-image studies, in which hospitalization risk/rate during a period of OAP treatment was compared with a subsequent period of LAI treatment in the same patients, demonstrated significant superiority of LAIs over OAPs.20 Effect sizes were large for preventing hospitalization (risk ratio = 0.43) and decreasing the number of hospitalizations (rate ratio = 0.38). However, mirror image studies are vulnerable to critical methodological limitations including expectation bias and regression to the mean.18
Given inconsistent results and different biases inherent in RCTs and mirror image studies, nonrandomized, but parallel comparison cohort studies of LAIs and OAPs are a third design to explore the comparative effectiveness of LAIs and OAPs. We, therefore, conducted a meta-analysis of cohort studies that provided information about hospitalization or relapse-related data.
Methods
The meta-analysis followed MOOSE guidelines for reporting meta-analyses of observational studies.21
Study Selection
We selected cohort studies with hospitalization of LAIs and OAPs in adults with schizophrenia and related disorders. Cohort studies, by definition involve a design where samples are followed prospectively (though some cohort studies examine data retrospectively) and subsequent status/outcome evaluations are conducted to determine differences between a priori defined groupings. In our case, we collected studies that followed patients who initiated LAIs or OAPs and provided hospitalization data. We included both prospective and retrospective cohort studies. The former refers to studies in which 2 groups were started and followed prospectively, while the latter refers to studies in which the investigator collected data from past records, but the 2 cohorts were assessed longitudinally in a parallel manner from the point of cohort inception.
Data Sources
We conducted a search without language restrictions, using MEDLINE/PubMed, Cochrane library, PsycINFO, and CINAHL from database inception (last search: December 3, 2016), for cohort studies of patients with schizophrenia and related disorders with a prospective observation period of ≥24 weeks. We also searched for unpublished studies, such as conference proceedings and clinical trial registries (http://clinicaltrials.gov/). Search terms included synonyms of (1) antipsychotic(s) AND (2) schizophrenia and related disorders AND (3) depot, (long-acting) injection(s), microsphere, decanoate, palmitate, enanthate, monohydrate. Hand searches of reference lists of relevant publications were also conducted. When multiple reports referred to the same study or overlapping patient populations (eg, nationwide cohort studies with different publication years, but overlapping study year[s]), we included the newer or more extensive report.
Data Extraction
Data were extracted independently by ≥2 reviewers (T.K., K.H., M.N., C.U.C.) experienced in conducting literature searches and data extraction. Authors and companies were contacted to provide missing information and unpublished data. Disagreements were resolved by consensus. Foreign language papers were translated by bilingual speakers, and data extraction was double checked by at least 2 investigator (T.K., K.H., M.N.) using Google Translate (http://translate.google.com/).
Primary outcome was set as hospitalization rate. Secondary outcomes included hospitalization risk, all-cause discontinuation and total hospitalization days. We also compared severity and/or chronicity of the patients on LAIs vs OAPs, as in cohort studies, it was expected that as compared to patients on OAPs, patients on LAIs are likely to be have more severe or persistent conditions.
Data Synthesis
All data were double-entered into and meta-analyzed with Comprehensive Meta-Analysis Version 3 (BioStat) using a random effects model, as heterogeneity among studies was expected.22 For categorical variables, we computed rate ratio and/or risk ratio, with their 95% CIs, as the effect size, with values <1 indicating superiority of LAIs and values >1 indicating superiority of OAPs. Hospitalization rate was computed as the number of hospitalizations divided by person-years at risk. The rate ratios were calculated as the ratio of rates for LAIs vs OAPs. Hospitalization risk was computed as the number of patients who had ≥1 hospitalization divided by the number of patients at risk. The risk ratio was then calculated as the ratio of risk for LAIs vs OAPs. Numbers-needed-to-treat (NNTs) with 95% CIs were calculated for categorical outcomes by dividing 1 by the risk difference.
Reporting of hospitalization-related outcomes differed widely and rate ratio was calculated as described below. Some studies reported the mean number of hospitalization during the study period. In such cases, we calculated the number of hospitalizations by multiplying the mean number of hospitalizations and the number of patients. Similarly, we calculated patient-year by multiplying the duration of the study and the patients’ number. Some studies stopped the follow-up when patients had their first hospitalization with information of the length of time from baseline to hospitalization. In such cases, we counted the number of hospitalization and added up the follow-up length. Regarding risk ratio, there was no standardization of the observation time. For example, when a study reported only the proportion of patients who had ≥1 hospitalization during the study period, without reporting when the relapse occurred, we were only able to calculate risk ratio. In cases where we could extract both rate and risk, we used the data for rate ratio and risk ratio outcomes. To derive the effect size of patients’ illness severity/chronicity, we meta-analytically compared relevant clinical characteristics of patients on LAIs and OAPs. These included prior number of hospitalizations, prior hospitalization days, illness duration, and proportion of hospitalized patients in the last year. These parameters were examined separately and pooled. When reporting the pooled comparative severity/chronicity parameters, we used the variable reported by the most studies following the hierarchical order of frequency described above to avoid study overlaps and to reduce heterogeneity. For continuous variables related to prior hospitalization and illness duration at study entry, we computed Hedges’ g with 95% CIs as the effect size, with values <0 indicating superiority of LAIs or indicating that LAI patients were less severely/chronically ill, and with values >0 indicating inferiority of LAIs or that LAI patients were more severely/chronically ill. Heterogeneity was only inspected when there were ≥2 studies in an analysis. With regard to the heterogeneity, τ2, I2, Q, and P values are reported.23
We also conducted subgroup analyses in order to identify potential methodological biases or subpopulations in which primary outcome differed. Subgroup analyses were based on (1) country, (2) region (North-America, Western Europe, Asia or others), (3) publication year (published before 2000, from 2000 to 2009, 2010 or later), (4) pharmaceutical sponsorship, (5) data source (single institution, multiple institutions, large database studies, including nationwide registration and insurance databases), (6) LAI medication group (FGA, SGA, mix), (7) OAP medication group (FGA, SGA, mixed), (8) informed consent (Obtained/Not obtained), (9) study design (prospective vs retrospective), (10) statistical adjustment of differences in baseline patient characteristics (Yes/No), (11) study sample size (N = <100, 100–499, 500–999, ≥1000), (12) clozapine patients (Included/Not included), (13) analysis method (intention to treat (ITT) vs observed cases (OC)), (14) study quality score; Newcastle-Ottawa scale24 (high [score ≥8] vs low [score <8]), (15) same medication allocation (FGA-LAI vs FGA-OAP, SGA-LAI vs SGA-OAP, and (16) follow-up duration (median split, ie, >12 mo vs ≤12 mo).
The Newcastle-Ottawa quality scale captures representativeness of the exposed cohort; selection of the unexposed cohort; ascertainment of exposure; outcome of interest not present at start of study; control for important factor/additional factor; assessment of outcome; follow-up long enough for outcomes to occur; and adequacy of follow-up.
Egger’s regression test25 followed by Duval and Tweedie’s trim and fill method26 were used to assess publication bias. In this large, exploratory set of analyses, no adjustments were made to the P-values for the multiple comparisons; therefore, the P values should be interpreted with caution.
Results
Search and Study Characteristics
The literature search yielded 9498 citations. We identified 42 cohort studies with 101 624 participants diagnosed with schizophrenia followed for ≥6 months (supplementary figure 1). Study, patient, illness and treatment characteristics are summarized in table 1 (for additional details, see supplementary table 1).27–68 Altogether, 27 studies had a retrospective database design (n = 70 165) and 15 studies were prospective (n = 31 459). The number of patients per study ranged from 50 to 14 610 (median = 522), and the mean study duration was 18.6 ± 10.0 (range = 6–48, median = 12) months (duration: ≤1 y = 23 studies, ≥ 2 y = 15 studies). There were 14 studies with FGA LAIs (33.3%); 9 with mixed FGA and SGA LAIs (21.4%); 10 with risperidone LAI (23.8%); 6 with paliperidone LAI (14.3%); 1 with haloperidol LAI (2.4%); and 1 with risperidone and paliperidone LAIs (2.4%). There were 29 studies with FGA and SGA OAPs, (70.7%); 6 with any SGA OAP (12.2%); 3 with risperidone OAP (7.3%); 1 with any FGA OAP (2.4%); 1 with clozapine (2.4%); and 1 with haloperidol OAP (2.4%). The search yielded 15 studies that reported number of hospitalizations and 33 reported hospitalization risk. Six studies reported both outcomes (supplementary figure 1).
Table 1.
Study, Patient, Illness and Treatment Characteristics
| Study/Country | n a | Design/Data Source | F/U Duration (M) | Inclusion Criteria | Mean Age (SD) (y.o.) | Information Regarding Severity/Chronicity | Medication |
|---|---|---|---|---|---|---|---|
| LAI (n)b | |||||||
| OAP (n)b | |||||||
| Babiker,27 ‘87/ Canada | 1235 | Retro/MHSB and SPDP | 24 | SCZ Pts (ICD-9) who were discharged, not affective disorder. | Total 41.1 (15.8) | First admission 30.4%, Maintenance Therapy 69.6% | FGA LAI (368) |
| FGA (508) | |||||||
| Barrio et al28 ‘13/ Spain | 52 | Retro /Single center | 24 | Recent-onset SCZ (<2Y) who started RLAI, continued the medication and were followed up for ≥2Y (LAI group). Consecutively selected SCZ, matched by age and sex who started OAP after their first psychotic episode, were followed up for ≥2Y with appropriate adherence. | RLAI 26.9 (6.7) | NR | (26) |
| Any OAP 27.4 (7.5) | (26)c | ||||||
| Baser et al29 ‘15/ United States | 670 | Retro/Multi center | 12 | Aged ≥18 and <65 on the index date; Continuous health plan enrollment for 24M prior to the index date (baseline period) and for 12M after the index date; At least one pharmacy claim for an OAP or LAI in the baseline period; No LAI use between the first 2 claims for the index therapy, no claims for PP in the 24M baseline period. Individuals in the OAP cohort were also required to have no claims for their index OAP product for 6M prior to the index date and no claims for PP in the 12M follow-up period. | PP 51.3 (9.9) | Prior # Hp: 2.1 (3.3) Prior # Hp days: 30.7 (56.2) | (335) |
| OAP 51.2 (10.3) | Prior # Hp: 2.2 (3.0) Prior # Hp days: 44.1 (118.5) | (335) | |||||
| Bellido et al30 ‘08/ Spain | 60 | Pro/Single center | 12 | OP of SCZ or SCZAD (ICD-10 and DSM-IV), no Hp in the previous 6M, no other mental disease | Total 34.5 (8.9) | DOI ≥15Y for 68.3% of pts, Prior # Hp <5 for 76.7% of pts | Any LAI (35) |
| Any OAP (25) | |||||||
| Bitter et al31 ‘13/ Hungary | 9567 | Retro /NHIF | 12 | SCZ (ICD-10), started a new AP monotherapy, no prescription of the same medication within the previous 6 M, no other AP prescription after 30 days from inclusion. | RLAI 45.6 | NR | (1095) |
| OAP 48.2 | RIS (2480), OLA (1633), QUE (1587), AMI (920), CLO (790), ARI (601), ZIP (461) | ||||||
| Calabresi and Marchetti32 ‘83/ Italy | 56 | Retro /Single center | 12 | Chronic OP with SCZ on long term treatment, not first episode patients, not borderline personality disorder | FLU LAI 39.0 | Chronic patients | (18) |
| Any OAP 37.5 | (26) | ||||||
| Chan et al33 ‘15/ Taiwan | 379 | Retro /Single center | 12 | At least 18 years of age, recruited at the same regional hospital, E-Da Hospital, Schizophrenia (DSM IV-TR) | RLAI 33.8 | # of Hps in the last year 29/43 | (43) |
| OAP 39.4 | # of Hps in the last year 108/336 | (336), RIS, QUE, OLA, AMI, ZIP, PAL, FLU, CLO, SUL, ZOT, HAL | |||||
| Chue et al34 ‘05/ Canada | 137 | Retro /Multi center | NR | SCZ who initiated on RLAI or a new oral atypical AP | RLAI 45.3 | NR | (63) |
| OAP 39.1 | RIS (36), OLA (31), QUE (5), Other OAP (2) | ||||||
| Ciudad et al35 ‘12/ Spain | 597 | Pro / Multi center | 12 | OP, SCZ (DSM-IV) who required any modification in the treatment based on the risk of non-adherence, not already on LAI medication | Total 40.1 (11.1) | DOI years (SD): 15.2 (10.0) Onset of disease: 24.9 years old Prior # Hp ≤ 4 for 58.3% | RIS (79), ZUC (7), FLU (6) |
| OAP (505) | |||||||
| Conley et al36 ‘03/ United States | 411 | Pro / Multi center | 12 | SCZ(DSM-IV), started AP and discharged on the same medication | FLU 39.9 (9.4), HAL 35.1 (8.9) | Prior # Hp: 1.0 (1.2) | FLU (59), HAL (59) |
| RIS 38.0 (14.5), OLA 39.7 (13.7), CLO 36.9 (9.4) | Prior # Hp: 1.3 (1.6) | RIS (149), OLA (103), CLO (41) | |||||
| Conlon et al37 ’02/ Ireland | 69 | Pro / Multi center | 24 | SCZ(DSM-IV) who had been using the same FGA depot for ≥ 3M prior to the study. | FGA LAI 49 (12) | DOI years: 24 (8.0), >10 years of depot use: 59% | (36) |
| RIS 51 (11) | DOI years: 24 (9.5), >10 years of depot use: 76% | (33) | |||||
| Grimaldi-Bensouda et al38 ‘12/ France | 1859d | Pro / Multi center | 12 | SCZ (DSM-IV), ambulatory or hospitalized for <92 consecutive days. | RLAI 36.7 (10.7) | Prior # Hp days: 60.8 | (489) |
| Non-RLAI 38.6 (11.2) | Prior # Hp days: 45.2 | (1370)d | |||||
| Gutwinski et al39 ‘07/ Germany | 233 | Retro/Multi center | 36 | Aged ≥16 and <81 on the index date; SCZ pts were identified at the time of rehospitalization, and duration of relapse free interval was determined. | Total 41.4 | NR | FGA (77) |
| SGA (156) | |||||||
| Haro et al40 ‘07/ International | 7728 | Pro/Multi center | 36 | SCZ who were initiating or changing AP (as monotherapy), who presented within the normal course of OP care | FGA LAI 42.1 (12.2) | DOI years: 12.3 (10.4), % of first treatment: 6.0 | (348) |
| OAP 39.7 | DOI years: 10.6 (10.8), % of first treatment: 12.4 | OLA (4247), RIS (1549), QUE (583), CLO (274), AMI (256), FGA (471) | |||||
| Hoiberg and Nielsen41 ‘06/ Norway | 123 | Retro /Single center | 12 | SCZ (ICD-10) | FGA LAI 41.6 | Prior # Hp: 16.3 | (47) |
| OAP 38.2 | Prior # Hp: 8.1 | FGA (17), SGA (59) | |||||
| Huang et al42 ‘13/ Taiwan | 14 610 | Pro/Taiwan NHRI | 12 | SCZ or SCZAD (ICD-9-CM code 295). Pts admitted frequently were retrieved for analysis. Frequent admission was defined as Hp for SCZ treatment at least twice in an acute psychiatric ward within 2 consecutive years. | Total 38.4 (12.8) | NR | NR |
| Ibach and Schreiner43 ‘08/ NR | 230 | Pro/Multi center | 12 | Recently diagnosed SCZ (ICD-10), presence of symptomatology for ≥1Y and <5Y | RLAI 34.4 | DOI years: 2.8 (1.8) | (113) |
| OAP 34.3 | DOI years: 2.4 (1.5) | ARI (25), OLA (20), QUE (21), ZIP (19), RIS (18), AMI (14) | |||||
| Ju et al44 ‘14/ Taiwan | 1755 | Retro /PIMC | 12 | Pts who joined the psychiatric home care program within 60 days after hospital discharge between 2004 and 2009 were initially included in this study. | Any LAI 41.1 (10.1) | NR | (810) |
| Any OAP 41.8 (12.1) | (945) | ||||||
| Kelin et al45 ‘11/ International | 406 | Pro/Multi center | 12 | OP, SCZ (DSM-IV) who had ≥2 clinical worsening requiring Hp or increased level of care in 24M before study, at risk of nonadherence, required a switch of their primary OAP, not treatment resistant | LAI 35.8 (12.1) | Prior # Hp: 1.1 (1.2) | HAL (8), ZUC (5), PIP (5), Other FGA (3), RLAI (22) |
| OAP 37.4 (10.0) | Prior # Hp: 1.1 (1.5) | HAL (10), TRI (10), Other FGA (18), OLA (93), AMI (55), RIS (48), ARI (47), Other SGA (82) | |||||
| Kim et al46 ‘08/ South Korea | 50 | Pro/Single center | 24 | AP-naïve OP with first-episode SCZ or SCZAD (DSM-IV), stable for ≥4W before the baseline in an OP setting. | RLAI 32.5 (10.6) | DOI years: 1.5 (1.5), Stable for ≥4W | (22) |
| RIS 31.0 (10.1) | DOI years: 2.2 (3.1), Stable for ≥4W | (28) | |||||
| Lafeuille et al47 ‘13/ United States | 3828 | Retro /PREMIER | 30 | Adult SCZ, who received OAP during Hp and who received OAP or LAI at the time of second Hp (relapse). | LAI 42.1 | Prior # Hp days: 16.3 (19.4) | RLAI or PP (1032) |
| Any OAP 42.4 | Prior # Hp days: 16.0 (22.3) | (2796) | |||||
| Liu et al48 ‘15/ Taiwan | 92 | Retro /Single center | 36 | Pts who also have not previously received more than 3 consecutive LAI doses, and who continued to receive OAP medications throughout index admission. | LAI 37.8 (10.5) | Prior # Hp: 3.0 (1.9) | RLAI (15), Flupentixol decanoate (20) FLU (12) |
| OAP 37.4 (12.4) | Prior # Hp: 3.5 (2.6) | (45) | |||||
| Marchiaro et al49 ‘05/ Italy | 60 | Retro /Single center | 24 | OP with SCZ who completed 2Y treatment on OAP or LAI. | LAI 40.7 (8.57) | Prior # Hp: 6.63 (3.76) DOI years: 15.8 (9.16) | HAL (14), FLU (10), ZUC (6) |
| OAP 39.4 (10.79) | Prior # Hp: 6.50 (5.39) DOI years: 15.9 (9.48) | RIS (13), OLA (12), QUE (5) | |||||
| Marcus et al50 ‘15/ United States | 3768 | Retro/Medicade | 6 | OPs with SCZ who were aged ≥18 years, who had no use of CLO prior to Hp, and who had received an OAP or LAI within 30 days of hospital discharge. | LAI 37.5 (13.8) | Prior # Hp days: 12.8 (12.0) | FLU (45), HAL (112), RIS (81), PAL (102) |
| OAP 38.0 (12.9) | Prior # Hp days: 8.9 (9.2) | (3428) | |||||
| Moore et al51 ‘98/ United States | 118 | Retro/Multi center | 24 | All pts discharged with prescription of depot HAL, depot FLU or oral RIS. | NR | NR | FLU (29), HAL (14) |
| RIS (75) | |||||||
| Offord et al52 ’13 United States | 3669 | Retro/Multi center | 12 | Pts with Dx of ICD-9-CM code 295.X, who initiated LAI or OAP and had ≥ 12M continuous medical and prescription drug coverage prior to and after the initiation. Pts commercially insured or Medicare insured. | LAI 48.6 (18.2) | Prior # Hp: 0.97 (1.17), Prior # Hp days: 12.1 (17.3) | FLU, HAL or RIS (541) |
| OAP 43.1 (20.2) | Prior # Hp: 0.35 (0.62), Prior # Hp days: 3.21 (6.52) | RIS, OLA, QUE, ARI, PAL, CLO, HAL, PER, or FLU (3128) | |||||
| Olivares et al53 ‘09/ Spain | 1622 | Pro/Multi center | 24 | Pts who initiated treatment with or changed AP to RLAI or OAP. | RLAI 38.4 (11.2) | DOI years: 12.6 (9.5) | (1345) |
| OAP 37.0 (10.8) | DOI years: 10.9 (9.7) | (277) | |||||
| Pesa et al54 ‘15/ United States | 5183 | Retro/Medicade | 12 | Pts newly started on PP or OAP as of the index date. Pts were required to have continuous enrollment 6M before and 12M after the index date, be at least 18 y.o. at index date | LAI 38.8 (12.3) | # of Hps in the last year LAI: 363/984 | PP (984) |
| OAP 41.6 (12.9) | # of Hps in the last year OAP:1796/4199 | OAP (4199) | |||||
| Pinto et al55 ‘00/ Portugal | 183 | Retro/Single center | 36 | At least 2Y of illness admitted to the Hp | FGA LAI 37.5 (11.6) | DOI years > 2Y | (98) |
| OAP 38.5 (11.4) | (85) | ||||||
| Remington and Khramov56 ‘01/ Canada | 66 | Retro/Single center | 18 | SCZ (DSM-IV) stabilized for 3-6M on one of the followings: an oral or depot FGA, CLO or RIS, then maintained on the same treatment for ≥18M | FGA LAI 36.5 | Prior # Hp: 11.1 (9.3), DOI years: 13.7 (8.1) | (18)e |
| OAP 32.4 | Prior # Hp: 7.0 (8.9), DOI years: 9.7 (8.1) | FGA (18)f, CLO (15), RIS (15) | |||||
| San et al57 ‘13/ Spain | 1646 | Pro/Multi center | 12 | All consecutive SCZ or SCZAD (DSM-IV) admitted to and discharged from short-stay or acute-care psychiatric units. DOI ≥2Y. Having medical history data for the previous 3Y. | Total 38.2 (11.1) | Prior # Hp: 2.7 (2.2), DOI of 57.2% was> 10 years Pts were admitted to and discharged from short-stay or acute-care psychiatric units. | SGA (723), FGA (109) |
| FGA (45), SGA (567) | |||||||
| Schreiner et al58 ‘14/ International | 1084 | Pro/Multi center | 12 | ≥18 y.o. with a diagnosis of SCZ for whom ≥ 6M of retrospective clinical records Eligible Pts were required to have newly initiated or switched to RLAT or an OAP within 2W prior to study enrolment. | RLAI 42.5 (12.9) | DOI years: 11.5 (10.2) | (561) |
| OAP 41.9 (13.3) | DOI years: 11.0 (10.4) | OAP total (522); RIS (126) PAL (94), ARI (84), QUE (59), OLA(54), ZIP (31), FGA (28) | |||||
| Tavcar et al59 ‘00/ Slovenia | 447 | Pro/Single center | 12 | SCZ or SCZAD (ICD-10) discharged with AP | FGA LAI 38.6 (10.8) | Prior # Hp: 5.7 (5.6) DOI years: 10.4 (9.0) % first time Hp: 19.6 % previously receiving LAI: 70.8 | (332) |
| OAP FGA 40.1 (15.1) OAP SGA 40.6 (13.0) | Prior # Hp: 6.6 (6.5) DOI years: 11.7 (10.2) % first time Hp: 21.6 % previously receiving LAI: 19.2 | FGA (82), SGA (43) | |||||
| Tiihonen et al60 ‘11/ Finland | 2588 | Pro/FNHDR | 24 | All Finnish SCZ (ICD-10) who had their first Hp and had not collected AP within 6M before Hp | Total 37.8 (13.7) | Patients who had their first Hp. | RLAI (119)g, PER (109)g, ZUC (52)g, HAL (18)g |
| OLA (904)g, RIS (581)g, CLO (412)g, QUE (217)g, PER (63)g, HAL (13)g, ZUC (12)g, OtherOAP (82)g, PolyPharm (321)g | |||||||
| Valevski et al61 ‘12/ Israel | 720 | Retro/Single center | 12 | SCZ admitted consecutively, not readmitted ≤5 days following discharge, not treated with a combination of FGA and SGA | LAI 31.3 (11.9) | Prior # Hp: 2.4 (4.3) DOI years: 7.3 (9.1) | FLU (118), HAL (87) ZUC (87), Other FGA (1) |
| CLO 30.8 (12.5) | Prior # Hp: 2.2 (2.5) DOI years: 8.0 (8.8) | CLO (74) | |||||
| Varner et al62 ‘01/ United States | 153 | Retro/Single center | 48 | Acutely hospitalized psychotic pts (primarily with SCZ) who received depot or oral HAL prior to discharge | Total 35.9 (10.8) | Prior # Hp: 4.3 (4.6) Acutely hospitalized | HAL (95) |
| Prior # Hp: 3.0 (2.7) Acutely hospitalized | HAL (58) | ||||||
| Voss et al63 ‘15/ United States | 218 | Retro/Medicade | 12 | SCZ pts (ICD-9) switching from RLAI to PP or oral AP medication. | LAI 40.4 (13) | LAI Prior # Hp: 2.63 | PP (109) |
| OAP 42.2 (13) | OAP Prior # Hp:2.69 | OAP (109) | |||||
| Werneck et al64 ‘11/ Brazil | 242 | Retro/Single center | 36 | SCZ (ICD-10) discharged from the participating Hp | LAI 45 (12) | Prior # Hp: 3.4 (4.8) Prior # Hp days: 55 (46) DOI years: 20.0 (8.0) | HAL (16), PIP (3), ZUC (1) |
| OAP 38 | Prior # Hp: 2.3 (2.7) Prior # Hp days: 46 (37) DOI years: 16.6 (9.12) | RIS (63), CLO (59), HAL (41), OLA (26), ZIP (13), AMI (10), TRI (6), CPZ (2), ARI (1), PEN (1) | |||||
| Xiao et al65 ‘15/ United States | 13 126 | Retro/Medicade | Mean F/U LAI: 10.1 OAP: 10.9 | Adult Pts with a SCZ diagnosis, who received PP or OAP since July 1, 2009. | PP 40.3 (12.7) | NR | PP (952) |
| OAP 45.3 (13.6) | OAP (12 174) | ||||||
| Xiao et al66 ‘16/ United States | 11 654 | Retro/Medicade | 12 | Adult Pts (age ≥18years old) with a SCZAD diagnosis, who received PP or OAP since January 1, 2010. | PP 42.6 (31.8) | Prior # Hp: 2.1 (9.1) | PP (5589) |
| OAP 43.0 (9.7) | Prior # Hp:1.9 (2.6) | OAP (6065) | |||||
| Young-Xu et al67 ‘16/ US | 10 290 | Retrospective/VHA | 12 | Adult Pts (age ≥18years old) who had at least 2 SCZ diagnoses during the observation period and at least 2 dispensings of PP or OAA within 90 days between January 1, 2010, and October 31, 2014. | PP 53.4 (17.2) | PP Prior # Hp: 2.2 (2.6) | PP (5052) |
| OAP 53.0 (9.8) | OAP Prior # Hp: 2.2 (1.7) | OAP (5238) | |||||
| NCT 0189498468 China | 640 | Pro/NR | 6 | Total course of disease no more than 5Y. According to physician’s discretion, participants need to be changed to RLAI and other atypical anti-psychotic drug |
RLAI 27.4 (9.44) | NR | (396) |
| OAP 32.2 (11.6) | (243) | ||||||
| Region: North America (studies = 15, n = 54 526), Western Europe (studies = 13, n = 9148), Asia (studies = 6, n = 17 526), ROTW (studies = 4, n = 10 976), International (studies = 3, n = 9218), NR (study = 1, n = 230). | Study Design: RETROSP (studies = 27, n = 70 165), PROSP (studies = 15, n = 31 459). Data source: Multi center (studies = 15, n = 20 479), Single center (studies = 14, n = 2683), Large database (studies = 12, n = 77 822), NR (studies = 1, n = 640). F/U duration: Mean = 18.6 ± 10.0M (range6-48), ≤1Y = 24studies, ≥2Y = 15studies, Patient-Years = 115 141. |
Mean age: 39.5 ± 5.2 y.o. The number of patients per study: Median=522 (range 50–14 610). LAI: Any FGA (studies = 14), FGA and SGA mixed (studies = 9), Risperidone (studies = 10), Paliperidone (study = 6), Haloperidol (study = 1), Risperidone and Paliperidone (study = 1), Unclear (study = 1). OAP: FGA and SGA mixed (studies = 29), Any SGA (studies = 6), Risperidone (studies = 3), Any FGA (studies = 1), Clozapine (study = 1), Haloperidol (study = 1), Unclear (study = 1). |
|||||
Note: AMI, amisulpride; AP, antipsychotic; ARI, aripiprazole; CLO, clozapine; CPZ, chlorpromazine; DOI, duration of illness; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders – fourth edition; FGA, first generation antipsychotic; FLU, fluphenazine; FNHDR, the Finnish National Hospital Discharge Register; F/U, follow-up; HAL, haloperidol; Hp, hospital, hospitalization; ICD, International Classification of Diseases; LAI, long acting injectable; M, months; MHSB, the Mental Health Services Branch; NHIF, the National Health Insurance Fund; NHRI, National Health Research Institutes; NR, not reported; OAP, oral antipsychotic; OLA, olanzapine; OP, outpatient; PAL, paliperidone; PANSS, the Positive and Negative Syndrome Scale; PEN, penfluridol; PER, perphenazine; PIMC, Psychiatric Inpatient Medical Claims Data; PIP, pipotiazine; PP, paliperidone palmitate; PREMIER, the Premier Perspective Comparative Hospital Database; Pro, prospective cohort study; Pt, patient; QUE, quetiapine; Retro, retrospective cohort study; RIS, risperidone; RLAI, risperidone long acting injection; ROTW, rest of the world; SCZ, schizophrenia; SCZPD, schizophreniform disorder; SPDP, the Saskatchewan Prescription Drug Plan; SCZAD, schizoaffective disorder; SGA, second generation antipsychotic; SUL, sulpiride; TRI, trifluoperazine; VHA, the Veterans Health Administration; W, weeks; Y, year; y.o., years old; ZIP, ziprasidone; ZUC, zuclopenthixol; ZOT, zotepine.
aOriginal study sample size.
bNumber of patients analyzed.
cAntipsychotics at baseline in the oral group included OLA 12, RIS 11, AMI 1, ARI 1, and QUE 1. At endpoint, antipsychotics in the oral group included OLA 2, RIS 6, CLO 11, ZIP 1, ARI 4, and PAL 2.
dNumber of patients in original study was shown. Subpopulation was used for the analysis.
eFluphenazine decanoate 4; flupenthixol decanoate 7; haloperidol decanoate 4; pipotiazine palmitate 3.
fTrifluoperazine 8, pimozide 4, loxapine 3, methotrimeprazine 1; perphenazine 1; thioridazine 1.
gPerson-years.
Primary Outcome: Hospitalization Rate
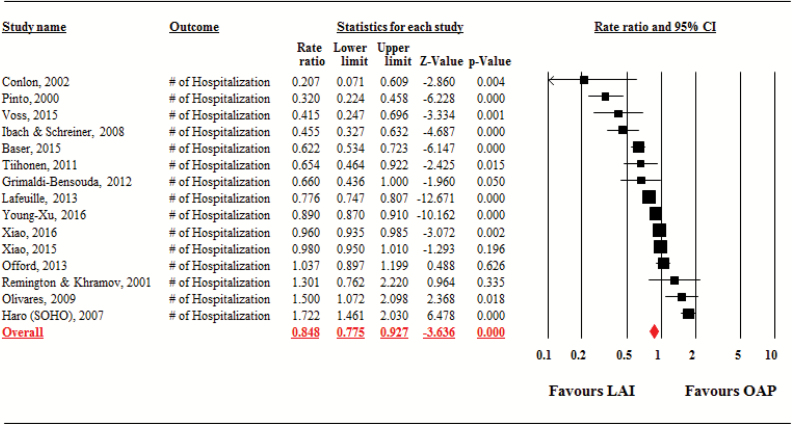
Pooled together, the hospitalization rate was significantly lower with LAIs compared to with OAPs (studies = 15, person-years = 68 009, rate ratio = 0.85, 95% CI = 0.78–0.93, P < .001; NNT = 6, 95% CI = 4–17, based on 11 studies with raw rate information). Significant heterogeneity was observed across studies (τ2 = 0.02, I2 = 94.9%, Q = 272.6, df = 14, P < .001; figure 1).
Fig. 1.
Hospitalization Rate. Note: LAI, long-acting injectable antipsychotic; OAP, oral antipsychotic.
Secondary Outcomes: Hospitalization Risk, All-Cause Discontinuation, and Hospitalization Days
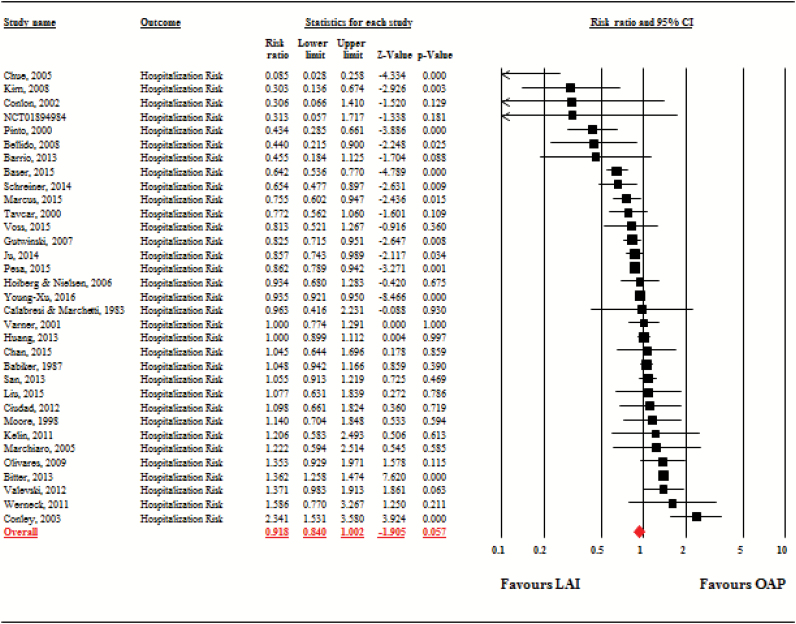
The hospitalization risk with LAIs was not superior to OAPs (studies = 33, n = 51 733, risk ratio = 0.92, 95% CI = 0.84–1.00, P = .06). The risk ratio varied significantly across studies (τ2 = 0.03, I2 = 84.6%, Q = 207.4, df = 32, P < .001; figure 2).
Fig. 2.
Hospitalization Risk. Note: LAI, long-acting injectable antipsychotic; OAP, oral antipsychotic.
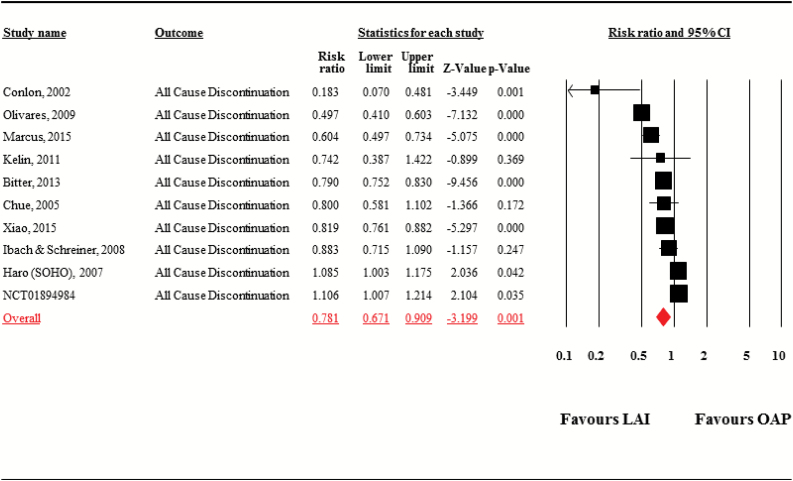
LAIs were associated with significantly lower risk of all-cause discontinuation than OAPs (studies = 10, n = 37 293, risk ratio = 0.78, 95% CI = 0.67–0.91, P = .001; heterogeneity, τ2 = 0.04, I2 = 93.0%, Q = 128.6, df = 9, P < .001, NNT = 10, 95% CI = 6–25) (figure 3). LAIs did not separate from OAPs regarding number of hospitalization days (studies = 11, n = 21 328, Hedges’ g = −0.05, 95% CI = −0.16 to 0.06, P = .39; heterogeneity, τ2 = 0.02, I2 = 84.8%, Q = 65.9, df = 10, P < .001; supplementary figure 2).
Fig. 3.
All-cause discontinuation. Note: LAI, long-acting injectable antipsychotic; OAP, oral antipsychotic.
Subgroup Analyses
Superiority of LAIs over OAPs regarding hospitalization rate was confirmed in approximately half (24/50) of the subpopulations and treatment groups (supplementary table 2). Subgroups in which LAIs demonstrated significantly lower hospitalization rates than OAPs included study publication year ≥2010 (P < .001), academic sponsorship (P = .021), large database studies (P < .001), and no need for informed consent (P < .001). Significant superiority of LAIs was also demonstrated in studies using retrospective databases (P < .001), statistical adjustment for differences in baseline patient characteristics (P < .001), intent-to-treat analyses (P < .001), higher study quality score (P < .001), and follow-up duration of 6–12 months (P < .001). Regarding the LAI class, SGA-LAIs were statistically superior to OAPs (studies = 9, n = 47 114, rate ratio = 0.83, 95% CI = 0.76–0.90, P < .001; heterogeneity, τ2 = 0.01, I2 = 95.2%, Q = 166.5, df = 8, P < .001), although this was not observed for FGA-LAIs (P = .43) and mixed LAI subgroups (P = .47).
Patient Illness Severity/Chronicity
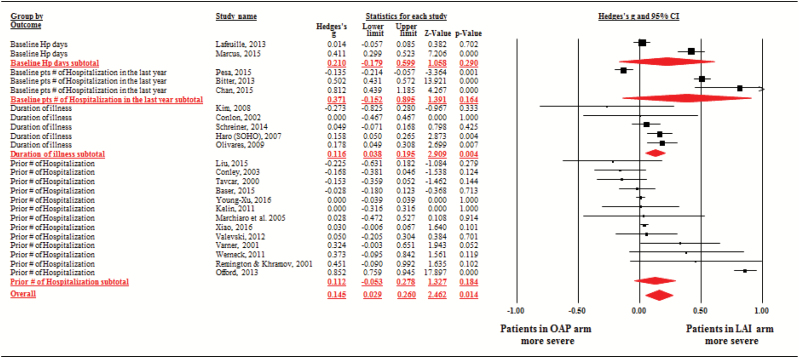
When comparing reported clinical characteristics between LAIs and OAPs regarding each severity/chronicity information, illness duration was longer in LAI-treated patients (studies = 11, n = 12 146, Hedge’s g = 0.10, 95% CI = 0.00–0.20, P = .046; heterogeneity, τ2 = 0.010, I2 = 48.2%, Q = 19.3, df = 10, P = .036), whereas LAIs groups had no significant difference in proportions of patients who had past hospitalization, in baseline hospitalization days and in prior number of hospitalization (studies = 3, n = 15 129, Hedge’s g = 0.37, 95% CI = −0.15 to 0.90, P = .164; heterogeneity, τ2 = 0.202, I2 = 98.7%, Q = 148.5, df = 2, P < .001, studies = 7, n = 13 045, Hedge’s g = 0.20, 95% CI = −0.17 to 0.57, P = .282; heterogeneity, τ2 = 0.235, I2 = 98.2%, Q = 329.7, df = 6, P < .001, and studies = 13, n = 28 529, Hedge’s g = 0.11, 95% CI = −0.05 to 0.28, P = .18; heterogeneity, τ2 = 0.072, I2 = 96.1%, Q = 306.0, df = 12, P < .001, respectively). When synthesizing these illness severity/chronicity information, patients receiving LAIs were more severely/chronically ill than patients receiving OAPs (studies = 23, n = 61 806, Hedges’ g = 0.15, 95% CI = 0.03–0.26, P = .01; heterogeneity, τ2 = 0.062, I2 = 95.8%, Q = 524.5, df = 22, P < .001; figure 4).
Fig. 4.
Patient severity/chronicity—oral antipsychotic groups vs long-acting injectable groups. Note: LAI, long-acting injectable antipsychotic; OAP, oral antipsychotic.
Publication Bias
The funnel-plot to assess publication bias was asymmetrical (supplementary figure 3). Subsequently, we conducted the trim-and-fill method to adjust for potential publication biases. Imputing missing studies did not change the result (original rate ratio = 0.85, 95% CI = 0.78–0.93 vs adjusted rate ratio = 0.91, 95% CI = 0.83–0.99).
Discussion
We report a comprehensive meta-analysis of parallel group nonrandomized cohort studies comparing LAIs and OAPs for the treatment of schizophrenia. Previously Kirson et al19 conducted a similar meta-analysis of 13 studies examining the effects of study design on comparative effectiveness of LAIs only based on RCTs and cohort studies published after 2000. Because that prior analysis treated hospitalization, all-cause discontinuation, and relapse as one outcome and allowed data overlap, we believe that our analysis of 42 studies is both more inclusive and methodologically rigorous. Based on our results, LAIs were superior to OAPs in decreasing the hospitalization rate, in other words, number of hospitalizations per unit time. Moreover, patients on LAIs were less likely to discontinue treatment. Superiority of LAIs over OAPs was not observed in some of the secondary outcomes including risk of hospitalization risk (although trend-level significance was observed) and number of hospital days. However, it was noteworthy that as compared with patients on OAPs those on LAIs had clinical characteristics consistent with greater severity and chronicity.
We selected hospitalization rate as the primary outcome because unlike hospitalization risk, the rate adjusts for duration of follow-up. Because patients on LAIs were less likely to discontinue treatment than their counterparts on OAPs they were likely to be observed for a longer time. Failing to control for follow-up time could therefore have biased results in favor of OAPs. Moreover, unlike risk ratios, rate ratios do not have ceiling effects. As compared to risk ratios, therefore, rate ratios more precisely represent the differential treatment effects. Given the advantage of LAIs over OAPs regarding hospitalization rate, it appears that LAIs are comparatively more effective in patients at risk for multiple hospitalizations.69 However, we did not find a significant difference between LAIs and OAPs regarding the number of inpatient days. This finding is noteworthy, because the number of inpatient days made an important contribution to overall treatment costs days. However, as discussed further below, since patients on LAIs seemed more severely ill, having similar numbers of inpatient days as the OAP patients may represent a positive outcome for LAIs.
Cohort studies and mirror image studies include patients whose medication choice is determined in real-world clinical settings without study-related alterations in treatment ecology. In contrast with mirror image studies, cohort studies have no predetermined order effect and time effects occur concurrently, as both LAI and OAP groups are followed in parallel. Nevertheless, like mirror image studies, cohort studies are prone to expectation biases, as there is no blinding. This might introduce bias if treatment decisions (eg, whether to hospitalize or not) are influenced by knowledge of route of antipsychotic administration. Furthermore, a particular disadvantage of cohort studies, as observed in the meta-analysis, is a systematic channeling bias in that patients who are more severely ill or prone to poor illness insight and non-adherence are more likely to be selected by clinicians to receive LAIs, whereas patients who are perceived to be at lower risk for relapse and hospitalization are more likely treated with OAPs.
SGA-LAIs, but not FGA-LAIs, were superior to OAPs with respect to hospitalization rate. This finding might be due to better tolerability of SGA-LAIs than FGA-LAIs. However, a recent Danish study reported that FGA-LAIs and risperidone-LAI do not differ with regard to time to hospitalization, all-cause discontinuation, and duration of hospitalization.70 A head-to-head RCT comparing haloperidol and paliperidone once monthly also found no differences regarding all-cause treatment failure and other relapse and hospitalization related outcomes.71 Moreover, superiority of SGA-LAIs over OAPs is the opposite of the subgroup analyses in our meta-analysis of RCTs17 where FGA-LAIs, but not SGA-LAIs separated significantly from OAPs. Thus, based on these inconsistencies, more high-quality head-to-head trials in representative patients are needed that compare FGA-LAIs and SGA-LAIs with OAPs, such as the currently ongoing European Long-acting Antipsychotics in Schizophrenia Trial (EULAST).72
Results of the present meta-analysis were highly heterogeneous. We conducted multiple sensitivity/meta-regression analyses in order to identify potential treatment effect moderators. In addition to the superiority of SGA-LAIs, several significant moderators of superiority of LAIs but not of OAPs were identified. For variables associated with significantly lower hospitalization rates, the identified moderators strengthened the finding of superiority of LAIs, as they each related to either a more generalizable patient sample or more naturalistic data ascertainment and a lower likelihood of bias including academic sponsorship, statistical adjustment for differences in baseline patient characteristics, intent-to-treat analyses, and higher Newcastle-Ottawa scale score.
These considerations above highlight the complexities and difficulties of evaluating the comparative effectiveness of a treatment that consists of the same molecular entity in different formulations where non-adherence is the targeted mediating factor of favorable outcomes. As argued before,18 since RCTs, mirror-image studies and cohort studies have different strengths and weaknesses, the best design may actually be a large pragmatic trial that retains random assignment but minimizes barriers for participation and alters clinical care as little as possible. Such considerations are supported by a recent analysis of explanatory vs pragmatic features of RCTs comparing LAIs and OAPs which found that studies with more pragmatic features were more likely to identify advantages of LAIs over OAPs.73 Unlike other recent RCTs, 3 recent studies, which focused on patients in early stages of their illness as well as at particularly high risk for non-adherence, found LAIs to be superior to OAPs regarding relapse, treatment failure or rehospitalization.14–16 Along with 2 ongoing pragmatic trials in first-episode and early-phase schizophrenia patients,72,74 these new studies advance our understanding of this complex comparative effectiveness issue.
Limitations
A key shortcoming of this meta-analysis is that the cohort studies were nonrandomized and therefore prone to a selection bias regarding the clinician’s choice treatment with either LAIs or OAPs. Although some studies adjusted for such baseline differences, only 6 out of 42 studies did so, and some other studies did so for hazard ratio or odds ratio, which we could not use for the meta-analytic synthesis in this study due to insufficient data.
Second, for all outcomes that we examined, results were significantly heterogeneous, meaning that effects varied significantly across the meta-analyzed studies which suggests that the studies differed regarding design, population, and treatment variables. By conducting sensitivity analyses, we identified several moderators that strengthened or weakened group differences between LAIs and OAPs. Although based on the data it is not possible to determine the most important moderator, as most of the significant moderators drove the effect in favor of LAIs and related to greater generalizability and higher quality of the results. As a side note, patient characteristics that may have led to LAI superiority in recent RCTs, ie, recent onset schizophrenia and/or incarcerated patients, were not reported in the selected studies. Therefore, we were unable to examine their effects in subgroup analyses. Third, the indicators that we used as proxies for illness severity/chronicity, such as number of past hospitalizations, inpatient days in the past year, and proportion of patients admitted in the last year, may not necessarily capture the true severity/chronicity of the patients, although there were limited options in the reported data. We also note that illness severity and chronicity are not necessarily directly related to each other, as in some cases longer illness duration and chronicity may reduce the risk of hospitalization. Fourth, the secondary outcome, hospitalization days may not be a meaningful outcome to assess the comparative effectiveness of LAIs vs OAPs because it can be influenced by variables, such as patient insurance, legal, or housing status. Moreover, despite their clinical importance, quality of life and functional status were not included in our meta-analysis because none of the studies reported these outcomes. Fifth, the number of studies for rate ratio calculation (N = 15) was relatively small compared to risk ratio (N = 33). Nevertheless, as discussed previously, we believe that rate ratio is superior to risk ratio in terms of accounting for follow-up length and avoiding the ceiling effect of limiting the outcome to one possible hospitalization per patient, although reducing the risk of multiple hospitalizations is even more relevant. Future cohort studies should include hospitalization rate as an outcome. Finally, data on treatment adherence, psychopathology and adverse effects were too sparse to allow for meaningful meta-analysis. Such outcomes should be reported in future cohort studies. Given these limitations, future cohort studies should include detailed assessments of pre-baseline illness severity, chronicity and insight, as well as medication attitude and adherence and adjust for any between-group imbalances.
In summary, in a meta-analysis of cohort studies, LAIs were superior to OAPs regarding reducing hospitalization rate and treatment discontinuation, whereas LAIs were not superior to OAPs regarding hospitalization risk and hospitalization days. These results occurred even though patients on LAIs were more severely and/or chronically ill than were patients on OAPs. Whether or not advantages over OAPs are larger with SGA-LAIs requires further investigation, but will be relevant given the cost differences between SGA-LAIs and FGA-LAIs.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported in part by The Zucker Hillside Hospital Advanced Center for Interventions and Services Research for the Study of Schizophrenia (P30MH090590) from the National Institute of Mental Health, Bethesda, MD, United States. The sponsor had no influence on the design, data acquisition, data analysis, data interpretation or writing of the report.
Supplementary Material
Acknowledgments
We thank Dr Galling and Dr Molteni for the translation of German and Italian articles, respectively. T.K. has received speaker’s honoraria from Abbvie, Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, Otsuka and Pfizer. He has received grant support from the Byoutaitaisyakenkyukai Fellowship (Fellowship of Astellas Foundation of Research on Metabolic Disorders) and Eli Lilly Fellowship for Clinical Psychopharmacology. K.H. and M.N. are employees of Sumitomo Dainippon Pharma, Japan. M.O. is principal investigator on a grant to Columbia University from Sunovion Pharmaceuticals. J.M.K. has received honoraria for lectures and/or consulting from Alkermes, Bristol Myers Squibb, Eli Lilly, Forrest Labs, Forum, Genentech, Intracellular Therapies, Janssen, Johnson and Johnson, Lundbeck, Merck, Neurocrine, Novartis, Otsuka, Pfizer, Reviva, Roche, Sunovion, and Teva. He has received grant support from NIMH, CMS, Genentech, Johnson and Johnson, and Otsuka. He is a shareholder of MedAvante, LB Pharmacueticals and Vanguard Research Group. C.U.C. has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Actavis, Actelion, Alexza; Alkermes, Bristol-Myers Squibb, Cephalon, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Merck, Otsuka, Pfizer, ProPhase, Reviva, Roche, Sunovion, Supernus, Takeda, Teva, and Vanda. He has received grant support from the American Academy of Child and Adolescent Psychiatry, the Bendheim Foundation, Bristol-Myers Squibb, the National Institute of Mental Health, Novo Nordisk A/S, Otsuka, Takeda and the Thrasher Foundation. In the last 3 years S.L. has received honoraria for lectures from EliLilly, Lundbeck (Institute), Pfizer, Janssen, BMS, Johnson and Johnson, Otsuka, Roche, SanofiAventis, ICON, Abbvie, AOP Orphan, Servier; for consulting/advisory boards from Roche, Janssen, Lundbeck, EliLilly, Otsuka, TEVA; for the preparation of educational material and publications from Lundbeck Institute and Roche. EliLilly has provided medication for a clinical trial led by S.L. as principal investigator.
References
- 1. Lieberman JA, Perkins D, Belger A et al. . The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. [DOI] [PubMed] [Google Scholar]
- 2. van Haren NE, Hulshoff Pol HE, Schnack HG et al. . Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. [DOI] [PubMed] [Google Scholar]
- 3. Robinson D, Woerner MG, Alvir JM et al. . Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. [DOI] [PubMed] [Google Scholar]
- 4. Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry. 2003;160:1209–1222. [DOI] [PubMed] [Google Scholar]
- 5. Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–447. [DOI] [PubMed] [Google Scholar]
- 6. Leucht S, Tardy M, Komossa K et al. . Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–2071. [DOI] [PubMed] [Google Scholar]
- 7. Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents?Am J Psychiatry. 2002;159:103–108. [DOI] [PubMed] [Google Scholar]
- 8. Velligan DI, Wang M, Diamond P et al. . Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58:1187–1192. [DOI] [PubMed] [Google Scholar]
- 9. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;52:S63–S67. [DOI] [PubMed] [Google Scholar]
- 10. Rosenheck RA, Krystal JH, Lew R et al. . Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011;364:842–851. [DOI] [PubMed] [Google Scholar]
- 11. Buckley PF, Schooler NR, Goff DC et al. . Comparison of SGA oral medications and a long-acting injectable SGA: the PROACTIVE study. Schizophr Bull. 2015;41:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kane JM, Detke HC, Naber D et al. . Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167:181–189. [DOI] [PubMed] [Google Scholar]
- 13. Macfadden W, Ma YW, Thomas Haskins J, Bossie CA, Alphs L. A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry. 2010;7:23–31. [PMC free article] [PubMed] [Google Scholar]
- 14. Subotnik KL, Casaus LR, Ventura J et al. . Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schreiner A, Aadamsoo K, Altamura AC et al. . Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169:393–399. [DOI] [PubMed] [Google Scholar]
- 16. Alphs L, Benson C, Cheshire-Kinney K et al. . Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76:554–561. [DOI] [PubMed] [Google Scholar]
- 17. Kishimoto T, Robenzadeh A, Leucht C et al. . Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66:S37–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirson NY, Weiden PJ, Yermakov S et al. . Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74:568–575. [DOI] [PubMed] [Google Scholar]
- 20. Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–965. [DOI] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells G, Shea B, O’Connell D et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses 2015. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 24, 2016.
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 27. Babiker IE. Comparative efficacy of long-acting depot and oral neuroleptic medications in preventing schizophrenic recidivism. J Clin Psychiatry. 1987;48:94–97. [PubMed] [Google Scholar]
- 28. Barrio P, Batalla A, Castellví P et al. . Effectiveness of long-acting injectable risperidone versus oral antipsychotics in the treatment of recent-onset schizophrenia: a case-control study. Int Clin Psychopharmacol. 2013;28:164–170. [DOI] [PubMed] [Google Scholar]
- 29. Baser O, Xie L, Pesa J, Durkin M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18:357–365. [DOI] [PubMed] [Google Scholar]
- 30. Bellido I, López C, Gómez-Luque A. Depot antipsychotics in outpatients with schizophrenia improved compliance and reduced the incidence of relapses. Methods Find Exp Clin Pharmacol. 2008; 30:137–41. [Google Scholar]
- 31. Bitter I, Katona L, Zámbori J et al. . Comparative effectiveness of depot and oral second generation antipsychotic drugs in schizophrenia: a nationwide study in Hungary. Eur Neuropsychopharmacol. 2013;23:1383–1390. [DOI] [PubMed] [Google Scholar]
- 32. Calabresi M, Marchetti G. [The long-term pharmacological treatment of schizophrenic patients: Comparing effects resulting from daily administered neuroleptics and “long acting.”]. Rivista Sperimentale di Freniatria e Medicina Legale delle Alienazioni Mentali. 1983;107:1205–1223. [Google Scholar]
- 33. Chan HW, Huang CY, Feng WJ, Yen YC. Risperidone long-acting injection and 1-year rehospitalization rate of schizophrenia patients: a retrospective cohort study. Psychiatry Clin Neurosci. 2015;69:497–503. [DOI] [PubMed] [Google Scholar]
- 34. Chue P, Lam A, Chandra K, Luong D, Camacho F. Hospitalization and medication use in schizophrenia patients receiving risperidone longacting injectable or oral atypical antipsychotic medication. Value in Health. 2005;8:A202. [Google Scholar]
- 35. Ciudad A, San L, Bernardo M et al. . Relapse and therapeutic interventions in a 1-year observational cohort study of nonadherent outpatients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:245–250. [DOI] [PubMed] [Google Scholar]
- 36. Conley RR, Kelly DL, Love RC, McMahon RP. Rehospitalization risk with second-generation and depot antipsychotics. Ann Clin Psychiatry. 2003;15:23–31. [DOI] [PubMed] [Google Scholar]
- 37. Conlon L, Fahy TJ, OToole R, Gilligan J, Prescott P. Risperidone in chronic schizophrenia: a detailed audit, open switch study and two-year follow-up of patients on depot medication. Eur Psychiatry. 2002;17:459–465. [DOI] [PubMed] [Google Scholar]
- 38. Fe Bravo-Ortiz M, Gutiérrez-Casares JR, Rodríguez-Morales A, García MA, Hidalgo-Borrajo R. Influence of type of treatment on the well-being of Spanish patients with schizophrenia and their caregivers. Int J Psychiatry Clin Pract. 2011;15:286–295. [DOI] [PubMed] [Google Scholar]
- 39. Grimaldi-Bensouda L, Rouillon F, Astruc B et al. . Does long-acting injectable risperidone make a difference to the real-life treatment of schizophrenia? Results of the Cohort for the General study of Schizophrenia (CGS). Schizophr Res. 2012;134:187–194. [DOI] [PubMed] [Google Scholar]
- 40. Gutwinski S, Müller P, Koller M. [Intervals between hospitalisations in schizophrenia patients under antipsychotics in depot-form versus oral second generation antipsychotics]. Psychiatr Prax. 2007;34:289–291. [DOI] [PubMed] [Google Scholar]
- 41. Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D. Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17:235–244. [DOI] [PubMed] [Google Scholar]
- 42. Høiberg MP, Nielsen B. Antipsychotic treatment and extrapyramidal symptoms amongst schizophrenic inpatients. Nord J Psychiatry. 2006;60:207–212. [DOI] [PubMed] [Google Scholar]
- 43. Huang SS, Lin CH, Loh el-W, Yang HY, Chan CH, Lan TH. Antipsychotic formulation and one-year rehospitalization of schizophrenia patients: a population-based cohort study. Psychiatr Serv. 2013;64:1259–1262. [DOI] [PubMed] [Google Scholar]
- 44. Ibach B, Schreiner A. Long-term treatment with long acting injecatble risperidone and oral second generation antipsychotics in patients with schizophrenia (LARA): an interim-analysis. Int J Neuropsychopharmacol. 2008;11:157. [Google Scholar]
- 45. Ju PC, Chou FH, Lai TJ et al. . Long-acting injectables and risk for rehospitalization among patients with schizophrenia in the home care program in Taiwan. J Clin Psychopharmacol. 2014;34:23–29. [DOI] [PubMed] [Google Scholar]
- 46. Kelin K, Lambert T, Brnabic AJ et al. . Treatment discontinuation and clinical outcomes in the 1-year naturalistic treatment of patients with schizophrenia at risk of treatment nonadherence. Patient Prefer Adherence. 2011;5:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim B, Lee SH, Choi TK et al. . Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1231–1235. [DOI] [PubMed] [Google Scholar]
- 48. Lafeuille MH, Laliberté-Auger F, Lefebvre P, Frois C, Fastenau J, Duh MS. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu CC, Shan JC, Chiang CL et al. . Initiating long-acting injectable antipsychotics during acute admission for patients with schizophrenia–A 3-year follow-up. J Formos Med Assoc. 2015;114:539–545. [DOI] [PubMed] [Google Scholar]
- 50. Marchiaro L, Rocca P, LeNoci F et al. . Naturalistic, retrospective comparison between second-generation antipsychotics and depot neuroleptics in patients affected by schizophrenia. J Clin Psychiatry. 2005;66:1423–1431. [DOI] [PubMed] [Google Scholar]
- 51. Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21:754–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moore DB, Kelly DL, Sherr JD, Love RC, Conley RR. Rehospitalization rates for depot antipsychotics and pharmacoeconomic implications: comparison with risperidone. Am J Health Syst Pharm. 1998;55:S17–S19. [DOI] [PubMed] [Google Scholar]
- 53. Offord S, Wong B, Mirski D, Baker RA, Lin J. Healthcare resource usage of schizophrenia patients initiating long-acting injectable antipsychotics vs oral. J Med Econ. 2013;16:231–239. [DOI] [PubMed] [Google Scholar]
- 54. Olivares JM, Rodriguez-Morales A, Diels J et al. . Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry. 2009;24:287–296. [DOI] [PubMed] [Google Scholar]
- 55. Pinto LC, Paiva A, Chainho J, Filipe DR. [Treatment of schizophrenic patients: Depot neuroleptics versus oral neuroleptics: a three year comparative trial.] Psicologia: Revista da Associacao Portuguesa Psicologia. 2000;14:45–50. [Google Scholar]
- 56. Remington G, Khramov I. Health care utilization in patients with schizophrenia maintained on atypical versus conventional antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:363–369. [DOI] [PubMed] [Google Scholar]
- 57. San L, Bernardo M, Gómez A, Martínez P, González B, Peña M. Socio-demographic, clinical and treatment characteristics of relapsing schizophrenic patients. Nord J Psychiatry. 2013;67:22–29. [DOI] [PubMed] [Google Scholar]
- 58. Schreiner A, Svensson A, Wapenaar R et al. . Long-acting injectable risperidone and oral antipsychotics in patients with schizophrenia: results from a prospective, 1-year, non-interventional study (InORS). World J Biol Psychiatry. 2014;15:534–545. [DOI] [PubMed] [Google Scholar]
- 59. Tavcar R, Dernovsek MZ, Zvan V. Choosing antipsychotic maintenance therapy–a naturalistic study. Pharmacopsychiatry. 2000;33:66–71. [DOI] [PubMed] [Google Scholar]
- 60. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168:603–609. [DOI] [PubMed] [Google Scholar]
- 61. Valevski A, Gilat Y, Olfson M, Benaroya-Milshtein N, Weizman A. Antipsychotic monotherapy and adjuvant psychotropic therapies in schizophrenia patients: effect on time to readmission. Int Clin Psychopharmacol. 2012;27:159–164. [DOI] [PubMed] [Google Scholar]
- 62. Varner RV, Hays JR, Wagner AL, Averill P. Outcome comparison of patients receiving oral or depot neuroleptic medication. Psychol Rep. 2001;89:169–174. [DOI] [PubMed] [Google Scholar]
- 63. Voss EA, Ryan PB, Stang PE, Hough D, Alphs L. Switching from risperidone long-acting injectable to paliperidone long-acting injectable or oral antipsychotics: analysis of a Medicaid claims database. Int Clin Psychopharmacol. 2015;30:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Werneck AP, Hallak JC, Nakano E, Elkis H. Time to rehospitalization in patients with schizophrenia discharged on first generation antipsychotics, non-clozapine second generation antipsychotics, or clozapine. Psychiatry Res. 2011;188:315–319. [DOI] [PubMed] [Google Scholar]
- 65. Xiao Y, Muser E, Lafeuille MH et al. . Impact of paliperidone palmitate versus oral atypical antipsychotics on healthcare outcomes in schizophrenia patients. J Comp Eff Res. 2015;4:579–592. [DOI] [PubMed] [Google Scholar]
- 66. Xiao Y, Muser E, Fu DJ et al. . Comparison of Medicaid spending in schizoaffective patients treated with once monthly paliperidone palmitate or oral atypical antipsychotics. Curr Med Res Opin. 2016;2:1–11. [DOI] [PubMed] [Google Scholar]
- 67. Young-Xu Y, Duh MS, Muser E et al. . Impact of Paliperidone Palmitate Versus Oral Atypical Antipsychotics on Health Care Resource Use and Costs in Veterans with Schizophrenia. J Clin Psychiatry. 2016;77:e1332–e1341. [DOI] [PubMed] [Google Scholar]
- 68. The US National Institutes of Health Clinical Trials Registry. ClinicalTrials.gov Identifier: NCT01894984, An Observational Study to Evaluate Efficacy and Safety of Risperidone Long-Acting Injection for Treatment of Schizophrenia. http://www.clinicaltrials.gov. Accessed September 18, 2015. [Google Scholar]
- 69. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nielsen J, Jensen SO, Friis RB, Valentin JB, Correll CU. Comparative effectiveness of risperidone long-acting injectable vs first-generation antipsychotic long-acting injectables in schizophrenia: results from a nationwide, retrospective inception cohort study. Schizophr Bull. 2015;41:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McEvoy JP, Byerly M, Hamer RM et al. . Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014;311:1978–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. The US National Institutes of Health Clinical Trials Registry. ClinicalTrials.gov Identifier: NCT02146547, European Long-acting Antipsychotics in Schizophrenia Trial (EULAST). http://www.clinicaltrials.gov. Accessed September 18, 2015. [Google Scholar]
- 73. Bossie CA, Alphs LD, Correll CU. Long-acting injectable versus daily oral antipsychotic treatment trials in schizophrenia: pragmatic versus explanatory study designs. Int Clin Psychopharmacol. 2015;30:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. The US National Institutes of Health Clinical Trials Registry. ClinicalTrials.gov Identifier: NCT02360319, Comparison of a Long-acting Injectable Antipsychotic vs Clinician’s Choice Early in Treatment to Break the Cycle of Relapse in Early Phase Schizophrenics (PRELAPSE). http://www.clinicaltrials.gov. Accessed September 18, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.