Abstract
The high prevalence of nicotine dependence contributes to excess mortality in schizophrenia. Cue reactivity, or the encounter of drug-related cues or contexts, triggers craving, drug-seeking, and relapse. Prior functional magnetic resonance imaging (fMRI) research indicates that individuals with schizophrenia have blunted neural responses to rewarding stimuli in association with more severe negative symptoms. The objectives of this study are to determine if smokers with schizophrenia have altered neural reactivity to smoking cues compared with non-psychiatrically ill smokers and to evaluate the influence of negative symptoms on cue reactivity. Twenty smokers with schizophrenia and 19 control smokers underwent fMRI while viewing smoking-related and neutral cues. The primary analysis was group comparison of Smoking-Neutral contrast using whole-brain analysis (Pcorrected < .05). Smokers with schizophrenia had significantly greater baseline carbon monoxide levels and longer duration of smoking, suggesting more nicotine use. While both groups had greater brain reactivity to smoking vs neutral cues, smokers with schizophrenia had significantly decreased cue reactivity (Smoking-Neutral) compared to controls in bilateral frontal midline regions. There were significant negative correlations between negative symptoms and frontal midline reactivity. Despite greater nicotine use, smokers with schizophrenia exhibited decreased smoking cue-induced neural reactivity in frontal midline regions, suggesting that increased smoking and low cessation rates in schizophrenia are not primarily driven by responses to smoking-related cues. The finding of negative correlations between cue reactivity and negative symptoms is consistent with previous research demonstrating decreased neural responses to rewarding cues, particularly in patients with negative symptoms.
Keywords: functional magnetic resonance imaging, smoking, nicotine, cue reactivity, negative symptoms, superior frontal gyrus
Introduction
The prevalence of nicotine dependence in schizophrenia is at least 3-fold higher than in the general population,1 contributing to high rates of smoking-related mortality in schizophrenia.2 The relationship between smoking and schizophrenia is complex and multifactorial as previous research demonstrates improved cognitive performance, reduced negative affect and normalization of sensory gating with nicotine use in individuals with schizophrenia.3–5 Shared neurobiological abnormalities in schizophrenia and nicotine dependence6 likely contribute to increased smoking and low cessation rates in smokers with schizophrenia.7
Cue reactivity is a phenomenon in which encountering drug-related cues or contexts triggers craving, drug-seeking, and relapse. It can be assessed with subjective reports of craving and changes in brain activation measured with functional magnetic resonance imaging (fMRI) when smokers are exposed to smoking-related cues. Since individuals with schizophrenia have been reported to have higher levels of craving 15 minutes after smoking a cigarette compared to control smokers in the absence of cues,8 subjective cue-induced craving may be limited by ceiling effects. Neural activation to smoking cues provides an objective measure of brain function that may be less prone to ceiling effects. Neural cue reactivity is correlated with subjective reports of craving and predicts relapse in non-psychiatrically ill smokers.13–15
Rewarding aspects of drugs of abuse include hedonic (“liking”) and motivational properties associated with reward (“wanting”). Dopamine is thought to specifically mediate the attribution of motivating properties of reward-related stimuli, or “incentive salience.”11 The attribution of incentive salience to previously neutral cues associated with nicotine use is an essential component of the establishment and maintenance of nicotine dependence and is mediated by phasic dopaminergic release induced by activation of presynaptic nicotinic acetylcholine receptors on dopaminergic neurons in the mesoaccumbens system. Drug-related cues evoke striatal phasic dopaminergic release12 associated with craving and compulsive drug-use, independent of drug administration. fMRI studies in nonpsychiatrically ill smokers demonstrate cue-related activations in regions including ventral striatum, ventromedial prefrontal cortex (vmPFC), dorsomedial prefrontal cortex, posterior cingulate cortex (PCC), anterior insula and dorsal anterior cingulate.9,13,14 Likewise, striatal presynaptic hyperdopaminergic activity (ie, elevated dopamine synthesis capacity and release) is a highly replicated positron emission tomography finding in schizophrenia.15,16 As cue reactivity is mediated by striatal phasic dopaminergic release, individuals with schizophrenia may be prone to exaggerated dopamine responses to smoking cues. Indeed, the only prior study of smoking cue reactivity in schizophrenia found increased vmPFC activation in smokers with schizophrenia.17
However, behavioral studies of subjective craving to smoking cues are not consistent with enhanced cue reactivity; studies have found no significant differences in cue-induced craving between smokers with and without schizophrenia.18–20 In contrast to numerous studies documenting exaggerated striatal dopamine release, one study identified blunted dopamine release in individuals with schizophrenia with comorbid substance abuse, including nicotine dependence, compared to controls.21 Since exaggerated striatal responses are not found in participants with schizophrenia with co-occurring substance dependence, smokers with schizophrenia may not have increased cue reactivity relative to controls. Multiple fMRI studies in schizophrenia demonstrate blunted neural responses to salient cues. Decreased ventral striatal activation to rewarding cues is reported in unmedicated patients22 and predicts severity of negative symptoms,26,27 consistent with the conceptualization of negative symptoms as stemming from impaired reward processing. Smokers with schizophrenia with more affective flattening have decreased reward-related learning.24 Such findings suggest hypofunction of the reward system in schizophrenia, especially in patients with prominent negative symptoms, and are consistent with the hypothesis that smokers with schizophrenia may have decreased responses to smoking cues. Thus, if found, decreased cue reactivity may be another example of impaired reward-related learning in schizophrenia.
In our own previous work, neural reactivity to smoking cues was positively correlated with functional connectivity between areas commonly activated in cue reactivity tasks (dorsal anterior cingulate and insula): the greater the connectivity, the greater the cue reactivity in nonpsychiatrically ill smokers.25 We also found that insular-cingulate connectivity was negatively correlated with nicotine dependence severity in smokers with and without schizophrenia. However, connectivity between these regions was decreased in smokers with schizophrenia compared to control smokers at all levels of nicotine dependence severity.26 Since insular-cingulate connectivity predicts neural reactivity to smoking cues, smokers with schizophrenia may have decreased cue reactivity compared to control smokers.
Building upon our prior work, we test the hypotheses that (1) smokers with schizophrenia will have decreased neural responses to smoking vs neutral cues relative to control smokers; (2) decreased neural responses to smoking cues will be associated with greater negative symptoms. Importantly, our hypotheses contradict the prior study that found increased cue reactivity in smokers with schizophrenia.17 Previous work suggests heterogeneity in response to smoking cues among patients with schizophrenia. Fonder et al, found that only half of smokers with schizophrenia were reactive to cues as measured by self-reported craving.18 If our hypotheses are met, differences in study design and clinical characteristics of our sample from that of Potvin et al may help understand this heterogeneity. If alternatively, we find increased cue reactivity compared to controls, our findings will provide important replication of prior work.
Methods
Participants
Twenty smokers with schizophrenia (n = 17) or schizoaffective disorder (n = 3) were recruited from a database of participants who previously participated in research at McLean Hospital. Nineteen control smokers were recruited from the community. Subjects were 18–55 years old and gave informed consent approved by McLean Hospital IRB prior to study procedures. Participants were matched on gender and nicotine dependence severity (Fagerstrom Test for Nicotine Dependence [FTND]).27 Inclusion criteria included daily cigarette use ≥ 6 months, FTND ≥ 4 and expired air carbon monoxide (CO) breath test ≥ 10. Diagnosis of schizophrenia or schizoaffective disorder in patients and absence of Axis I diagnoses in controls was confirmed by Structured Clinical Interview for DSM-IV (SCID).28 Two smokers with schizophrenia were not taking medications. The remaining 18 were on a stable dose of antipsychotic medication (n = 3 clozapine, n = 15 atypical antipsychotic medications). Subjects were excluded if they reported current substance abuse or substance abuse within the past 6 months as assessed with SCID substance abuse modules and were required to have a negative urine toxicology test.
Demographic, smoking and clinical characteristics of the 2 groups are presented in table 1. Participants with schizophrenia were significantly older than controls (t = −2.6, P = .01). Although groups were matched on FTND, smokers with schizophrenia had significantly greater baseline CO (t = −2.1, P = .04) and lifetime cigarette use (t = −2.2, P = .04).
Table 1.
Demographic and Clinical Characteristics
| Normal Control Smokers (n = 19) | Schizophrenia Smokers (n = 20) | t | P | |
|---|---|---|---|---|
| Demographic | ||||
| Age (mean ± SD) | 31.5 ± 5.8 | 38.5 ± 10.1 | −2.6 | .01* |
| Gender (Female/Male) | 10/9 | 9//11 | χ2 = 0.2 | .63 |
| Education (y) | 14.4 ± 2.4 | 13.0 ± 2.4 | 1.8 | .08 |
| Smoking characteristics | ||||
| Baseline CO | 20.1 ± 11.4 | 29.2 ± 15.0 | −2.1 | .04* |
| Cigarettes per day | 13.1 ± 3.8 | 15.7 ± 8.0 | −1.3 | .19 |
| FTND | 5.4 ± 1.5 | 5.6 ± 1.1 | −0.4 | .68 |
| Lifetime cigarette use (pack years) | 9.2 ± 5.2 | 15.1 ± 10.8 | −2.2 | .04* |
| Symptom scales and antipsychotic medication | ||||
| SANS total score | __ | 39.7 ± 23.3 | __ | __ |
| BPRS psychosis | __ | 8.8 ± 5.9 | __ | __ |
| BPRS negative | __ | 8.1 ± 4.2 | __ | __ |
| Chlorpromazine equivalents (mg/day) | __ | 526.3 ± 479.0 | __ | __ |
| D2 receptor occupancy (%) | __ | 64.9 ± 26.4 | __ | __ |
Note: CO, carbon monoxide; FTND, Fagerstrom Test for Nicotine Dependence, SANS, Scale for Assessment of Negative Symptoms; BPRS, Brief Psychiatric Rating Scale.
*Statistically significant P < .05.
MRI Data Acquisition
Participants were scanned on a 3-T Siemens Trio scanner with a 32-channel head coil at McLean Imaging Center. Multi-planar rapidly acquired dual echo gradient-echo structural images used the following parameters: TR = 2.1 s, TE = 3.3 ms, slices = 128, matrix = 256 × 256, resolution = 1.0 × 1.0 × 1.33 mm. Functional MR images for the smoking cue task were acquired over 37 axial, interleaving slices parallel to the AC-PC line using a gradient echoplanar imaging sequence (159 volumes per run; TR = 2 s, TE = 30 ms; flip angle 75°; field of view 224 × 224 mm2; image matrix 64 × 64; slice thickness = 3.5 mm; voxel dimensions 3.5 mm isotropic).
Smoking Cues Task
To standardize time since last cigarette, participants smoked a cigarette 1.5 hours prior to scanning. We used an event-related design in which subjects viewed pictures of smoking and neutral cues. Images were obtained from the International Smoking Image Series29 and from our prior work.9,25,30 Images were converted to gray scale to control for visual characteristics. Smoking cues were comprised of 3 categories: people smoking, people holding cigarettes and smoking-related items such as cigarettes. Each neutral cue was matched for visual content to a smoking image (eg, person with pen in mouth, hand holding paintbrush and neutral items such as pens).
To ensure subjects were paying attention, an occasional target stimulus (picture of animal) was presented and subjects were required to press a button in response to the target. Five runs of equal length with 12 smoking cues, 12 neutral cues and 2 target images per run were presented. No individual image was repeated. Image type was presented in a pseudo-randomized fashion with no more than 2 of an image type presented in a row for 4 seconds per image. A jittered delay between sequential image presentation was employed during which a fixation cross appeared for 6–14 seconds (2-second increments). We excluded runs where participants did not correctly identify targets with a button press, resulting in excluding 1 run from 2 participants with schizophrenia.
Assessments
We assessed craving using the Questionnaire of Smoking Urges (QSU) before and after the cue reactivity task.31 Symptom severity in patients was measured using the Scale for Assessment of Negative Symptoms (SANS) and Brief Psychiatric Rating Scale (BPRS).32,33 Total SANS score was the primary measure. BPRS positive symptom score was calculated as sum of scores for unusual thought content, conceptual disorganization, hallucinatory behavior and grandiosity. BPRS negative symptom score was calculated as sum of scores for blunted affect, emotional withdrawal and motor retardation.
Statistical Analyses
Craving Data.
Paired t tests were used within each group separately to compare Post vs Pre-task craving to determine if each group had a change in cue reactivity attributable to the task. To compare craving between groups, we used ANCOVA with change in craving (Post-Pre) as dependent variable, and group, age, gender, education, and smoking variables (cigarettes/day, baseline CO, FTND, and pack years) as independent variables.
Smoking Cues Task.
Analyses were conducted using FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). To allow for signal stabilization, the first 4 volumes were removed. Pre-processing included motion correction with rigid-body transformations (middle volume as reference), skull stripping and creation of brain mask, slice time correction, spatial smoothing with Gaussian kernel full-width half maximum 6 mm and high-pass temporal filtering (Gaussian-fitted least squares straight line fitting with sigma = 100 s). An in-house program detected artifacts from motion/intensity spiking (spike = ≥ 100 voxels exhibiting ≥ 2% difference of timepoint from mean intensity) by including these timepoints as regressors.9,25,30 Subject EPI data were co-registered to the anatomical image, which was spatially normalized to the MNI 152 template.
Individual subject data were analyzed using the general linear model with regressors for Smoking, Neutral and Target stimuli, 6 motion parameters and spike timepoints. Stimulus waveforms were convolved with gamma variate hemodynamic response function (normalization of probability density function of single gamma function). Primary contrasts of interest were Smoking-Neutral and Neutral-Smoking. Whole-brain group analysis was performed with FMRIB’s Local Analysis of Mixed Effects (FLAME). One-sample t tests were performed for each group separately to identify regions significantly activated by smoking cues. Independent sample t tests were used to compare groups for Smoking-Neutral and Neutral-Smoking contrasts. To control for multiple comparisons, we used a cluster-defining threshold of z = 3.1 (P = .001) to define contiguous clusters with Pcorrected < .05 (minimum cluster size 161 voxels). Between-group analyses controlled for age.
To evaluate whether antipsychotic medication usage contributed to group differences, we calculated Pearson correlation coefficients between activation in regions identified by between-group analyses and chlorpromazine equivalent dose (CED)34 and D2 receptor occupancy.35
Clinical Correlations.
We extracted mean contrast parameters from Smoking-Neutral contrast for each cluster identified with significant between-group differences (average across all voxels in each region). Pearson correlation coefficients were calculated between these neural measures and negative symptoms using total SANS score. We performed correlations with BPRS positive and negative scores as supportive evidence. Tests of hypotheses were 2-sided with significance level of .05 (uncorrected for multiple comparisons).
Results
Craving Data
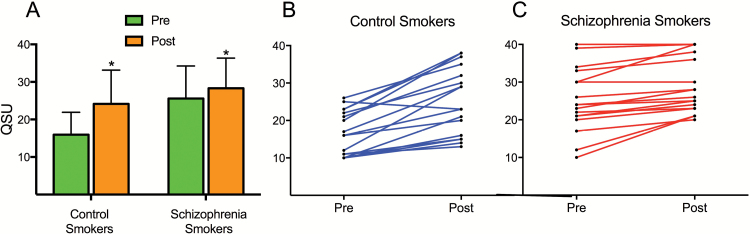
Smokers with schizophrenia had significantly greater craving than control smokers prior to the task (t = −4.0, P < .001; figure 1). Both groups had significant increases in craving after the task (paired t test, Post-Pre-task: both P < .001). After controlling for age, gender, education, and smoking variables, control smokers had significantly greater increased craving than smokers with schizophrenia (F1,29 = 8.56, P = .007). Figure 1C demonstrates a ceiling effect as some smokers with schizophrenia had maximal baseline craving (highest score = 40). We repeated the analysis in subjects with a baseline craving score < 30 to remove subjects with possible ceiling effect (n = 6, all in schizophrenia group); smokers with schizophrenia still had significantly decreased change in craving than controls (F1,23 = 4.7, P = .04). Therefore, it is unlikely that ceiling effect was entirely responsible for blunted increases in craving in smokers with schizophrenia.
Fig. 1.
(A) Smokers with schizophrenia had higher baseline craving compared with control smokers (P ≤ .001). Both groups had greater craving after (Post) the task compared to before (Pre) the task (P ≤ .001). Control smokers had greater cue-induced increases in craving compared with smokers with schizophrenia (P = .007). QSU = Questionnaire of Smoking Urges. (B–C) Comparison of individual participant craving levels Pre and Post cue reactivity task for controls (B) and smokers with schizophrenia (C). Blunted increases in craving compared to control smokers may in part be due to ceiling effect due to maximal baseline craving in some participants. However, blunted increases in craving were seen at all levels of baseline craving.
Since prior research indicates that antipsychotic medications may decrease cue-induced craving,36 we performed correlations between various craving measures and CED and D2 receptor occupancy. There was a trend towards a positive correlation between baseline craving and CED (r = .41, P = .08), a finding inconsistent with medication-related reductions in craving. There were no significant correlations between D2 receptor occupancy and craving measures.
Smoking Cues Task
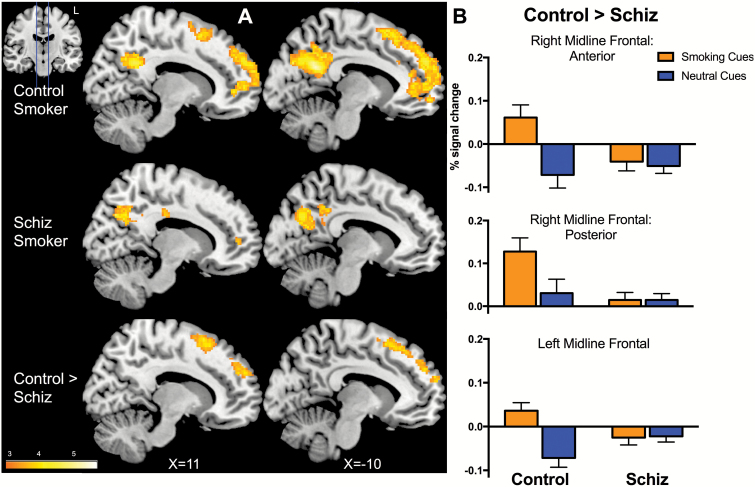
For the Smoking-Neutral contrast, both groups had significant activations in PCC, medial prefrontal cortex, middle temporal and inferior parietal gyri, consistent with activations reported in the literature (table 2; figure 2A).13
Table 2.
Smoking-Neutral Contrast
| Brain Region | Side | Volume (# Voxels) | Z Value | Peak Activity: MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Control smokersa | ||||||
| Medial prefrontal cortex, superior frontal gyrus, anterior cingulate cortex | Bilateral | 6534 | 4.71 | −14 | 52 | 30 |
| Posterior cingulate cortex/precuneus | Bilateral | 4395 | 5.36 | −4 | −52 | 24 |
| Angular/supramarginal gyrus/middle temporal/inferior parietal lobule | L | 3483 | 5.29 | −48 | −60 | 30 |
| Angular/supramarginal/middle temporal gyri | R | 1370 | 5.03 | 58 | −62 | 4 |
| Inferior temporal gyrus | R | 840 | 4.5 | 52 | −10 | −38 |
| Cerebellum | R | 793 | 4.4 | 46 | −78 | −40 |
| Cerebellum | L | 388 | 4.19 | −32 | −80 | −36 |
| Inferior/ superior temporal gyri | L | 378 | 4.41 | −48 | 16 | −16 |
| Inferior frontal gyrus | R | 349 | 4.8 | 56 | 26 | 2 |
| Schizophrenia Smokersa | ||||||
| Medial prefrontal cortex | R | 198 | 4.46 | 2 | 62 | 0 |
| Posterior and middle cingulate cortex/precuneus | Bilateral | 2118 | 5.67 | 2 | −44 | 22 |
| Middle temporal gyrus | L | 619 | 5.07 | −46 | −74 | 4 |
| Inferior parietal lobule/angular gyrus | L | 210 | 4.08 | −36 | −64 | 44 |
| Middle temporal/angular gyri | R | 942 | 4.83 | 52 | −60 | 6 |
| Superior parietal lobule | L | 155 | 4.5 | −36 | −52 | 60 |
| Control > Schizophreniab | ||||||
| Left midline frontal: Superior frontal gyrus (BA8/9/10) | L | 716 | 4.32 | −8 | 22 | 54 |
| Right midline frontal, posterior: superior frontal gyrus (BA8) | R | 299 | 4.44 | 10 | 16 | 62 |
| Right midline frontal, anterior: Superior frontal gyrus (BA9/10) | R | 283 | 4.14 | 14 | 56 | 28 |
Note: aOne sample t test for Smoking-Neutral contrast within each group. There were no significant findings for Neutral-Smoking contrast.
bIndependent sample t test for Control > Schizophrenia comparison for Smoking-Neutral contrast. There were no significant findings for Schizophrenia > Control comparison for Smoking-Neutral contrast and there were no significant group differences for Neutral-Smoking contrast.
Fig. 2.
(A) Activated brain regions in Smoking-Neutral contrast for control smokers (top) and smokers with schizophrenia (middle) included posterior cingulate and medial prefrontal cortices (Pcorrected < .05, one-sample t tests within each group). Group differences for the Smoking-Neutral contrast (Control > Schizophrenia, A bottom, B) involved anterior and posterior clusters in right and left frontal midline regions (Pcorrected < .05, independent sample t test). (B) Control smokers had greater activation for smoking vs neutral cues. In contrast, smokers with schizophrenia showed similar levels of activation for smoking and neutral cues in frontal midline regions.
Between-group analysis revealed 3 clusters where control smokers had greater activation than smokers with schizophrenia: right anterior frontal midline, right posterior frontal midline and a left frontal midline region that involved corresponding regions in the left hemisphere. These regions encompassed bilateral superior frontal gyri (SFG; table 2; figure 2A). Whereas control smokers had increased activation to smoking vs neutral cues in frontal midline regions, smokers with schizophrenia had negligible dynamic variability to smoking and neutral cues (figure 2B).
We repeated analyses removing subjects with possible ceiling effect in craving (baseline craving score ≥ 30); group differences in the 3 clusters remained significant. There were no significant correlations between cluster activations and CED or D2 receptor occupancy. We performed analyses to demonstrate that between-group differences were not due to smoking variables and repeated analyses after removing patients with schizoaffective disorder (n = 3) or patients taking clozapine (n = 3) (supplementary material).
Clinical Correlations
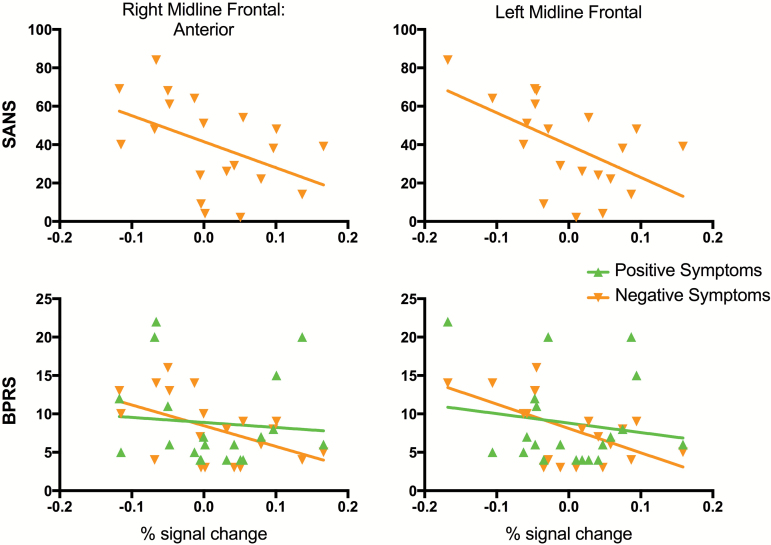
We found significant negative correlations between total negative symptoms (SANS) and activation of right anterior (r = −.46, P = .04) and left (r = −.55, P = .01) frontal midline regions. Confirming findings with the SANS, we found significant negative correlations between BPRS negative symptoms for right anterior (P = −.50, P = .02) and left (r = −.57, P = .009) frontal midline regions. Importantly, there were no significant correlations for BPRS positive symptoms (figure 3).
Fig. 3.
Negative correlations between negative symptoms and brain regions (% signal change averaged across each region) shown to have lower reactivity to the Smoking-Neutral contrast in smokers with schizophrenia. Top: Negative correlations with Scale for the Assessment of Negative Symptoms (SANS) for right anterior (r = −.46, P = .04) and left (r = −.55, P = .01) midline frontal regions. Bottom: Negative correlations were found with Brief Psychiatric Rating Scale (BPRS) negative symptoms (r = −.50 and r = −.57 for right anterior and left midline frontal regions, respectively; P < .02) but not for positive symptoms (P > .50).
We found a significant negative correlation between negative symptoms and change in craving (r = −.62, P = .005), such that smokers with schizophrenia with greater negative symptoms had lower cue-induced increases in craving. We performed additional analyses to demonstrate that negative correlations between negative symptoms and brain activations were not related to craving or medication usage (supplementary material).
Discussion
Both smokers with and without schizophrenia had smoking cue-induced craving and brain activations in PCC and medial PFC, areas previously identified in cue reactivity studies.15,16,38,39 Using an event-related design, we report novel findings that (1) in comparison to controls, smokers with schizophrenia showed decreased cue reactivity in bilateral prefrontal midline regions; (2) cue-induced activation of these regions was negatively correlated with negative symptoms.
Although groups were matched on FTND, smokers with schizophrenia had greater baseline CO levels and lifetime cigarette use. Despite signs suggestive of greater nicotine use, our findings indicate that enhanced cue-reactivity may not be driving increased nicotine dependence severity and low quit rates characteristic of smokers with schizophrenia, as patients had lower reactivity in frontal midline regions and blunted increases in craving to smoking cues relative to controls.
Negative correlations between negative symptoms and prefrontal midline regions were robust and significant as they were identified with both SANS and BPRS negative symptom scores and did not correlate with BPRS positive symptoms. Previous research indicates that smokers have greater negative symptoms than nonsmokers with psychotic disorders.41,42 Smoking at age 14 predicts greater negative symptoms at disease onset.41 Nicotine dependence severity predicts greater negative, but not positive, symptoms.42 Therefore, since studies collectively suggest that smoking behaviors are more severe in patients with greater negative symptoms, the findings of negative correlations between negative symptoms and cue-induced craving and smoking-related SFG activation strengthen our conclusion that enhanced cue reactivity is not driving increased smoking in schizophrenia.
Prefrontal cortex hypofunction is thought to underlie negative symptoms, supported by neuroimaging findings of hypofrontality associated with negative symptoms.43 The bilateral prefrontal midline regions exhibiting decreased responsiveness to smoking cues in the schizophrenia group spanned the SFG, a region consistently activated in studies of smoking cue reactivity.13,30,37,38 Interestingly, a recent study demonstrated a robust and specific association of left SFG thickness and schizophrenia genetic risk.44 Previous studies report decreased left SFG volume in schizophrenia45 with more pronounced decrements in patients with deficit schizophrenia.46 SFG activation is observed in response to reward-dependent skill acquisition and working memory performance (performance-driven monetary reward vs unrewarded comparisons).47–49 Segarra et al reported decreased SFG activation in subjects with schizophrenia in response to unexpected monetary rewards in comparison to healthy subjects: reduced SFG was associated with decreased task-related motivation.50 These findings suggest the SFG has a prominent role in goal-directed motivation, of direct relevance to negative symptoms.
SFG activation to cues in smokers with schizophrenia was decreased compared to controls and was characterized by a lack of variability to smoking and neutral cues. In contrast to many fMRI studies of salient stimuli in schizophrenia in which patients have greater activation to neutral cues than controls,51,52 this pattern is more consistent with studies of anhedonia. Pizzagalli et al found similar levels of caudate activation to monetary rewards vs no reward in patients with depression; caudate volume was negatively correlated with anhedonia.53
To our knowledge, one prior study of cue reactivity in schizophrenia has been published (also in medicated patients), which reported increased vmPFC cue reactivity compared to nonpsychiatrically ill smokers and a negative correlation between vmPFC activation and cue-elicited cravings in patients.17 Our results contradict those of Potvin, as we found no significant differences in medial PFC smoking cue reactivity between smokers with and without schizophrenia. There are several notable differences between Potvin et al and our study. We had a higher proportion of female patients. Previous research has found decreased cue-induced activation in female compared to male subjects.54 However, groups were matched for gender composition; controlling for gender did not alter our findings. The Potvin study employed a block design, whereas we used an event-related design. Blocked designs are prone to habituation effects, and it is possible that neural activation within a block may habituate to successive stimuli. Patients with schizophrenia have reduced habituation to fMRI stimuli;55 therefore, the relative increase in smoking cue-induced activation may have been due to reduced habituation to smoking stimuli in the schizophrenia group compared to controls. Another explanation for increased vmPFC cue reactivity in their study was a trend for higher nicotine dependence severity (FTND) in the schizophrenia group, while our study matched groups on FTND. In previous studies, nicotine dependence severity is associated with greater neural responses to smoking cues.56,57
While only 3 patients in our sample were prescribed clozapine, the majority of patients in Potvin’s study were on clozapine. It is unlikely that clozapine use itself explained greater vmPFC activation to smoking cues, as they did not find a significant correlation between clozapine dose and vmPFC activation. Prior studies demonstrate reduced smoking in patients on clozapine compared to other antipsychotics.58,59 Patients with schizophrenia and comorbid cannabis use disorders on clozapine showed reduced cannabis cue-induced activation compared to patients on risperidone.60 The higher proportion of patients treated with clozapine suggests the Potvin sample had more treatment-refractory patients. As one study reported decreased responsiveness to antipsychotic medication in heavier smokers with schizophrenia,61 vmPFC reactivity to smoking cues may be enhanced in treatment-refractory patients. Patients in our study may have had more severe negative symptoms, as reduced SFG cue-reactivity was stronger in patients with prominent negative symptoms. Collectively, these differences show that heterogeneity of individuals with schizophrenia should be considered when evaluating smoking cue-reactivity. In the present work, negative symptoms impact cue-reactivity and most likely contributed to variability between current and prior work.
Limitations
All but 2 smokers with schizophrenia were on antipsychotic medications. Controlling for CED/D2 receptor occupancy did not alter our findings. Studying cue reactivity in medicated patients is an important clinical problem, as smoking rates are high regardless of medication status.62,63 Some smokers with schizophrenia had maximal levels of subjective craving at baseline; the relative blunting of task-related craving may be partially due to ceiling effects. Including participants on clozapine and with schizoaffective disorder was another limitation, given the possibility of decreased cue reactivity in these subgroups; excluding these participants did not alter our main findings.
Conclusion
Smokers with schizophrenia exhibited cue reactivity as demonstrated by increased neural responses to smoking cues in PCC and medial PFC and increased craving after the cue reactivity task. However, we found blunted increases in craving and decreased brain activation to smoking vs neutral cues in midline frontal regions in association with negative symptoms, suggesting that increased rates of smoking and low quit rates in smokers with schizophrenia compared to smokers in the general population is not primarily driven by cue reactivity.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by NIDA K01DA029645 (A.C.J.), NIMH K23MH110564, NARSAD Young Investigator Award, Brain and Behavior Research Foundation, Pope-Hintz Fellowship Award, McLean Hospital, and Dupont-Warren Fellowship Award, Harvard Medical School (L.V.M.).
Supplementary Material
Acknowledgment
All authors declare no conflicts of interest.
References
- 1. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. [DOI] [PubMed] [Google Scholar]
- 2. Kelly DL, McMahon RP, Wehring HJ et al. . Cigarette smoking and mortality risk in people with schizophrenia. Schizophr Bull. 2011;37:832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonard S, Mexal S, Freedman R. Smoking, genetics and schizophrenia: evidence for self medication. J Dual Diagn. 2007;3:43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mann-Wrobel MC, Bennett ME, Weiner EE, Buchanan RW, Ball MP. Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophr Res. 2011;126:277–283. [DOI] [PubMed] [Google Scholar]
- 5. Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. [DOI] [PubMed] [Google Scholar]
- 6. Moran LV, Sampath H, Kochunov P, Hong LE. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyons MJ, Bar JL, Kremen WS et al. . Nicotine and familial vulnerability to schizophrenia: a discordant twin study. J Abnorm Psychol. 2002;111:687–693. [DOI] [PubMed] [Google Scholar]
- 8. Lo S, Heishman SJ, Raley H et al. . Tobacco craving in smokers with and without schizophrenia. Schizophr Res. 2011;127:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janes AC, Pizzagalli DA, Richardt S et al. . Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. [DOI] [PubMed] [Google Scholar]
- 11. Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boileau I, Dagher A, Leyton M et al. . Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Engelmann JM, Versace F, Robinson JD et al. . Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Janes AC, Ross RS, Farmer S et al. . Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addict Biol. 2015;20:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howes OD, Kambeitz J, Kim E et al. . The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howes OD, Montgomery AJ, Asselin MC et al. . Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 17. Potvin S, Lungu O, Lipp O et al. . Increased ventro-medial prefrontal activations in schizophrenia smokers during cigarette cravings. Schizophr Res. 2016;173:30–36. [DOI] [PubMed] [Google Scholar]
- 18. Fonder MA, Sacco KA, Termine A et al. . Smoking cue reactivity in schizophrenia: effects of a nicotinic receptor antagonist. Biol Psychiatry. 2005;57:802–808. [DOI] [PubMed] [Google Scholar]
- 19. Freeman TP, Stone JM, Orgaz B, Noronha LA, Minchin SL, Curran HV. Tobacco smoking in schizophrenia: investigating the role of incentive salience. Psychol Med. 2014;44:2189–2197. [DOI] [PubMed] [Google Scholar]
- 20. Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2008;10:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson JL, Urban N, Slifstein M et al. . Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry. 2013;18:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juckel G, Schlagenhauf F, Koslowski M et al. . Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. [DOI] [PubMed] [Google Scholar]
- 23. Arrondo G, Segarra N, Metastasio A et al. . Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol. 2015;6:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutra SJ, Stoeckel LE, Carlini SV, Pizzagalli DA, Evins AE. Varenicline as a smoking cessation aid in schizophrenia: effects on smoking behavior and reward sensitivity. Psychopharmacology (Berl). 2012;219:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janes AC, Farmer S, Peechatka AL, Frederick Bde B, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 28. First M, Spitzer R, Gibbon M et al. . Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version (SCID). New York, NY: Biometric Research Department: New York State Psychiatric Institute; 2002. [Google Scholar]
- 29. Gilbert D, Rabinovich N.. International Smoking Image Series (with Neutral Counterparts). Version 1.2. Carbondale, IL: Integrative Neurosciene Laboratory, Department of Psychology, Southern Illinois University; 1999. [Google Scholar]
- 30. Janes AC, Frederick Bd, Richardt S et al. . Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 32. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 33. Overall J, Gorham D. The brief psychiatric rating scale. Psychological Reports 1962;10:799–812. [Google Scholar]
- 34. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. [DOI] [PubMed] [Google Scholar]
- 35. Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33:675–681. [DOI] [PubMed] [Google Scholar]
- 36. Hutchison KE, Rutter MC, Niaura R, Swift RM, Pickworth WB, Sobik L. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175:407–413. [DOI] [PubMed] [Google Scholar]
- 37. David SP, Munafò MR, Johansen-Berg H et al. . Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper J, Mancuso SG, Borland R, Slade T, Galletly C, Castle D. Tobacco smoking among people living with a psychotic illness: the second Australian Survey of Psychosis. Aust N Z J Psychiatry. 2012;46:851–863. [DOI] [PubMed] [Google Scholar]
- 40. Iasevoli F, Balletta R, Gilardi V, Giordano S, de Bartolomeis A. Tobacco smoking in treatment-resistant schizophrenia patients is associated with impaired cognitive functioning, more severe negative symptoms, and poorer social adjustment. Neuropsychiatr Dis Treat. 2013;9:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mäkinen J, Miettunen J, Jääskeläinen E, Veijola J, Isohanni M, Koponen H. Negative symptoms and their predictors in schizophrenia within the Northern Finland 1966 Birth Cohort. Psychiatry Res. 2010;178:121–125. [DOI] [PubMed] [Google Scholar]
- 42. Patkar AA, Gopalakrishnan R, Lundy A, Leone FT, Certa KM, Weinstein SP. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190:604–610. [DOI] [PubMed] [Google Scholar]
- 43. Andreasen NC, Rezai K, Alliger R et al. . Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. [DOI] [PubMed] [Google Scholar]
- 44. Lee PH, Baker JT, Holmes AJ et al. . Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol Psychiatry. 2016;21:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohtani T, Levitt JJ, Nestor PG et al. . Prefrontal cortex volume deficit in schizophrenia: a new look using 3T MRI with manual parcellation. Schizophr Res. 2014;152:184–190. [DOI] [PubMed] [Google Scholar]
- 46. Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ. Gray-matter abnormalities in deficit schizophrenia. Schizophr Res. 2010;120:63–70. [DOI] [PubMed] [Google Scholar]
- 47. Dayan E, Hamann JM, Averbeck BB, Cohen LG. Brain structural substrates of reward dependence during behavioral performance. J Neurosci. 2014;34:16433–16441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hager OM, Kirschner M, Bischof M et al. . Reward-dependent modulation of working memory is associated with negative symptoms in schizophrenia. Schizophr Res. 2015;168:238–244. [DOI] [PubMed] [Google Scholar]
- 49. Pochon JB, Levy R, Fossati P et al. . The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Segarra N, Metastasio A, Ziauddeen H et al. . Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. 2016;41:2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anticevic A, Corlett PR. Cognition-emotion dysinteraction in schizophrenia. Front Psychol. 2012;3:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Potvin S, Tikàsz A, Mendrek A. Emotionally neutral stimuli are not neutral in schizophrenia: a mini review of functional neuroimaging studies. Front Psychiatry. 2016;7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pizzagalli DA, Holmes AJ, Dillon DG et al. . Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wetherill RR, Young KA, Jagannathan K et al. . The impact of sex on brain responses to smoking cues: a perfusion fMRI study. Biol Sex Differ. 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Williams LE, Blackford JU, Luksik A, Gauthier I, Heckers S. Reduced habituation in patients with schizophrenia. Schizophr Res. 2013;151:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smolka MN, Bühler M, Klein S et al. . Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl). 2006;184:577–588. [DOI] [PubMed] [Google Scholar]
- 58. McEvoy JP, Freudenreich O, Wilson WH. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;46:125–129. [DOI] [PubMed] [Google Scholar]
- 59. Procyshyn RM, Ihsan N, Thompson D. A comparison of smoking behaviours between patients treated with clozapine and depot neuroleptics. Int Clin Psychopharmacol. 2001;16:291–294. [DOI] [PubMed] [Google Scholar]
- 60. Machielsen MWJ, Veltman DJ, van den Brink W et al. . Comparing the effect of clozapine and risperidone on cue reactivity in male patients with schizophrenia and a cannabis use disorder: a randomized fMRI study [published online ahead of print March 25, 2017]. Schizophr Res. doi:10.1016/j.schres.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 61. Rajkumar AP, Chitra C, Bhuvaneshwari S et al. . Clinical predictors of response to clozapine in patients with treatment resistant schizophrenia. Psychopharmacol Bull. 2011;44:51–65. [PMC free article] [PubMed] [Google Scholar]
- 62. de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ. Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital. Schizophr Res. 2002;56:55–65. [DOI] [PubMed] [Google Scholar]
- 63. Salokangas RK, Honkonen T, Stengård E, Koivisto AM, Hietala J. Cigarette smoking in long-term schizophrenia. Eur Psychiatry. 2006;21:219–223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.