Abstract
Mismatch negativity (MMN) and P300 event-related potential (ERP) reductions in schizophrenia (SZ) reflect preattentive and attention-mediated auditory processing deficits, respectively. Although both have been linked to cognitive deficits in SZ, their relative contributions to real-world functioning are unclear. We sought to determine the functional significance of disrupted auditory processing in SZ by examining MMN and P300 in typically disabled low-functioning patients and in patients with high levels of independent role functioning. MMN to auditory deviants and P300 to infrequent auditory target and nontarget novel stimuli were assessed in 20 high-functioning SZ patients (HF-SZ), 17 low-functioning patients (LF-SZ), and 35 healthy comparison (HC) subjects. There was a group effect on MMN and P300 amplitudes across stimulus types. MMN was significantly diminished in LF-SZ compared to HF-SZ and HC, and HF-SZ demonstrated comparable MMN to HC. In contrast, P300 was significantly reduced in both LF-SZ and HF-SZ compared to HC. Logistic regression suggested independent sensitivity of MMN to functioning in SZ over and above P300 measures. Neither MMN nor P300 were associated with positive or negative symptom severity. Results replicate MMN and P300 abnormalities in SZ, and also suggest that the neural mechanisms associated with the preattentive detection of auditory deviance are most compromised in patients with functional disability. MMN may index pathophysiological processes that are critical for optimal functioning in SZ.
Keywords: auditory information processing, functional outcome, event-related potentials, electroencephalography, psychosis
Introduction
Despite successful treatment of psychotic symptoms, disability in occupational, residential, and social functioning persists for many patients with schizophrenia (SZ). Cognitive dysfunction is a core feature of SZ and a robust predictor of functional outcomes.1,2 Evidence that antipsychotic medications provide limited benefit for cognitive impairments3–6 has motivated interest in the development of interventions directly targeting cognition, which is guided by efforts to understand the neural correlates of disrupted cognitive systems in SZ. EEG-based event-related potential (ERP) components are commonly used to characterize the neurophysiological basis of early information processing impairments that may contribute to higher-order cognitive dysfunction and associated functional disability in SZ. In particular, auditory mismatch negativity (MMN) and P300 reductions probe the range of early information processing impairments common in SZ, as they have been linked to cognition and reflect preattentive and early attention-mediated auditory processing deficits, respectively.
MMN is associated with auditory deviance detection and elicited by infrequent deviant stimuli occurring within a series of repeated standard sounds.7,8 Because deviance detection requires the short-term online formation of a memory trace of preceding stimuli that have been “standard” in the auditory processing stream, MMN is considered to reflect sensory echoic memory.7,9 MMN has been localized to both auditory cortex and frontal lobes8 and appears to arise from distinct neural generators within these regions8,10–14 depending on the deviant sound feature (eg, pitch, duration, intensity).8,15 Additionally, MMN depends on glutamatergic neurotransmission at NMDA receptors, based on evidence from pharmacological challenge studies with NMDA receptor antagonists.16–22 Importantly, MMN is considered to be preattentive, as it is elicited automatically despite engagement in an unrelated primary task and instructions to ignore the simultaneously presented auditory stimuli.7,23,24 Moreover, MMN is largely unaffected by top-down information processing,7,25,26 allowing the examination of auditory processing dysfunction in disorders such as SZ without the confounding influence of attentional and motivational deficits that affect performance on higher-order cognitive tasks.27 MMN amplitude reductions have been consistently observed in SZ28,29 and are associated with impaired cognition.30–32 Evidence of reduced MMN amplitudes in individuals at clinical high-risk for psychosis, particularly in those who ultimately transition to full psychosis, suggests that compromised MMN may reflect vulnerability to illness progression.33–39
In contrast to MMN, P300 is an attention-dependent ERP following behaviorally relevant infrequent deviant stimuli interspersed among frequent “standard” stimuli during an oddball task.40 P300 is thought to reflect controlled attentional resource allocation,40–43 contextual updating of working memory,44,45 and stimulus salience processing,46,47 and prefrontal cortex and temporal-parietal junction have been implicated in its generation.48,49 Two subcomponents of P300 are evident depending on task conditions: P3b and P3a are elicited by infrequent target stimuli and infrequent non-target novel distractor stimuli, respectively.40,50–53 While P3a reflects bottom-up attention orienting and has a frontocentral scalp maximum,40,53–56 P3b reflects top-down attentional allocation and is maximal at parietal regions.40,51,52 Like MMN, auditory P300 amplitude reductions are widely replicated in SZ,57–59 evident in the clinically high-risk state,60–63 and associated with cognitive impairments.64–66
Given links between early information processing deficits and cognitive impairment in SZ, more recent work has begun to establish relationships between these neurophysiological measures and functional outcomes. Associations between MMN and skills-based measures of psychosocial functioning and clinician-based global assessment of functioning and independent living ratings have been reported by several studies.32,67–71 Fewer studies have examined the relationship between P300 and functioning, although associations between P3a and psychosocial functioning ratings,31,70,72 and between P3b and a functioning performance assessment,66 have been reported. These correlational studies may be limited, however, by a restricted range of functional abilities represented by typically recruited SZ samples. Additionally, common clinician-rated outcome measures confound functional disability with psychiatric symptom severity, capture only a single domain of functioning, or do not account for contextual factors that may influence functional performance.73 Furthermore, previous work has not examined the relative contributions of MMN and P300 deficits to functional outcomes.
The present study sought to examine whether indices of auditory processing deficits in SZ, including both the preattentive MMN and the later attention-mediated auditory P300, are associated with role functioning in a sample of SZ patients representing a wide range of functional outcomes. To achieve this aim, we prospectively recruited patients with high levels of functioning or more typical poor functioning using a multidimensional measure of functional disability that rates position, performance, and support in several domains.73 We hypothesized that MMN deficits would be more prominent in lower functioning patients based on prior evidence.32,67–71 However, given the link between higher-order cognitive dysfunction and functional outcomes in SZ1,2 and the relatively greater contributions of attention-mediated cognitive processes to P300 relative to MMN, we reasoned that P300 would be at least as sensitive to variation in functional outcomes as MMN. Accordingly, we predicted that patients with greater functional disability would demonstrate reduced MMN and P300 compared to patients with high levels of independent role functioning, and that both MMN and P300 would contribute independently to the prediction of patients’ functional status.
Methods
Participants
Thirty-seven individuals with SZ (n = 31) or schizoaffective disorder (n = 6) and 35 healthy comparison subjects (HC) were evaluated via structured interview.74 Exclusion criteria included history of substance dependence or abuse within the past year, significant medical or neurological illness, or history of head injury with loss of consciousness. Additional exclusion criteria for HC participants included a past or current DSM-IV Axis I disorder or having a first-degree relative with a psychotic disorder. The study was approved by the institutional review boards of Yale University and the University of California, San Francisco, and participants provided written informed consent.
Functional and Clinical Assessment
A clinical psychologist, psychiatrist, or clinically trained research assistant rated SZ patients’ level of functional disability using the Multidimensional Scale of Independent Functioning (MSIF).73 The MSIF assesses role position, support, and performance in work, education, and residential environments over the past month. Based on ratings on each dimension within each environment, global ratings are made on a scale from 1 (“essentially normal role functioning”) to 7 (“totally disabled”). SZ patients were classified as high functioning (HF-SZ) if they received global MSIF ratings of 1–3 and generally had normal functioning without or with some support, or slightly below normal functioning but with minimal support. Patients rated 5–7 were classified as low functioning (LF-SZ) and were significantly disabled and generally unable to function with or without supports. Participants rated as 4 (“moderately disabled”) were excluded from participation. Data from 20 HF-SZ and 17 LF-SZ patients were included in the study.
Symptoms were rated using the Scale for the Assessment of Negative Symptoms (SANS)75 and the Scale for the Assessment of Positive Symptoms (SAPS)76 within 2 weeks of ERP assessment (mean ± SD = 3.70 ± 4.03 days). Demographically adjusted premorbid intellectual functioning was evaluated using the Wechsler Test of Adult Reading.77
MMN Paradigm
MMN was assessed using a multi-deviant paradigm consisting of 4 blocks, approximately 5 min each, with a fixed pseudorandom sequence of 600 tones, presented in counterbalanced order. In 2 blocks, 80% of the stimuli were standard tones (50 ms, 633 Hz), 10% were duration deviant tones (100 ms, 633 Hz), and 10% were pitch deviant tones (50 ms, 1000 Hz). In the other 2 blocks, 90% were standard tones (50 ms, 633 Hz) and 10% were double deviant tones (combined duration and pitch deviant; 100 ms, 1000 Hz). All tones had 5 ms rise/fall times and were presented with a 500 ms stimulus onset asynchrony. Participants were instructed to ignore auditory stimuli and perform a picture-word matching task that required button press responses.78
P300 Paradigm
During the oddball task, participants heard a random series of infrequent target tones (8.33%; 1000 Hz, 500 ms), frequent standard stimuli (83.33%; 20 Hz, 30 Hz, or 40 Hz click trains, 500 ms), and infrequent task-irrelevant novel distractor sounds (ie, a variety of natural and man-made sounds79; 8.33%; 175—250 ms). Stimuli averaged 80 dB SPL (C weighting) and were presented in 3 blocks with a 1250 ms stimulus onset asynchrony. Participants were asked to press a response key to target tones. Each of the 3 counterbalanced blocks included 15 targets, 15 novels, and 150 standards. In order to maximize signal-to-noise ratio, ERPs to the deviant stimuli were averaged across blocks. Trials with incorrect button presses were excluded from analysis, and there were no group differences in response accuracy, F(2, 69) = 1.65, P = .200. Time-frequency analyses of the auditory steady state response to click trains are presented separately (Ferri et al., in preparation).
Electroencephalographic Data Acquisition and Preprocessing
Participants sat in an acoustically shielded booth in front of a computer monitor and wore insert earphones (Etymotic Research, Inc.). EEG was recorded at 1000 Hz from 26 scalp electrodes, filtered between 0.05 and 200 Hz, and referenced to the right mastoid (Neuroscan SynAmps, Compumedics Neuroscan). Additional electrodes at the outer canthi of both eyes and above and below the left eye recorded eye movements and blinks (vertical and horizontal electro-oculogram; VEOG, HEOG). All electrode impedances were maintained below 10 kOhm.
EEG data from 9 lead sites were analyzed (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4). Continuous data were subjected to a low-pass filter of 30 Hz for MMN and 12 Hz for P300 and re-referenced offline to averaged mastoid electrodes. Data were separated into epochs time-locked to stimulus onset (550 ms with a 50 ms prestimulus baseline for MMN; 1000 ms with a 100 ms prestimulus baseline for P300). VEOG and HEOG data were used to correct for eye movements and blinks with a regression-based algorithm. After baseline correction (−50 to 0 ms for MMN; −100 to 0 ms for P300), epochs containing artifacts (voltages exceeding ±100 µV) were rejected. Separate averages were calculated for all trial types for each participant.
For MMN, standard waves were subtracted from deviant waves, and MMN amplitude was defined as the most negative peak in the difference waves between 90 and 290 ms for each deviant type. P300 was identified as the most positive peak 235—400 ms following stimulus onset. Because P3b and P3a have different topographies, different algorithms were implemented for automated peak-picking (supplementary material).80,81
Statistical Analysis
Group differences in MMN and P300 amplitudes were examined at midline electrodes using 3-way repeated-measures ANOVA models with a between-subjects factor of group (HF-SZ, LF-SZ, HC) and within-subjects factors of deviant type (MMN: duration, pitch, double; P300: target, novel) and lead (MMN: frontocentral Fz, Cz; P300: anterior-posterior Fz, Cz, Pz). Secondary analyses were performed at off-midline sites to assess potential hemispheric differences using 4-way group × deviant type × lead × hemisphere (left, right) ANOVAs. Greenhouse-Geisser corrections were applied to within-subjects effects with more than 2 levels, and the Benjamini and Hochberg procedure82 was used to correct for multiple comparisons. Midline analyses of effects involving the group factor and off-midline analyses showing hemisphere or group × hemisphere effects are described below. Other main effects and repeated-measures ANOVA models examining ERP latency effects are presented in supplementary material.
ERP amplitudes at maximal leads (ie, Fz for MMN, Cz for P3a, Pz for P3b) were used in regression and correlational analyses. To examine independent contributions to functioning, MMN and P300 were entered in hierarchical logistic regression models predicting SZ patient group (ie, LF-SZ vs HF-SZ).
Relationships between ERP amplitudes and SAPS and SANS global subscale scores were examined using Spearman rank-order correlations. An alpha level of P = .05, 2-tailed, was used for all statistical tests.
Results
Demographic Differences Between Groups
Demographic data are shown in table 1. Age and gender did not differ between groups. The distribution of handedness differed at a trend level, with LF-SZ having a greater proportion of left-handed participants than HC. HC participants completed more education and had higher predicted IQ scores than both HF-SZ and LF-SZ, whereas the SZ groups did not differ. Average parental socioeconomic status (PSES) was lower in the LF-SZ than HF-SZ and HC, whereas the HF-SZ and HC did not differ. Given these demographic differences, analyses were repeated using analysis of covariance (ANCOVA) with education, PSES, and predicted IQ as covariates. HF-SZ and LF-SZ did not differ in terms of chlorpromazine (CPZ) equivalent dosages of antipsychotic medications or duration of illness. However, LF-SZ patients experienced greater negative symptom severity than the HF-SZ group, and SZ group comparisons were repeated with SANS global total score as a covariate.
Table 1.
Demographic and Clinical Characteristics
| High Functioning Schizophrenia (n = 20) | Low Functioning Schizophrenia (n = 17) | Healthy Comparison (n = 35) | P | Follow-Up Tests | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Gender | .789 | |||||||
| Female | 4 | 20.00 | 5 | 29.41 | 8 | 22.86 | ||
| Male | 16 | 80.00 | 12 | 70.59 | 27 | 77.14 | ||
| Handednessa | .063 | |||||||
| Right | 19 | 95.00 | 14 | 82.35 | 34 | 97.14 | ||
| Left | 0 | 0.00 | 3 | 17.65 | 1 | 2.86 | ||
| Ambidextrous | 1 | 5.00 | 0 | 0.00 | 0 | 0.00 | ||
| Antipsychotic type | .642 | |||||||
| Atypical alone | 17 | 85.00 | 15 | 88.24 | ||||
| Typical alone | 2 | 10.00 | 2 | 11.76 | ||||
| None | 1 | 5.00 | 0 | 0.00 | ||||
| Diagnostic subtype | .486 | |||||||
| Paranoid | 10 | 50.00 | 10 | 58.82 | ||||
| Undifferentiated | 2 | 10.00 | 4 | 23.53 | ||||
| Residual | 3 | 15.00 | 1 | 5.88 | ||||
| Schizoaffective | 4 | 20.00 | 2 | 11.76 | ||||
| Catatonic | 1 | 5.00 | 0 | 0.00 | ||||
| M | SD | M | SD | M | SD | P | ||
| Age (years) | 32.60 | 10.64 | 37.47 | 7.61 | 34.57 | 8.88 | .275 | |
| Education (years) | 13.55 | 1.10 | 12.74 | 1.73 | 16.03 | 2.61 | <.001 | HF-SZ = LF-SZ, HC > LF-SZ,e HC > HF-SZe |
| Parental SESb | 31.43 | 15.07 | 44.24 | 16.09 | 31.96 | 13.31 | .011 | HF-SZ > LF-SZ,d HC > LF-SZ,d HF-SZ = HC |
| WTAR predicted FSIQc | 101.75 | 5.53 | 99.29 | 6.32 | 107.29 | 7.06 | <.001 | HF-SZ = LF-SZ, HC > LF-SZ,e HC > HF-SZd |
| CPZ equivalent dosages | 429.10 | 399.31 | 665.24 | 456.47 | .102 | |||
| Duration of illness (years) | 10.26 | 9.79 | 14.94 | 9.26 | .151 | |||
| MSIF global score | 2.10 | 0.72 | 5.65 | 0.49 | <.001 | |||
| SAPS global total score | 5.20 | 3.14 | 5.76 | 3.11 | .588 | |||
| SANS global total score | 4.70 | 2.66 | 8.94 | 4.75 | .004 | |||
Note: Numbers and percentages of participants are reported for gender, handedness, antipsychotic type, and diagnostic subtype, and were analyzed using Pearson chi-square tests. Group means (M) and standard deviations (SD) are reported for age, personal years of education, parental socioeconomic status (SES), Wechsler Test of Adult Reading (WTAR) full-scale intelligence quotient (FSIQ), chlorpromazine (CPZ) equivalent dosages, Multidimensional Scale of Independent Functioning (MSIF) global score, and Scale for the Assessment of Positive Symptoms (SAPS) and Scale for the Assessment of Negative Symptoms (SANS) global total scores, and were analyzed with one-way ANOVAs, followed by post hoc false discovery rate (FDR)-corrected tests to further parse group differences.
aThe Crovitz and Zener questionnaire83 was used to measure handedness.
bThe Hollingshead 4-factor index of parental socioeconomic status (SES)84 is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower values signify higher socioeconomic status.
cData missing for one healthy comparison participant.
d P < .01.
e P < .001.
ANOVA of MMN Amplitudes
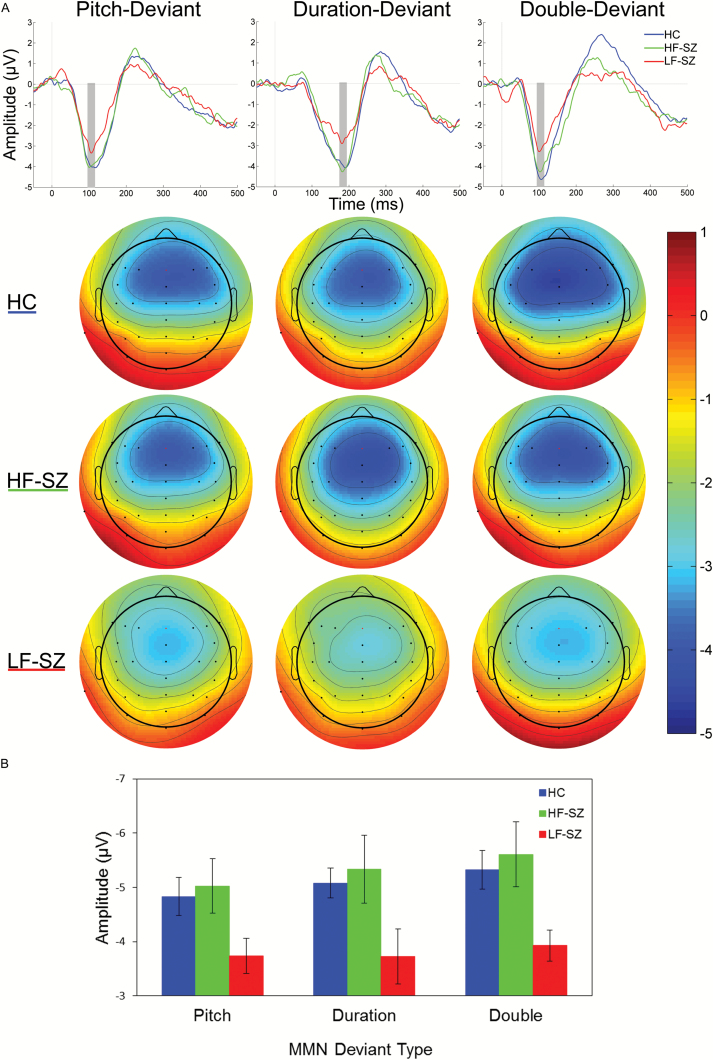
Grand average difference waves for each deviant type show smaller MMN amplitudes (ie, less negative) in LF-SZ than HF-SZ and HC (figure 1; mean amplitude values are shown in supplementary table S3). The ANOVA of midline MMN amplitudes (table 2) revealed a marginally significant group effect that was qualified by a significant group × frontocentral lead interaction, which indicated variation in the strength of group effects at frontal and central midline leads. Parsing this interaction demonstrated a group effect at Fz, with post hoc tests showing significantly reduced MMN amplitudes in LF-SZ patients compared to both HC (Cohen’s d = .92) and HF-SZ (d = .81), and no difference in MMN between the HF-SZ and HC. The group effect at central leads did not reach statistical significance. This pattern of results was unchanged when years of education, PSES, and IQ were included as covariates, and the difference in MMN between the LF-SZ and HF-SZ groups remained statistically significant after covarying for negative symptom scores.
Fig. 1.
(A) Mismatch negativity (MMN) difference waveforms, averaged at Fz, for healthy comparison (HC), high-functioning schizophrenia (HF-SZ), and low-functioning schizophrenia (LF-SZ) participants by deviant type. Scalp voltage topography maps, showing group means of MMN amplitudes around the peak latency ±10 ms (indicated by gray bars in waveform plots), are shown for each deviant type. (B) Column graph shows means and standard errors for MMN amplitudes at Fz.
Table 2.
ANOVA of Midline MMN Amplitudes
| Effect | df | F | P Value | Follow-Up Testsa |
|---|---|---|---|---|
| Group | 2, 69 | 3.01 | .056 | HF-SZ < LF-SZ,b HC < LF-SZ,b HF-SZ = HC |
| Deviant type (double, pitch, duration) | 2, 138 | 1.68 | .194 | |
| Frontocentral lead (frontal, central) | 1, 69 | 41.66 | <.001 | Fz < Czc |
| Group × deviant type | 4, 138 | 0.37 | .804 | |
| Group × frontocentral lead | 2, 69 | 5.65 | .005 | |
| Group effect in frontal leads | 2, 69 | 4.32 | .017 | HF-SZ < LF-SZ,d HC < LF-SZ,b HF-SZ = HC |
| Group effect in central leads | 2, 69 | 1.66 | .197 | |
| Frontocentral lead effect in HC | 1, 34 | 37.68 | <.001 | Fz < Czc |
| Frontocentral lead effect in LF-SZ | 1, 16 | 0.54 | .474 | |
| Frontocentral lead effect in HF-SZ | 1, 19 | 31.30 | <.001 | Fz < Czc |
| Group × deviant type × frontocentral lead | 4, 138 | 1.41 | .234 | |
| Deviant type × frontocentral lead | 2, 138 | 2.14 | .123 |
Note: More negative amplitude indicates larger MMN. ANOVA, analysis of variance; HF-SZ, high-functioning schizophrenia; LF-SZ, low-functioning schizophrenia; HC, healthy comparison.
aAll follow-up tests of statistically significant effects survived FDR correction for multiple comparisons.
b P < .05.
c P < .001.
d P < .01.
The ANOVA examining hemisphere effects on MMN demonstrated a similar pattern of group effects, but there were no group × hemisphere interaction effects indicative of hemispheric abnormalities in the SZ patients (supplementary table S1). A main effect of hemisphere was qualified by a frontocentral lead × hemisphere interaction, parsing of which revealed greater MMN amplitudes at right compared to left frontal leads across participants and deviant types.
ANOVA of P300 Amplitudes
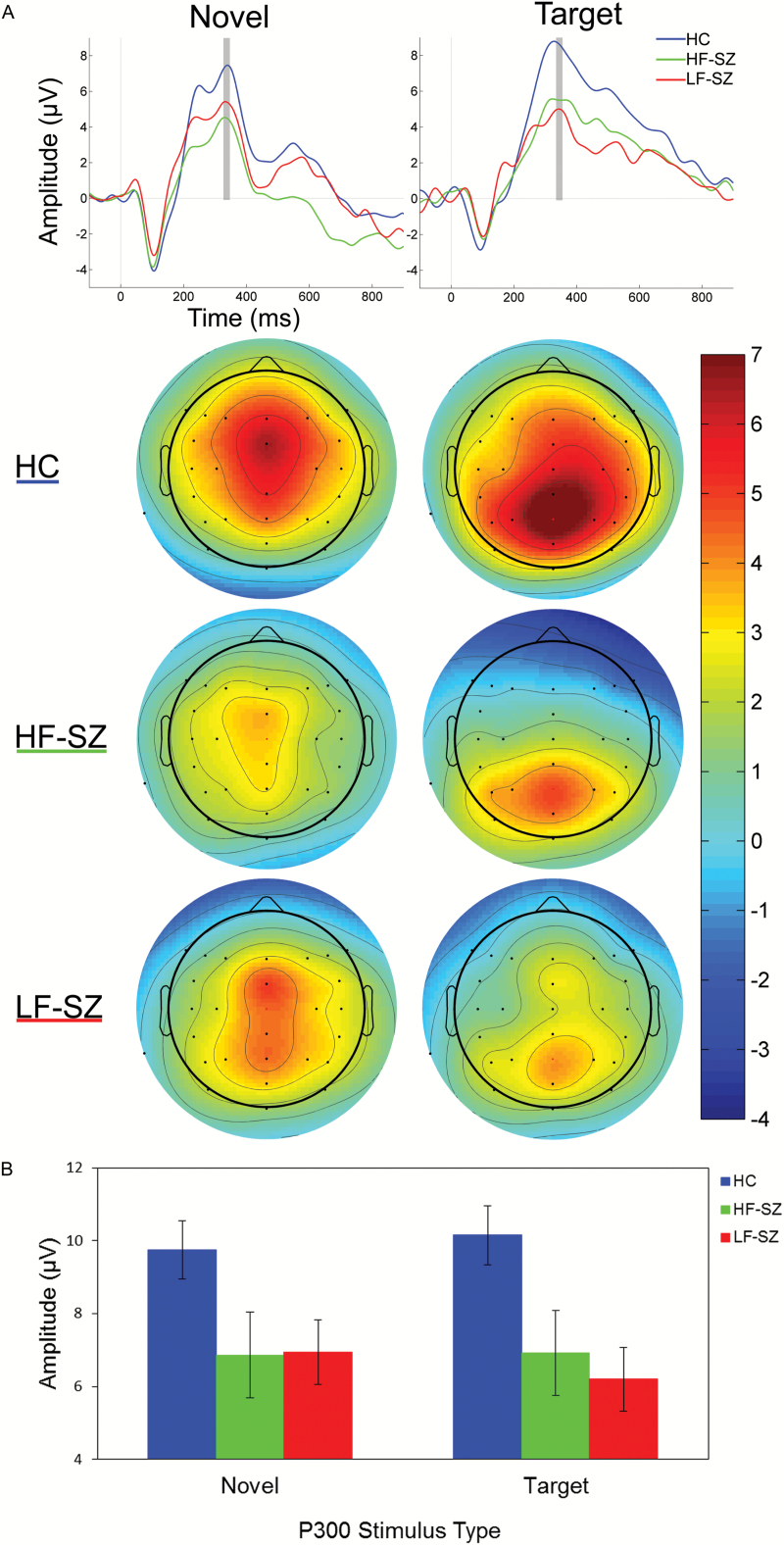
Grand average waveforms show reduced novelty P3a and target P3b amplitudes in both patient groups compared to HC (figure 2 and supplementary table S3). The ANOVA of midline P300 amplitudes indicated a significant effect of group, with post hoc tests demonstrating reduced P300 amplitudes in both LF-SZ and HF-SZ compared to HC (d = .76 and d = .75, respectively; table 3). This main effect was qualified by a group × deviant type × anterior-posterior lead interaction. Parsing this interaction did not reveal an absence of the group effect or a dependence of the group effect on P300 deviant type. Instead, this interaction appeared to capture group variation in the anterior-posterior topography of P3a and P3b (supplementary material). When education, PSES, and IQ were included as covariates, the group and anterior-posterior lead main effects remained statistically significant, while the interaction effects and main effect of deviant type were no longer significant.
Fig. 2.
(A) P300 waveforms, averaged at Cz for novel stimuli (P3a) and Pz for target stimuli (P3b) for healthy comparison (HC), high-functioning schizophrenia (HF-SZ), and low-functioning schizophrenia (LF-SZ) participants. Scalp voltage topography maps, showing group means of P300 amplitudes around the peak latency ±10 ms (indicated by gray bars in waveform plots), are shown for novel and target stimuli. (B) Column graph shows means and standard errors for novelty P3a amplitudes at Cz and target P3b amplitudes at Pz.
Table 3.
ANOVA of Midline P300 Amplitudes
| Effect | df | F | P Value | Follow-Up Testsa |
|---|---|---|---|---|
| Group | 2, 69 | 5.18 | .008 | HC > LF-SZ,b HC > HF-SZ,c LF-SZ = HF-SZ |
| Deviant type (novels, targets) | 1, 69 | 23.34 | <.001 | Novels > targetsd |
| Anterior–posterior lead (Fz, Cz, Pz) | 2, 138 | 16.19 | <.001 | Pz > Cz,c Pz > Fz,d Cz > Fzc |
| Group × deviant type | 2, 69 | 0.54 | .586 | |
| Group × anterior–posterior lead | 4, 138 | 0.30 | .835 | |
| Group × deviant type × anterior–posterior lead | 4, 138 | 2.82 | .038 | |
| Group × anterior–posterior lead for novels | 4, 138 | 0.23 | .878 | |
| Group × anterior–posterior lead for targets | 4, 138 | 1.20 | .315 | |
| Group × deviant type at Fz | 2, 69 | 0.86 | .429 | |
| Group × deviant type at Cz | 2, 69 | 0.41 | .668 | |
| Group × deviant type at Pz | 2, 69 | 2.34 | .104 | |
| Deviant type × anterior–posterior lead in HC | 2, 68 | 54.35 | <.001 | |
| Deviant type effect at Fz | 1, 34 | 27.10 | <.001 | Novels > targetsd |
| Deviant type effect at Cz | 1, 34 | 13.91 | .001 | Novels > targetsd |
| Deviant type effect at Pz | 1, 34 | 10.46 | .003 | Targets > novelsc |
| Anterior–posterior lead effect for novels | 2, 68 | 2.10 | .150 | |
| Anterior–posterior lead effect for targets | 2, 68 | 24.12 | <.001 | Pz > Cz,d Pz > Fz,d Cz > Fzc |
| Deviant type × anterior–posterior lead in HF-SZ | 2, 38 | 33.10 | <.001 | |
| Deviant type effect at Fz | 1, 19 | 20.29 | <.001 | Novels > targetsd |
| Deviant type effect at Cz | 1, 19 | 15.02 | .001 | Novels > targetsc |
| Deviant type effect at Pz | 1, 19 | 1.01 | .327 | |
| Anterior–posterior lead effect for novels | 2, 38 | 0.58 | .541 | |
| Anterior–posterior lead effect for targets | 2, 38 | 32.36 | <.001 | Pz > Cz,d Pz > Fz,d Cz > Fzb |
| Deviant type × anterior–posterior lead in LF-SZ | 2, 32 | 17.27 | <.001 | |
| Deviant type effect at Fz | 1, 16 | 10.02 | .006 | Novels > targetsc |
| Deviant type effect at Cz | 1, 16 | 13.61 | .002 | Novels > targetsc |
| Deviant type effect at Pz | 1, 16 | 0.10 | .760 | |
| Anterior–posterior lead effect for novels | 2, 32 | 1.16 | .321 | |
| Anterior–posterior lead effect for targets | 2, 32 | 14.29 | <.001 | Pz > Cz,b Pz > Fz,d Cz = Fz |
| Deviant type × anterior–posterior lead | 2, 138 | 84.45 | <.001 | |
| Deviant type effect at Fz | 1, 71 | 57.58 | <.001 | Novels > targetsd |
| Deviant type effect at Cz | 1, 71 | 40.93 | <.001 | Novels > targetsd |
| Deviant type effect at Pz | 1, 71 | 7.40 | .008 | Targets > novelsc |
| Anterior–posterior lead effect for novels | 2, 142 | 3.45 | .048 | Cz > Fz,b Cz > Pz,c Fz = Pz |
| Anterior–posterior lead effect for targets | 2, 142 | 60.77 | <.001 | Pz > Cz,c Pz > Fz,d Cz > Fzd |
Note: ANOVA, analysis of variance; HF-SZ, high-functioning schizophrenia; LF-SZ, low-functioning schizophrenia; HC, healthy comparison.
aAll follow-up tests of statistically significant effects survived FDR correction for multiple comparisons.
b P < .05.
c P < .01.
d P < .001.
The ANOVA examining hemisphere effects on P300 (supplementary table S2) demonstrated a similar pattern of group effects as observed in the midline analysis, with post hoc interrogation of the significant group effect demonstrating P300 amplitude reductions in both HF-SZ and LF-SZ compared to HC. There was not a main effect of hemisphere or a group × hemisphere interaction. Parsing of higher-order interactions involving hemisphere revealed a hemisphere effect in HC participants only and indicated greater target P3b amplitudes at right (C4), relative to left (C3), central leads.
Prediction of Functioning Status
Within patients, larger MMN amplitudes (averaged over deviant types at Fz) were modestly associated with smaller P300 amplitudes (novelty P3a at Cz: r = .33, P = .046; target P3b at Pz: r = .37, P = .025). In the first of 2 hierarchical logistic regression models, P3a and P3b amplitudes were entered as a block in step 1 and did not produce a prediction effect (χ2 = 0.61, P = .736), and neither P3a nor P3b produced a predictive increment over and above the other [P3a: Wald(1) = 0.35, P = .553, Exp(B) = 1.07; P3b: Wald(1) = 0.58, P = .447, Exp(B) = .92]. At step 2, entry of MMN amplitude significantly improved prediction of functional status [χ2 = 8.20, P = .004; P3a: Wald(1) = 0.38, P = .537, Exp(B) = 1.09; P3b: Wald(1) = 1.78, P = .182, Exp(B) = 0.80; MMN: Wald(1) = 5.77, P = .016, Exp(B) = 1.88] such that smaller MMN amplitude was associated with poorer functioning. In the second model, MMN was entered at step 1 and predicted functional status [χ2 = 5.87, P = .015; Wald(1) = 4.52, P = .034, Exp(B) = 1.59]. When entered at step 2, P300 failed to improve this prediction [χ2 = 2.95, P = .229; MMN: Wald(1) = 5.77, P = .016, Exp(B) = 1.88; P3a: Wald(1) = 0.38, P = .537, Exp(B) = 1.09; P3b: Wald(1) = 1.78, P = .182, Exp(B) = 0.80]. When MMN was the sole predictor, the results indicated that a one unit decrease in MMN amplitude (ie 1 µV less negative) increased the odds of being LF-SZ by 59%.
Correlations With Symptoms
Neither MMN nor P300 amplitudes were correlated with symptom ratings within LF-SZ or HF-SZ or the combined SZ sample.
Discussion
This study examined the relative contributions of preattentive and attention-dependent auditory processing impairments to functional disability in a sample of SZ patients that ranged considerably in their levels of independent role functioning. As expected, patients with poorer functioning showed MMN deficits compared to patients with high levels of independent functioning and healthy individuals. These results are consistent with observed correlations between MMN and measures of functional outcome in schizophrenia patients,32,67–70 and also support findings suggesting that MMN may be most closely associated with work and independent living domains of functioning.71 Our results also parallel previous findings demonstrating impaired basic tone discrimination in SZ patients residing in long-term care facilities, a setting that typically serves lower functioning patients, relative to outpatient first-episode and chronic patients.85 Given the lack of MMN reduction in the higher functioning group, we also extend previous reports by showing that patients without functional disability may exhibit normal MMN amplitudes. Furthermore, the absence of a group × deviant type interaction confirms prior work suggesting that the degree of MMN deficit does not significantly depend on the type of auditory deviance37,67,86–89 (but see Todd et al.90).
In contrast to some previous reports,31,66,70,72 we did not find an association between P300 and role functioning, as novelty P3a and target P3b amplitudes were reduced compared to healthy participants across both SZ groups, regardless of functional status. MMN, but not P300, predicted patients’ role functioning status even when covariation between MMN and P300 was statistically controlled. In some respects, this result is surprising, as P300 is thought to reflect higher-order cognitive functions that have been linked to functional outcomes in SZ.1,2 Moreover, P300 is generated by multiple distributed sources in frontal, temporal, and parietal cortices,48,91,92 reflecting multi-modal associative and integrative processes that would be expected to support successful management of the complex cognitive and behavioral demands associated with independent functioning. MMN, in contrast, is an automatic response to auditory deviance, requiring no cognitive effort to generate, and is subserved by a relatively limited number of neural generators in auditory and inferior frontal cortex.8,10–14,24
Accordingly, P300 would seem to be a more “face valid” indicator of the processes subsuming successful role functioning than MMN. Yet, our results suggest the opposite. One possible explanation is that the cognitive processes giving rise to P300 are subject to multiple influences, including motivation and effort, that may have compensatory or countervailing effects on the attentional and working memory deficits reflected by P300 reduction in SZ. These influences, in turn, may obscure any systematic relationship between P300 and functional outcomes. Processing reflected by MMN, on the other hand, is less susceptible to these influences because it is explicitly assessed with attention directed away from the auditory channel. As such, MMN may provide a more accurate assessment of the basic integrity of brain functioning that transcends its specific function in the auditory system, with MMN deficits reflecting a relatively broad neurophysiological constraint on an individual’s capacity for independent functioning. Indeed, recent analyses93 characterizing pathways from early auditory neurophysiological deficits (including MMN) to poor functional outcomes in SZ indicated that this relationship was mediated by impaired cognition. Moreover, the effects of early auditory processing deficits were not modality-specific, instead contributing to impairments in both auditory and visual neurocognitive domains.
These findings are also consistent with the proposed role of P300 as a trait marker of SZ and reports of robust abnormalities throughout the illness course.58,94–97 While deficits in MMN seem to be evident in the more typical subgroup of patients with lower levels of role functioning, P300 may have more general sensitivity to SZ irrespective of functioning, and therefore, may hold more promise as a candidate endophenotypic marker of the clinical diagnosis of SZ as currently defined. Although P300 varies somewhat with clinical state in SZ,97 P300 amplitude remains reduced in patients whose symptoms have improved97–104 and shows trait-like stability when assessed longitudinally.98,104,105 Given these reports, it is perhaps not surprising that P300 amplitude was reduced even in high functioning patients. In considering possible reasons for conflicting results with prior work,31,66,70,72 it is noteworthy that prior studies have used scales requiring a clinician to make broad judgments about functional outcomes or call for patients to respond to hypothetical situations to assess their understanding of what constitutes functionally adaptive behavior. In contrast, our criteria were based on objective indicators of residential, occupational, and educational functioning.73 Thus, it may be that P300 is sensitive to variation in cognitive responses to hypothetical functional challenges, but has less relevance to patients’ actual level of real-world functioning.
Recent meta-analytic reviews have concluded that first-episode SZ patients demonstrate less pronounced MMN deficits than chronic patients,29,106 and some studies have found normal MMN amplitudes in first-episode patients that subsequently decline over the first 1–2 years of illness,107,108 consistent with the possibility that MMN tracks disease progression. Although the present sample was comprised primarily of chronic patients, reanalysis after removal of first-episode participants (n = 4) maintained similar results, indicating that effects obtained were not due to a predominance of first-episode patients in the high-functioning group. These findings raise the possibility that sampling biases may partially explain inconsistent results, such that first-episode samples may typically have a greater proportion of higher functioning patients than chronic samples.
Similarly, converging evidence showing that MMN deficits predict subsequent conversion to full psychosis in clinically high-risk individuals37–39 may reflect greater cognitive and functional impairments in those at elevated risk for making this transition.109–111 Some have also reported that MMN abnormalities in first-episode patients may be confined to those with lower IQ.89,112 Inasmuch as low IQ is well-documented in SZ,113 the mediation of MMN deficits by premorbid IQ or role functioning impairments is best considered a reflection of the functional heterogeneity that is superimposed on the generally poor cognitive and functional trajectory typical of SZ, rather than constituting a confound that needs to be controlled. Regardless, neither years of education nor IQ accounted for group differences in MMN in the present sample, suggesting that intact MMN in patients with successful role functioning reflects more than intellectual ability per se. Furthermore, MMN and duration of illness were not correlated, which has also been reported by a recent meta-analysis.29 Together, these results suggest that MMN may better index functional disability than illness progression, and further suggest that functional status may be important to evaluate in MMN studies examining patients in other stages of illness, including the prodrome. Longitudinal studies will help determine whether MMN changes with functional status, or whether MMN deficits early in the illness reflect trait-like brain dysfunction that compromises later functional outcomes.
Interestingly, we also found that in patients, smaller MMN amplitudes were modestly associated with greater P3a and P3b amplitudes. This association should be interpreted with caution as it may reflect idiosyncrasies within our relatively small samples and therefore needs to be replicated in other larger samples. However, we speculate that the somewhat counterintuitive association observed might reflect the influence of later compensatory attentional strategies to overcome weaknesses in early automatic auditory deviance detection, particularly because the attentional systems reflected by the P3a and P3b are engaged later than the preattentive MMN.
Patients were taking antipsychotic medications when evaluated, which is a limitation of the present study. However, there were no dosage differences between patient groups so medication differences are unlikely to account for the absence of MMN deficits in the high functioning group. This is consistent with studies that failed to demonstrate an effect of antipsychotic medication on MMN.114–117 Further, evidence of P300 reductions in unmedicated patients97 and following medication withdrawal,118 as well as P300 improvement with antipsychotic medications,59,115–117 suggests that deficits would have been larger across patients if they were medication-free. In addition, although we were able to observe robust effects in a relatively small sample, confirmation of these results in other larger samples will be reassuring.
In conclusion, the present study suggests that MMN deficits, but not P300 deficits, are sensitive to independent role functioning disability in SZ. MMN may index relatively low-level pan-cortical neurophysiological dysfunction (eg, ubiquitous NMDA receptor dysfunction) that extends beyond the auditory cortex and automatic processing of auditory deviance, fundamentally limiting the potential of a patient with SZ to live and work independently.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by AstraZeneca for an investigator-initiated study (to D.H.M.), the National Institute of Mental Health (R01 MH-58262 to J.M.F.), and the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Sierra-Pacific Mental Illness Research, Education, and Clinical Center (H.K.H).
Supplementary Material
Acknowledgments
Dr Mathalon reports that he is a consultant for Boehringer Ingelheim, Alkermes, and Upsher-Smith Laboratories. Dr Jaeger was an employee of AstraZeneca during data collection.
References
- 1. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 2. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”?Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 3. Blyler CR, Gold JM. Cognitive effects of typical antipsychotic treatment: another look. In: Sharma T, Harvey P, eds. Cognition in Schizophrenia. New York: Oxford University Press; 2000:241–265. [Google Scholar]
- 4. Green MF, Marder SR, Glynn SM et al. . The neurocognitive effects of low-dose haloperidol: a two-year comparison with risperidone. Biol Psychiatry. 2002;51:972–978. [DOI] [PubMed] [Google Scholar]
- 5. Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–184. [DOI] [PubMed] [Google Scholar]
- 6. Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. [DOI] [PubMed] [Google Scholar]
- 7. Näätänen R, Teder W, Alho K, Lavikainen J. Auditory attention and selective input modulation: a topographical ERP study. Neuroreport. 1992;3:493–496. [DOI] [PubMed] [Google Scholar]
- 8. Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. [DOI] [PubMed] [Google Scholar]
- 9. Näätänen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005;42:25–32. [DOI] [PubMed] [Google Scholar]
- 10. Csépe V. On the origin and development of the mismatch negativity. Ear Hear. 1995;16:91–104. [DOI] [PubMed] [Google Scholar]
- 11. Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- 12. Giard MH, Lavikahen J, Reinikainen K et al. . Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: an event-related potential and dipole-model analysis. J Cogn Neurosci. 1995;7:133–143. [DOI] [PubMed] [Google Scholar]
- 13. Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cereb Cortex. 2005;15:545–551. [DOI] [PubMed] [Google Scholar]
- 14. Paavilainen P, Alho K, Reinikainen K, Sams M, Näätänen R. Right hemisphere dominance of different mismatch negativities. Electroencephalogr Clin Neurophysiol. 1991;78:466–479. [DOI] [PubMed] [Google Scholar]
- 15. Näätänen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clin Neurophysiol. 2004;115:140–144. [DOI] [PubMed] [Google Scholar]
- 16. Gunduz-Bruce H, Reinhart RM, Roach BJ et al. . Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry. 2012;71:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–116. [DOI] [PubMed] [Google Scholar]
- 18. Heekeren K, Daumann J, Neukirch A et al. . Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl). 2008;199:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knott V, Shah D, Millar A et al. . Nicotine, auditory sensory memory, and sustained attention in a human ketamine model of schizophrenia: moderating influence of a hallucinatory trait. Front Pharmacol. 2012;3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. [DOI] [PubMed] [Google Scholar]
- 21. Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. [DOI] [PubMed] [Google Scholar]
- 23. Fischer C, Morlet D, Bouchet P, Luaute J, Jourdan C, Salord F. Mismatch negativity and late auditory evoked potentials in comatose patients. Clin Neurophysiol. 1999;110:1601–1610. [DOI] [PubMed] [Google Scholar]
- 24. Näätänen R, Alho K. Generators of electrical and magnetic mismatch responses in humans. Brain Topogr. 1995;7:315–320. [DOI] [PubMed] [Google Scholar]
- 25. Sussman E, Winkler I, Wang W. MMN and attention: competition for deviance detection. Psychophysiology. 2003;40:430–435. [DOI] [PubMed] [Google Scholar]
- 26. Rinne T, Antila S, Winkler I. Mismatch negativity is unaffected by top-down predictive information. Neuroreport. 2001;12:2209–2213. [DOI] [PubMed] [Google Scholar]
- 27. Mathalon DH, Ford JM. Divergent approaches converge on frontal lobe dysfunction in schizophrenia. Am J Psychiatry. 2008;165:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. [DOI] [PubMed] [Google Scholar]
- 29. Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–217. [DOI] [PubMed] [Google Scholar]
- 31. Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:822–829. [DOI] [PubMed] [Google Scholar]
- 32. Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007;13:653–663. [DOI] [PubMed] [Google Scholar]
- 33. Näätänen R, Shiga T, Asano S, Yabe H. Mismatch negativity (MMN) deficiency: a break-through biomarker in predicting psychosis onset. Int J Psychophysiol. 2015;95:338–344. [DOI] [PubMed] [Google Scholar]
- 34. Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012;42:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. [DOI] [PubMed] [Google Scholar]
- 36. Shin KS, Kim JS, Kang DH et al. . Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009;65:1071–1078. [DOI] [PubMed] [Google Scholar]
- 37. Perez VB, Woods SW, Roach BJ et al. . Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol Psychiatry. 2014;75:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bodatsch M, Ruhrmann S, Wagner M et al. . Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. [DOI] [PubMed] [Google Scholar]
- 39. Shaikh M, Valmaggia L, Broome MR et al. . Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134:42–48. [DOI] [PubMed] [Google Scholar]
- 40. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isreal JB, Chesney GL, Wickens CD, Donchin E. P300 and tracking difficulty: evidence for multiple resources in dual-task performance. Psychophysiology. 1980;17:259–273. [DOI] [PubMed] [Google Scholar]
- 42. Kramer AF, Strayer DL. Assessing the development of automatic processing: an application of dual-task and event-related brain potential methodologies. Biol Psychol. 1988;26:231–267. [DOI] [PubMed] [Google Scholar]
- 43. Ravden D, Polich J. Habituation of P300 from visual stimuli. Int J Psychophysiol. 1998;30:359–365. [DOI] [PubMed] [Google Scholar]
- 44. Donchin E, Coles MGH. Is the P300 component a manifestation of context updating. Behav Brain Sci. 1988;11:357–427. [Google Scholar]
- 45. Johnson R., Jr A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. [DOI] [PubMed] [Google Scholar]
- 46. Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. [DOI] [PubMed] [Google Scholar]
- 47. Sutton S, Tueting P, Zubin J, John ER. Information delivery and the sensory evoked potential. Science. 1967;155:1436–1439. [DOI] [PubMed] [Google Scholar]
- 48. Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol. 1998;106:156–164. [DOI] [PubMed] [Google Scholar]
- 49. Smith ME, Halgren E, Sokolik M et al. . The intracranial topography of the P3 event-related potential elicited during auditory oddball. Electroencephalogr Clin Neurophysiol. 1990;76:235–248. [DOI] [PubMed] [Google Scholar]
- 50. Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. [DOI] [PubMed] [Google Scholar]
- 51. Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. [DOI] [PubMed] [Google Scholar]
- 52. Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- 53. Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38:387–401. [DOI] [PubMed] [Google Scholar]
- 54. Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Brain Res Cogn Brain Res. 1998;7:41–48. [DOI] [PubMed] [Google Scholar]
- 55. Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. [DOI] [PubMed] [Google Scholar]
- 56. Goldstein A, Spencer KM, Donchin E. The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology. 2002;39:781–790. [PubMed] [Google Scholar]
- 57. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. [DOI] [PubMed] [Google Scholar]
- 58. Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- 59. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. [DOI] [PubMed] [Google Scholar]
- 60. Bramon E, Shaikh M, Broome M et al. . Abnormal P300 in people with high risk of developing psychosis. Neuroimage. 2008;41:553–560. [DOI] [PubMed] [Google Scholar]
- 61. Frommann I, Brinkmeyer J, Ruhrmann S et al. . Auditory P300 in individuals clinically at risk for psychosis. Int J Psychophysiol. 2008;70:192–205. [DOI] [PubMed] [Google Scholar]
- 62. Ozgürdal S, Gudlowski Y, Witthaus H et al. . Reduction of auditory event-related P300 amplitude in subjects with at-risk mental state for schizophrenia. Schizophr Res. 2008;105:272–278. [DOI] [PubMed] [Google Scholar]
- 63. van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res. 2005;77:309–320. [DOI] [PubMed] [Google Scholar]
- 64. Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47:171–179. [DOI] [PubMed] [Google Scholar]
- 65. Souza VB, Muir WJ, Walker MT et al. . Auditory P300 event-related potentials and neuropsychological performance in schizophrenia and bipolar affective disorder. Biol Psychiatry. 1995;37:300–310. [DOI] [PubMed] [Google Scholar]
- 66. Turetsky BI, Dress EM, Braff DL et al. . The utility of P300 as a schizophrenia endophenotype and predictive biomarker: clinical and socio-demographic modulators in COGS-2. Schizophr Res. 2015;163:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biol Psychiatry. 2012;71:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. [DOI] [PubMed] [Google Scholar]
- 69. Kawakubo Y, Kamio S, Nose T et al. . Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. [DOI] [PubMed] [Google Scholar]
- 70. Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010;67:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Light GA, Swerdlow NR, Thomas ML et al. . Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr Res. 2015;163:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jaeger J, Berns SM, Czobor P. The multidimensional scale of independent functioning: a new instrument for measuring functional disability in psychiatric populations. Schizophr Bull. 2003;29:153–168. [DOI] [PubMed] [Google Scholar]
- 74. First MB, Spitzer RL, Gibbon M, Williams J.. Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). New York: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 75. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1984. [Google Scholar]
- 76. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa; 1984. [Google Scholar]
- 77. Wechsler D. Wechsler Test of Adult Reading: WTAR. New York: Psychological Corporation; 2001. [Google Scholar]
- 78. Perez VB, Ford JM, Roach BJ et al. . Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2012;121:372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Friedman D, Simpson G, Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30:383–396. [DOI] [PubMed] [Google Scholar]
- 80. Mathalon DH, Hoffman RE, Watson TD, Miller RM, Roach BJ, Ford JM. Neurophysiological distinction between schizophrenia and schizoaffective disorder. Front Hum Neurosci. 2010;3:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mathalon DH, Ahn KH, Perry EB Jr et al. . Effects of nicotine on the neurophysiological and behavioral effects of ketamine in humans. Front Psychiatry. 2014;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 83. Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- 84. Hollingshead AB. Four-Factor Index of Social Status. New Haven: Yale University; 1975. [Google Scholar]
- 85. Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility?Arch Gen Psychiatry. 2000;57:1149–1155. [DOI] [PubMed] [Google Scholar]
- 86. Hay RA, Roach BJ, Srihari VH, Woods SW, Ford JM, Mathalon DH. Equivalent mismatch negativity deficits across deviant types in early illness schizophrenia-spectrum patients. Biol Psychol. 2015;105:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. [DOI] [PubMed] [Google Scholar]
- 88. Todd J, Whitson L, Smith E, Michie PT, Schall U, Ward PB. What’s intact and what’s not within the mismatch negativity system in schizophrenia. Psychophysiology. 2014;51:337–347. [DOI] [PubMed] [Google Scholar]
- 89. Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. [DOI] [PubMed] [Google Scholar]
- 90. Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. [DOI] [PubMed] [Google Scholar]
- 91. Kügler CF, Taghavy A, Platt D. The event-related P300 potential analysis of cognitive human brain aging: a review. Gerontology. 1993;39:280–303. [DOI] [PubMed] [Google Scholar]
- 92. Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–479. [DOI] [PubMed] [Google Scholar]
- 93. Thomas ML, Green MF, Hellemann G et al. . Modeling deficits from early auditory information processing to psychosocial functioning in schizophrenia. JAMA Psychiatry. 2017;74:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ford JM, Pfefferbaum A, Roth W. P3 and schizophrenia. Ann N Y Acad Sci. 1992;658:146–162. [DOI] [PubMed] [Google Scholar]
- 95. McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J. Event-related potentials in schizophrenia: their biological and clinical correlates and a new model of schizophrenic pathophysiology. Schizophr Res. 1991;4:209–231. [DOI] [PubMed] [Google Scholar]
- 96. Pritchard WS. Cognitive event-related potential correlates of schizophrenia. Psychol Bull. 1986;100:43–66. [PubMed] [Google Scholar]
- 97. Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47:434–449. [DOI] [PubMed] [Google Scholar]
- 98. Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. Br J Psychiatry. 1987;150:154–160. [DOI] [PubMed] [Google Scholar]
- 99. Coburn KL, Shillcutt SD, Tucker KA et al. . P300 delay and attenuation in schizophrenia: reversal by neuroleptic medication. Biol Psychiatry. 1998;44:466–474. [DOI] [PubMed] [Google Scholar]
- 100. Hirayasu Y, Asato N, Ohta H, Hokama H, Arakaki H, Ogura C. Abnormalities of auditory event-related potentials in schizophrenia prior to treatment. Biol Psychiatry. 1998;43:244–253. [DOI] [PubMed] [Google Scholar]
- 101. Pass HL, Klorman R, Salzman LF, Klein RH, Kaskey GB. The late positive component of the evoked response in acute schizophrenics during a test of sustained attention. Biol Psychiatry. 1980;15:9–20. [PubMed] [Google Scholar]
- 102. St Clair D, Blackwood D, Muir W. P300 abnormality in schizophrenic subtypes. J Psychiatr Res. 1989;23:49–55. [DOI] [PubMed] [Google Scholar]
- 103. Rao KM, Ananthnarayanan CV, Gangadhar BN, Janakiramaiah N. Smaller auditory P300 amplitude in schizophrenics in remission. Neuropsychobiology. 1995;32:171–174. [DOI] [PubMed] [Google Scholar]
- 104. Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol Psychiatry. 1998;43:31–39. [DOI] [PubMed] [Google Scholar]
- 105. Mathalon DH, Ford JM, Rosenbloom M, Pfefferbaum A. P300 reduction and prolongation with illness duration in schizophrenia. Biol Psychiatry. 2000;47:413–427. [DOI] [PubMed] [Google Scholar]
- 106. Haigh SM, Coffman BA, Salisbury DF. Mismatch negativity in first-episode schizophrenia: a meta-analysis. Clin EEG Neurosci. 2017;48:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Devrim-Uçok M, Keskin-Ergen HY, Uçok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:179–185. [DOI] [PubMed] [Google Scholar]
- 108. Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cannon TD, Yu C, Addington J et al. . An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cornblatt BA, Carrión RE, Addington J et al. . Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 2012;38:1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Seidman LJ, Shapiro DI, Stone WS et al. . Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry. 2016;73:1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Salisbury DF, Polizzotto NR, Nestor PG et al. . Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophr Bull. 2017;43:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. [DOI] [PubMed] [Google Scholar]
- 114. Catts SV, Shelley AM, Ward PB et al. . Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. [DOI] [PubMed] [Google Scholar]
- 115. Schall U, Catts SV, Chaturvedi S et al. . The effect of clozapine therapy on frontal lobe dysfunction in schizophrenia: neuropsychology and event-related potential measures. Int J Neuropsychopharmacol. 1998;1:19–29. [DOI] [PubMed] [Google Scholar]
- 116. Umbricht D, Javitt D, Novak G et al. . Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. [DOI] [PubMed] [Google Scholar]
- 117. Umbricht D, Javitt D, Novak G et al. . Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–725. [DOI] [PubMed] [Google Scholar]
- 118. Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36:153–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.