Abstract
Background
Striatal dopamine (DA) synthesis capacity and release are elevated in schizophrenia (SCZ) and its putative prodrome, the clinical high risk (CHR) state. Striatal DA function results from the activity of midbrain DA neurons projecting mainly from the substantia nigra (SN). Elevated stress-induced DA release in SCZ and CHR was observed in the striatum; however, whether it is also elevated in the SN is unclear. The current study aims to determine whether nigral DA release in response to a validated stress task is altered in CHR and in antipsychotic-naïve SCZ. Further, we explore how DA release in the SN and striatum might be related.
Methods
24 CHR subjects, 9 antipsychotic-naïve SCZ and 25 healthy volunteers (HV) underwent 2 positron emission tomography (PET) scans using the DA D2/3 agonist radiotracer, [11C]-(+)-PHNO, which allows simultaneous investigations of DA in the SN and striatum. Psychosocial stress-induced DA release was estimated as the percentage differences in BPND (%[11C]-(+)-PHNO displacement) between stress and sensory-motor control sessions.
Results
We observed a significant diagnostic group by session interaction, such that SCZ exhibited greater stress-induced [11C]-(+)-PHNO % displacement (25.90% ± 32.2%; mean ± SD), as compared to HVs (−10.94% ± 27.1%). Displacement in CHRs (−1.13% ± 32.2%) did not differ significantly from either HV or SCZ.
Conclusion
Our findings suggest that elevated nigral DA responsiveness to stress is observed in antipsychotic-naïve SCZ.
Keywords: dopamine, substantia nigra, psychosis, positron emission tomography, clinical high risk, striatum
Introduction
Elevations in striatal dopamine (DA) synthesis capacity and release in response to a challenge (DA drugs or stress) are key features in schizophrenia (SCZ).1–3 Increased striatal DA synthesis capacity4,5 and release3,6 are also observed in individuals at clinical high risk (CHR) for psychosis, suggesting a pre-morbid altered neurochemical state. CHR is a well-defined cohort with attenuated psychotic symptoms and/or genetic risks and recent functional deterioration7 with a conversion rate 25%–35% over 24–36 months.8,9
The observation of increased presynaptic DA synthesis and release in SCZ and CHR, raises the question of upstream functional changes of DA transmission. Striatum receives dopaminergic innervations from the midbrain, particularly from substantia nigra (SN) and ventral tegmental area (VTA). Evidence indicates that alterations in striatal DA function are particularly apparent in the associative subdivision of the striatum (AST),3,4,10 which predominantly receives projections from the pars compacta of SN (SNc).11–13 Thus, the increased AST DA synthesis and release in psychosis may reflect an increased dopaminergic function in the SN.
Increased nigral DA activity in SCZ is supported by both postmortem and in vivo evidence. Postmortem studies have reported elevated tyrosine hydroxylase activity14–16 and D2 receptor levels17 in the SN of SCZ compared to healthy volunteers (HV; but see18,19). Similarly, in vivo studies reported higher midbrain DA turnover (estimated by kloss of [18F]fluorodopamine)20 and DA synthesis capacity (measured by Kicer of [18F]-DOPA)14 in SCZ compared to HV. In vivo D2 receptor availability has been reported to be elevated in antipsychotic-free/naïve SCZ by one,21 but not all studies,22 relative to HV. Investigations using functional magnetic resonance imaging (fMRI) have observed elevated SN activity in SCZ during cognitive tasks23,24 and during presentation of motivationally neutral stimuli.25 A functional relationship of DA activity in SN and striatum may be relevant to clinical presentation as greater psychotic symptoms were associated with not only higher midbrain DA synthesis capacity14 but also greater SN-caudate functional connectivity.23 However, it is not known if, or under what conditions, alterations of DA function in striatum may relate to midbrain DA function. Whereas striatal DA synthesis capacity26 and release is apparently blunted in otherwise healthy cannabis users, midbrain DA release has surprisingly been reported to be elevated following a challenge with methylphenidate27 but not with amphetamine28 or stress.29
Despite evidence for clinically relevant changes in the SN DA system in SCZ, few studies have explored nigral DA release in humans, fewer still in CHR. Studies using [11C]-(+)-PHNO have investigated nigral DA release in response to amphetamine30,31 and in response to nicotine and a psychosocial stress29 in nonpsychotic participants. In vivo imaging evidence of a functional relationship between SN and striatum DA in healthy humans comes from a study that observed a negative association between baseline SN/VTA D2/3 receptor availability and amphetamine-induced DA release in striatum,32 suggesting a measurable contribution of SN/VTA autoreceptors in modulating downstream DA release. To date, only 1 study has examined nigral DA release in SCZ. Using the high-affinity D2/3 ligand [11C]-FLB-457, Slifstein et al33 reported decreased DA release in extra-striatal regions in response to an amphetamine challenge in SCZ, including a trend-level decrease in the midbrain (SN/VTA). However, the relationship between DA release capacity in the SN and in striatum could not be explored in that [11C]-FLB-457 study.
[11C]-(+)-PHNO is a DA D2/3 agonist ligand that permits a simultaneous investigation of DA release in the SN and striatum. The overall regional signal of [11C]-(+)-PHNO is a function of its differential affinities for D3 vs D2 receptors and the availability of each receptor subtype in a given region. In SN, the majority of the [11C]-(+)-PHNO signal is attributable to binding to D3 receptors (83%–100%), whereas in dorsal striatum D2 binding predominates (D3 fraction: 0%–26%).34 Like D2 receptors, somatodendritic DA D3 receptors expressed by midbrain DA neurons35,36 are thought to act as autoreceptors.37,38 As such, changes in [11C]-(+)-PHNO binding potential in the SN reflect changes in the concentration of extracellular DA, which acts on D2/3 autoreceptors to modulate SN/VTA DA neuron activity39–41 and, in turn, striatal DA release.42
Based on postmortem and in vivo evidence of increased DA synthesis and turnover rate in the SN,14–16 but see Slifstein et al,33 we postulated an increased nigral DA release in antipsychotic-naïve SCZ and individuals at CHR for psychosis in response to a psychosocial stress paradigm. We also explored the association between changes in [11C]-(+)-PHNO binding in SN and striatum, as well as the association between changes in SN [11C]-(+)-PHNO binding and psychotic symptoms in SCZ and attenuated psychotic symptoms in CHR.
Methods and Materials
Participants
This study combines and extends a cohort described in prior studies,3,6,29,43 comprising a total 24 CHR, 9 SCZ, and 25 matched HV. This report omits the data of 1 SCZ individual included in an earlier analysis (examining striatum)3 as we were unable to obtain a reliable estimate of [11C]-(+)-PHNO binding in the SN in both scans. Data for HV and CHR in striatum and SN have been reported previously and are presented here for comparison with SCZ SN data and to explore regional associations in [11C]-(+)-PHNO displacement. As described previously,3 CHR participants met criteria for prodromal syndromes based on the Structured Interview for Prodromal Syndromes (SIPS),44 and the SCZ participants met the diagnosis of SCZ or schizophreniform disorder. All CHR and SCZ patients were free of other axis I disorders except 3 CHR individuals and 1 SCZ patient (table 1). All patients were antipsychotic-naïve. All participants screened negative for drugs of abuse on urine drug screens conducted at enrollment and on positron emission tomography (PET) scan days, excepting identified cannabis-users, all of whom screened positive for cannabis (table 1). In the cannabis user groups, all HV cannabis users, 11 of 12 CHR cannabis users and of 4 SCZ cannabis users 2 met criteria for cannabis dependence and 2 were in partial remission at the time of the study. All subjects provided written, informed consent to participate.
Table 1.
Demographic and Clinical Variables
| HV (n = 25) | CHR (n = 24) | SCZ (n = 9) | ||
|---|---|---|---|---|
| Age (y) | 25.12 (4.45) | 23.63 (4.67) | 24.11 (5.33) | |
| Education (y) | 14.48 (2.00) | 13.67 (2.55) | 13.63 (2.07) | |
| Ethnicity 1/2/3/4/5 | 17/0/4/3/1 | 13/0/7/3/1 | 5/0/3/0/1 | |
| Sex | Male | 13 | 13 | 6 |
| Female | 12 | 11 | 3 | |
| Tobacco smoking status | Nonsmoker | 21 | 19 | 6 |
| Smoker | 4 | 5 | 3 | |
| Cannabis (current) | Nonuser | 12 | 12 | 5 |
| User | 13 | 12 | 4 | |
| Cocaine (history) | Nonuser | 23 | 18 | 9 |
| User | 2 | 6 | 0 | |
| Amphetamine (history) | Nonuser | 25 | 22 | 9 |
| User | 0 | 2 | 0 | |
| Ecstasy (history) | Nonuser | 21 | 18 | 9 |
| User | 4 | 6 | 0 | |
| Clinical Information and PET parameters | HV (n = 25) | CHR (n = 24) | SCZ (n = 9) | |
| SOPS-P | — | 11.91 (2.54)b | — | |
| SOPS-N | — | 8.26 (4.91)b | — | |
| PANSS-P | — | — | 19.33 (3.84) | |
| PANSS-N | — | — | 16.78 (4.12) | |
| Other diagnosis | Anxiety disorder | 0 | 0 | 1 |
| Othersa | 0 | 3 | 0 | |
| Medications used | Anti-depressant | 0 | 1 | 1 |
| Others | 0 | 2 | 0 | |
| Amount injected (SD) | Control task | 9.50 (1.36) | 9.57 (1.73) | 9.12 (1.47) |
| Stress task | 9.93 (0.73) | 9.79 (0.96) | 9.76 (1.54) | |
| Specific activity (SD) | Control task | 1161.94 (488.57) | 1107.33 (504.07) | 1015.02 (550.59) |
| Stress task | 1239.25 (443.04) | 1243.93 (595.66) | 1012.19 (290.99) | |
| Mass injected (SD) | Control task | 2.16 (0.67) | 2.42 (0.93) | 2.54 (0.87) |
| Stress task | 2.11 (0.67) | 2.42 (1.02) | 2.65 (0.67) | |
Note: HV, healthy volunteer; CHR, clinical high risk; SCZ, schizophrenia; Ethnicity (self-reported): 1: White; 2: Mixed/Multiple ethnic groups; 3: Asian/Asian Canadian; 4: Black/African/Caribbean/Black Canadian; 5: Other; PANSS-P and PANSS-N: Positive and Negative Syndrome Scale, positive subscale and negative subscale; SOPS-P and SOPS-N: Scale of Prodromal Symptoms, positive scale and negative scale.
aOther diagnoses includes: Major depression in remission (2), alcohol abuse (1).
bThe SOPS score of 1 CHR participant was not available.
Assessments
Psychopathology Measures.
As described previously,3 symptom severity in CHR individuals was assessed using the Scale of Prodromal Symptoms (SOPS), a part of the SIPS with excellent inter-rater reliability.44 In patients with SCZ, psychosis symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). In addition, all subjects were screened for any axis I psychopathology using the Structured Clinical Interview for DSM-IV conducted by a qualified psychiatrist (R.M.).
Montreal Imaging Stress Task.
The Montreal Imaging Stress Task (MIST) which has been validated in fMRI and PET studies,3,6,45–48 was used to induce psychological stress as described previously.3 Briefly, subjects performed a series of mental arithmetic tasks on a computer screen that displayed information about the total number of errors, expected average number of errors, time spent on the current problem, and performance feedback for each problem (correct, incorrect, or timeout). The stress condition of the MIST includes a time constraint that varies according to each individual’s performance producing only 20%–30% correct answers. In addition, subjects received negative verbal feedback from the same investigator (R.M.) following a script to ensure verbatim language was delivered to all subjects. The investigator providing feedback was aware of the group membership of participants. Prior to the stress-task, all subjects performed a Sensory Motor Control Task (SMCT) PET session (non-stress) with a similar arithmetic task but without time constraints or negative verbal feedback.
[11C]-(+)-PHNO PET Image Acquisition and Analyses.
All 58 subjects completed 2 PET scans (n = 116 PET scans), conducted about a week apart, roughly at the same time of the day. The procedures were described in greater detail previously.3 Briefly, the SMCT (control task) was performed during the first scan and the MIST (stress task) was performed during the second scan. Stress-induced DA release was quantified as [11C]-(+)-PHNO % displacement = (BPND SMCT - BPND MIST) / BPND SMCT) * 100%.
All scans were carried out using a high-resolution PET CT, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging). Each subject was administered ~333–370 MBq of [11C]-(+)-PHNO and scanned for 90 minutes. A CT transmission scan was acquired for attenuation correction.
PET images were reconstructed with a 2D filtered back-projection algorithm with a ramp filter at Nyquist cut-off frequency and rebinned into 31 time frames (comprising the background frame, followed by [11C]-(+)-PHNO injection and fifteen 60-second frames followed by fifteen 300-second frames).49 Images and Time Activity Curves (TACs) were systematically inspected visually for head movement, and corrected when motion was visible (33 out of 116 scans, including 10 HV: 15 scans, 10 CHR: 16 scans, and SCZ: 2 scans) using our standard motion-correction technique which relies on frame-to-frame realignment. Specifically, the method involves registration of the no-attenuation-corrected frames using the Automatic Image Registration (AIR) algorithm. The primary region of interest (ROI) was SN; the whole striatum and its functional subdivisions, including AST, limbic (LST), and sensorimotor striatum (SMST)50,51 were included for comparisons with our previous findings. The analyses were performed in each subject’s native PET image space. Time activity curves (TACs) from the SN and the other ROIs were obtained from the dynamic [11C]-(+)-PHNO PET images. [11C]-(+)-PHNO binding potential relative to Non-Displaceable compartment (BPND)52 was derived using the Simplified Reference Tissue Model53 with cerebellar cortex as the reference region (cerebellar vermis excluded) as previously described for [11C]-(+)-PHNO analyses.54,55 Voxel-wise images were generated using the Receptor Parametric Mapping (RPM) software,56 and spatially normalized to MNI template space using Statistical Parametric Mapping (SPM). A paired t test was performed in the MNI template space to compare BPND between SMCT vs MIST within each group (HV, CHR, and SCZ) at the voxel level. An explicit anatomical mask of the midbrain was applied to define the region of interest. Midbrain gray matter voxels extending from planes z = −4 to z = −14 on 6 consecutive transverse slices in stereotaxic space (2 mm, MNI space).30,57 False-discovery rate correction was used as implemented in SPM8 (www.fil.ion.ucl.ac.uk/spm).
Statistical Analysis.
All statistical analyses were performed in SPSS version 22.0 (IBM). Demographic measures were compared using 1-way ANOVA for continuous variables, or chi-square tests for categorical variables. Repeated-measures ANCOVA (RM-ANCOVA) was used to test the effects of psychosocial stress on the outcome measure [11C]-(+)-PHNO BPND, with clinical group as between-subject variable, session (SMCT, MIST) as within-subject variable, and concurrent cannabis use included as a covariate. A significant clinical group × session interaction indicated the group differences of stress-induced change in [11C]-(+)-PHNO binding in the SN, as exemplified by [11C]-(+)-PHNO % displacement. Effect sizes were calculated as partial η2. To elucidate the role of cannabis use in stress-induced changes in nigral [11C]-(+)-PHNO binding, a supplementary RM-ANCOVA was performed, with cannabis use as between-subject variable, session as within-subject variable, and clinical group allocation as a covariate.
Partial correlation of the 2 measures, (1) BPND during the SMCT session and (2) stress-induced DA release ([11C]-(+)-PHNO % displacement), were examined between SN and other striatal regions, covarying for cannabis use. Exploratory partial correlations between these 2 measures and clinical symptoms were also examined, covarying for cannabis use. Statistical significance was defined as P < .05 unless otherwise stated. Bonferroni correction was used for multiple comparisons for striatal subdivisions (AST, SMST and LST; adjusted P value threshold = .017).
Results
Demographic and Clinical Variables
All 3 groups were comparable in demographics (table 1). The stress task was effective in producing subject-tailored failure and elicited subjective stress responses. During the stress task, all subjects, controlling for group allocation and cannabis use, exhibited higher overall perceived stress relative to the control task (F = 133.419, df = 1,104, P < .0001). During the stress task participants reported being less calm (F = 47.855, df = 1,104, P < .0001), satisfied (F = 98.067, df = 1,104, P < .0001), relaxed (F = 34.893, df = 1,104, P < .0001), and pleasant (F = 30.147, df = 1,104, P < .0001) but more strained (F = 47.854, df = 1,104, P < .0001), tense (F = 54.899, df = 1,104, P < .0001), upset (F = 131.499, df = 1,104, P < .0001), and confused (F = 41.964, df = 1,104, P < .0001) compared to the control session. Participants in all groups performed significantly worse during the stress task (number of errors 38.62, 34.96, and 32.00 for HV, CHR, and SCZ, respectively) relative to the control task (F = 277.212, P < .0001).
[11C]-(+)-PHNO Binding (BPND) Across Control and Stress Sessions
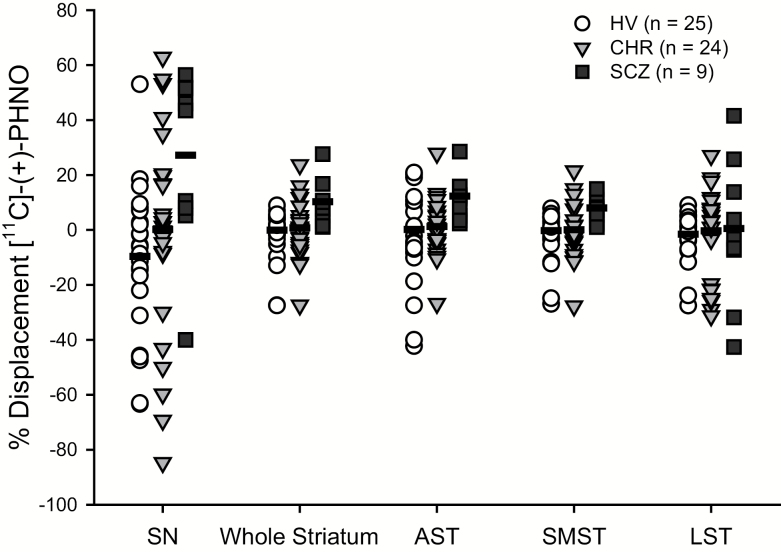
The main effect of group on nigral BPND was not significant (F = 0.269, df = 2,54, P = .765), but the session effect was significant (F = 12.396, df = 1,54, P = .001), with lower nigral BPND in the stress session than in the control session. As hypothesized, we observed a significant group × session interaction in the SN (F = 6.896, df = 2,54, P = .002), such that the % [11C]-(+)-PHNO displacement was −10.94% ± 27.1% (mean ± SD) in HV, −1.13% ± 32.2% in the CHR, and 25.90% ± 32.2% in SCZ (figure 1). Follow-up paired comparisons (Bonferroni corrected for 3 comparisons, adjusted α-level = .017) confirmed a significant group × session interaction between HV and SCZ (F = 15.250, df = 1,31, P < .001), with greater displacement in SCZ; and a trend towards a significant group × session interaction between CHR and SCZ (F = 5.093, df = 1,30, P = .031), with greater displacement in SCZ. The group × session interaction between HV and CHR was not significant. Similar group × session interactions were observed across the striatum (F = 4.362, df = 2,54, P = .018), where SCZ had higher stress-induced [11C]-(+)-PHNO displacement. The group × session interaction remained significant after controlling for tobacco (SN: F = 6.487, P = .003; striatum: F = 5.01, P = .01), or gender (SN: F = 6.484, P = .003; striatum: F = 4.745, P = .0127). Removal of individuals that reported past recreational use of drugs other than cannabis (table 1) did not significantly affect the primary outcome measures: overall group × session interaction in SN (F = 7.275, P = .002) and whole striatum (F = 4.577, P = .015). Post hoc analyses revealed that the effect was driven by the % [11C]-(+)-PHNO displacement in dorsal subregions of the striatum, including the AST (F = 6.131, df = 1,54, P = .004) and SMST (F = 3.384, df = 2,54, P = .041), but not in the ventral LST subregion (F = 0.173, df = 2,54, P = .842).
Fig. 1.
Significant effect of clinical group on stress-induced [11C]-(+)-PHNO displacement in the substantia nigra (SN) (F = 6.896, df = 2,54, P = .002) and the whole striatum (F = 4.362, df = 2,54, P = .018), including associative striatum (AST) (F = 6.131, df = 1,54, P = .004), sensorimotor striatum (SMST) (F = 3.384, df = 2,54, P = .041), but not in the limbic striatum (LST) (F = 0.173, df = 2, 54, P = .842). HV, healthy volunteer; CHR, clinical high risk; SCZ, schizophrenia.
In the second analysis examining the effect of cannabis use in SN, the main effect of cannabis use on nigral BPND across sessions was not significant (F = 2.089, P = .154), but the session effect was significant (F = 9.284, P = .004). We observed a significant cannabis use × session interaction (F = 8.785, P = .004), with greater [11C]-(+)-PHNO % displacement in non-cannabis users (10.90%) relative to users (−13.22%) across groups. The effect of cannabis use on [11C]-(+)-PHNO displacement did not differ between diagnostic groups (F = 0.529, P = .592).
Voxel-Based Analyses
In line with the ROI analyses outcome, we observed 2 significant clusters bilaterally in the midbrain with significant decreases in BPND in the SCZ group suggesting a significant DA release in the stress condition (Montreal Neurological Institute coordinates: 6, −26, 4, tmax = 5.01, cluster size = 82, P = .008; and −10, −20, −10; tmax = 3.03, cluster size = 53, P = .032, FDR cluster corrected; supplementary figure 1C). Employing the more conservative FWE correction led to similar results: Montreal Neurological Institute coordinates: 6, −26, 4, tmax = 5.01, cluster size = 82, P = .009; and −10, −20, −10; tmax = 3.03, cluster size = 53, P = .073. No significant differences were observed between the conditions in the HV (supplementary figure 1A) or CHR groups (supplementary figure 1B).
Correlational Analyses of BPND SMCT and Displacement in SN and Striatum
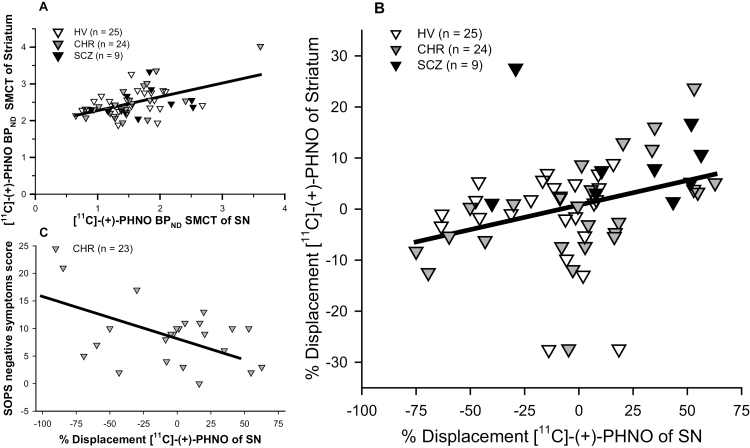
[11C]-(+)-PHNO BPND SMCT in the SN and whole striatum were positively correlated across all subjects (r = .519, P < .001). Significant correlations were also observed in all the striatal subdivisions (AST: r = .511, P < .001; SMST: r = .429, P = .001; LST: r = .471, P < .001, with adjusted threshold P < .017). In the subgroup analyses, correlations between SN SMCT BPND and the striatal subregions were observed only in the CHR group (Whole striatum: r = .768, P < .001; AST: r = .735, P < .001; SMST: r = .663, P = .001; LST: r = .726, P < .001, with adjusted threshold P < .017) but not in the SCZ or HV groups (table 2, figure 2A). The significant correlation in CHR was observed primarily in non-cannabis users (r = .895, P < .001) but not in cannabis users (r = .470, P = .123).
Table 2.
Correlations Table for BPND in the Control Session (BPND SMCT) and Displacement in SN With Striatum and its Subregion AST, Controlled for Cannabis Use
| Correlations of [11C]-(+)-PHNO BPND SMCT Between SN and Striatum | ||||
|---|---|---|---|---|
| SN BPND SMCT | ||||
| All Groups | HV | CHR | SCZ | |
| AST BPND SMCT | r = .511 | r = .114 | r = .735 | r = .138 |
| P < .001 | P = .595 | P < .001 | P = .745 | |
| Whole striatum BPND SMCT | r = .519 | r = .181 | r = .768 | r = −.071 |
| P < .001 | P = .397 | P < .001 | P = .866 | |
| Correlations of [11C]-(+)-PHNO % displacement between SN and striatum | ||||
| SN %displacement | ||||
| All groups | HV | CHR | SCZ | |
| AST %displacement | r = .306 | r = −.054 | r = .368 | r = .064 |
| P = .021 | P = .801 | P = .084 | P = .881 | |
| Whole striatum %displacement | r = .290 | r = −.010 | r = .382 | r = −.131 |
| P = .029 | P = .965 | P = .072 | P = .758 | |
Note: HV, healthy volunteer; CHR, clinical high risk; SCZ, schizophrenia; SN, Substantia nigra; AST, associative striatum; SMCT, Sensory Motor Control Task. Data presented across all groups and within each individual diagnostic group. For striatal subregions, Bonferroni correction was used for multiple comparisons with adjusted P value threshold = .017; P values below the threshold for significance are indicated in BOLD).
Fig. 2.
(A) [11C]-(+)-PHNO BPND in the control session (SMCT) in striatum and SN. A significant positive association was observed across all groups (r = .519, P < .001); (B) % Displacement of [11C]-(+)-PHNO, quantified as (BPND SMCT − BPND MIST) / (BPND SMCT) * 100%. A positive association of displacement in SN and striatum was observed across all groups (r = .290, P = .029). (C) [11C]-(+)-PHNO displacement in SN and SOPS negative symptoms scores. A significant negative association was observed in CHR (r = −.616, P = .002) (Bonferroni correction was used for multiple comparisons with adjusted P value threshold = .025). Correlations controlled for cannabis use. HV, healthy volunteer; CHR, clinical high risk; SCZ, schizophrenia; MIST, Montreal Imaging Stress Task; SMCT, Sensory Motor Control Task; SN, substantia nigra; SOPS: Scale of Prodromal Symptoms.
[11C]-(+)-PHNO displacement in the SN and whole striatum had a positive association across all subjects (r = .290, P = .029; figure 2B), similar associations were also observed in its dorsal subdivisions (AST: r = .306, P = .021; SMST r = .305, P = .021, with adjusted threshold P < .017) but not in the ventral subdivision (LST: r = .133, P = .326). The significant correlation of [11C]-(+)-PHNO displacement in the SN and in striatum was observed in non-cannabis users (striatum: r = .545, P = .002; AST: r = .543, P = .002; SMST: r = .388, P = .037; LST: r = .388, P = .037) but not in cannabis users (striatum: r = .224, P = .242; AST: r = .324, P = .087; SMST: r = .394, P = .034; LST: r = −.133, P = .493). Investigating diagnostic groups separately, the significant correlation in CHR was observed primarily in non-cannabis users (r = .646, P < .023) but not in cannabis users (r = −.033, P = .920).
Correlational Analyses of BPND SMCT and Displacement in SN With Clinical Positive and Negative Symptoms
In CHR, [11C]-(+)-PHNO BPNDSMCT in SN was not significantly correlated with SOPS positive (r = .171, P = .446) or negative symptoms score (r = −.292, P = .187). In contrast, [11C]-(+)-PHNO displacement in SN was negatively associated with SOPS negative symptoms score (r = −.616, P = .002, adjusted threshold P < .025 for 2 comparisons, positive and negative symptoms) but not (attenuated) positive symptoms scores (r = .100, P = .658), controlling for cannabis use (figure 2C). The significant correlation was observed primarily in non-cannabis users (r = −.886, P < .001) but not in cannabis users (r = −.028, P = .935).
In SCZ, [11C]-(+)-PHNO BPND SMCT in SN was not significantly correlated with PANSS positive (r = .540, P = .167) or negative symptoms scores (r = −.351, P = .395). [11C]-(+)-PHNO displacement in SN had trend towards a negative association with PANSS negative symptoms score (r = −.767, P = .026, adjusted threshold P < .025 for 2 comparisons, positive and negative symptoms), controlled for cannabis use, but not positive symptoms (r = .687, P = .060).
Discussion
To our knowledge, this is the first study to investigate stress-induced changes in nigral [11C]-(+)-PHNO binding in antipsychotic-naïve SCZ. We observed a significant diagnostic group by session interaction, such that SCZ exhibited greater stress-induced [11C]-(+)-PHNO % displacement (25.90%), as compared to HVs (−10.94%). Across all subjects, [11C]-(+)-PHNO % displacement in SN was correlated with displacement in the striatum, an effect observed primarily in cannabis nonusers across diagnostic groups, and within non-cannabis-using CHR. Exploratory analyses revealed an association of lower [11C]-(+)-PHNO % displacement in SN with greater negative symptoms in CHR and SCZ.
The present results are in agreement with the findings of increased midbrain DA synthesis capacity14 and DA turnover20 in SCZ. However, our results are at odds with a recent report by Slifstein et al33 of amphetamine-induced [11C]FLB-457 change (P = .10) in midbrain in SCZ. Differences between our studies may have contributed to the divergent observations, including composition of the SCZ cohorts (antipsychotic-naïve vs antipsychotic-free; recent onset vs chronic SCZ), challenge condition (amphetamine vs psychosocial stress), and radioligand (D3-preferring [11C]-(+)-PHNO vs D2/3 ligand [11C]FLB-457). Within the SN the signal from [11C]-(+)-PHNO34,58 and [11C]FLB-457 are thought to predominantly reflect binding to D3 and D2 receptors respectively, which may reflect DA release in histologically distinct subregions such as the D3-dense rostral pars reticulata (SNr) or D2- predominant pars compacta (SNc) or the VTA.59 Further, differences between stimulant and stress-induced DA release in extra-striatal regions have been observed previously, and may reflect the different mechanisms involved in direct pharmacologic action on DA neurons vs endogenous activation of stress-related pathways.60
Although we did not observe a significant change in [11C]-(+)-PHNO binding between CHR and HV, one explanation is that following stress, CHR cannabis users and nonusers exhibit changes in [11C]-(+)-PHNO binding that are opposite in direction.6 In addition, there is some evidence that individual differences including maternal care,61 personality traits,62 or repeated exposure to amphetamine63 may also influence stress-induced DA release in humans. Although the present sample includes individuals with exposure to recreational drugs other than cannabis, removal of these individuals from the analysis did not significantly affect the primary outcome measures. Finally, the lack of significant differences between CHR and HV may be related to the high variability in binding estimates observed in the SN, a relatively small ROI.
Based on the putative modulatory effect of D2/3 autoreceptor activation on nigral DA neuron activity,39–41 in HV we expected a negative association between the stress-induced [11C]-(+)-PHNO binding change in the SN and in the striatum. We did not find such association in HV. The positive association between [11C]-(+)-PHNO displacement in the SN and striatum in non-cannabis-using CHR, but not in HV or SCZ, provides a weak support of an altered balance of DA transmission in the SN and striatum in this putative prodromal stage of psychosis. Excessive nigral DA synthesis14 and turnover20 in psychosis might be expected to result in both excessive terminal (striatum) axonal and local (SN) somatodendritic DA release following stress-related activation of DA pathways. Future investigations may require a larger sample or should incorporate a measure of true baseline D2/3 receptor availability. Baseline SN D2/3 receptor availability has been reported to modulate striatal DA release, however our control condition itself may be expected to recruit DA activity64 and so does not permit estimation of a true baseline D2/3 availability.
In line with previous reports that cannabis users exhibit reduced DA release in striatum,6,27–29 cannabis users exhibited decreased nigral [11C]-(+)-PHNO displacement relative to nonusers, although in our sample the effect was not significantly associated with recency of cannabis exposure or cannabis use frequency (for details see:6,29). Our findings of decreased DA stress responsivity across somatodendritic (SN) and DA terminal fields (AST)6 in chronic cannabis users suggest that alterations to DA neurotransmission may extend beyond reduced DA synthesis and release capacity in striatum.26–28
As previously reported,6 in CHR cannabis nonusers the magnitude of changes in nigral [11C]-(+)-PHNO binding was negatively associated with attenuated negative but not positive symptoms. Our exploratory observation of a similar association in SCZ provides preliminary support of the result in CHR. These findings are consistent with a recent study in healthy cannabis-dependent subjects where lower DA release in AST was associated with higher ratings of negative symptoms.28
The current study has several limitations, many of which are inherent to neurochemical PET studies. The relatively low signal-to-noise ratio in SN produced higher variability of [11C]-(+)-PHNO BPND and [11C]-(+)-PHNO binding changes than in striatal regions, as previously reported.6,30,31 The test-retest variability of [11C]-(+)-PHNO BPND in SN was larger than in the striatum, ranging from 8.1% to 19% using different [11C]-(+)-PHNO paradigms,65,66 which is compatible with the larger variation we observed in SN. The negative displacement we found in HV may potentially be explained by test-retest variations. In contrast, the positive displacement in SCZ, is larger than the test-retest variation reported in the literature65,66 and can be conceivably interpreted as reflecting elevated DA release. Although automated delineation helps to refine this relatively small ROI more precisely, the spatial resolution of the PET may be compromised in participants with significant movement during PET scans. Additionally, the resolution of the scanner does not permit differentiation of histological subdivisions of SN (ie, SNr and SNc) and nearby structures such as VTA. Another limitation is that [11C]-(+)-PHNO signal in the SN reflects tracer binding to D3 receptors (100%)34; and may not generalize to D2 receptors studies in the same brain region. Although the current sample size provided sufficient power to detect group differences (partial η2 = 0.203, achieved power = 0.93), the relatively small sample size of the SCZ cohort (n = 9) limits interpretation of association findings in this subgroup. Finally, the history of substance use may have made the interpretation of the results difficult. However, we controlled by cannabis use, suggesting that it is not a major confounding factor regarding clinical group differences, and further reported significant cannabis effects in [11C]-(+)-PHNO % displacement in non-cannabis users (10.90%) relative to users (−13.22%).
In conclusion, our results of greater stress-induced [11C]-(+)-PHNO % displacement in SN in SCZ suggests that the elevated striatal DA responsivity to stress in SCZ extends to D3-rich regions in midbrain. The association between SN and striatal [11C]-(+)-PHNO binding changes in non-cannabis-using CHR may reflect an altered relationship between DA activity in SN with DA activity in striatum in this population, but needs further investigation to confirm. Chronic cannabis use is associated with reduced nigral DA response to stress in CHR and SCZ and may mask associations between nigrostriatal DA neurotransmission and clinical presentation.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by operating grants from the Canadian Institutes for Health Research (CIHR) and the Ontario Mental Health Foundation (OMHF). RM was supported by a New Investigator Award from CIHR and an OMHF New Investigator Fellowship.
Supplementary Material
Acknowledgments
R.M. has received speaker’s honoraria from Otsuka-Lundbeck Canada. No other disclosure is declared by all authors. We are grateful to Armando Garcia, Winston Stableford, Alvina Ng, Terry Bell, Ted Harris-Brandts and Peter Bloomfield.
References
- 1. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. [DOI] [PubMed] [Google Scholar]
- 3. Mizrahi R, Addington J, Rusjan PM et al. . Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–567. [DOI] [PubMed] [Google Scholar]
- 4. Howes OD, Montgomery AJ, Asselin MC et al. . Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 5. Howes OD, Bose SK, Turkheimer F et al. . Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizrahi R, Kenk M, Suridjan I et al. . Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22:283–303. [DOI] [PubMed] [Google Scholar]
- 8. Cannon TD, Cadenhead K, Cornblatt B et al. . Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fusar-Poli P, Bonoldi I, Yung AR et al. . Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 10. Kegeles LS, Abi-Dargham A, Frankle WG et al. . Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. [DOI] [PubMed] [Google Scholar]
- 11. Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. [DOI] [PubMed] [Google Scholar]
- 14. Howes OD, Williams M, Ibrahim K et al. . Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136:3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res. 2004;121:60–69. [DOI] [PubMed] [Google Scholar]
- 16. Toru M, Watanabe S, Shibuya H et al. . Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78:121–137. [DOI] [PubMed] [Google Scholar]
- 17. Owen R, Owen F, Poulter M, Crow TJ. Dopamine D2 receptors in substantia nigra in schizophrenia. Brain Res. 1984;299:152–154. [DOI] [PubMed] [Google Scholar]
- 18. Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry. 2012;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rice MW, Roberts RC, Melendez-Ferro M, Perez-Costas E. Mapping dopaminergic deficiencies in the substantia nigra/ventral tegmental area in schizophrenia. Brain Struct Funct. 2016;221:185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumakura Y, Cumming P, Vernaleken I et al. . Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kessler RM, Woodward ND, Riccardi P et al. . Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry. 2009;65:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–429. [DOI] [PubMed] [Google Scholar]
- 23. Yoon JH, Westphal AJ, Minzenberg MJ et al. . Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naive first episode schizophrenia. Schizophr Res. 2014;159:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoon JH, Minzenberg MJ, Raouf S, D’Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray GK, Corlett PR, Clark L et al. . Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–478. [DOI] [PubMed] [Google Scholar]
- 27. Volkow ND, Wang GJ, Telang F et al. . Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci U S A. 2014;111:E3149–E3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van de Giessen E, Weinstein JJ, Cassidy CM et al. . Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry. 2017;22:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizrahi R, Suridjan I, Kenk M et al. . Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-+-PHNO. Neuropsychopharmacology. 2013;38:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boileau I, Payer D, Chugani B et al. . In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry. 2014;19:1305–1313. [DOI] [PubMed] [Google Scholar]
- 31. Shotbolt P, Tziortzi AC, Searle GE et al. . Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buckholtz JW, Treadway MT, Cowan RL et al. . Dopaminergic network differences in human impulsivity. Science 2010;329:532–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slifstein M, van de Giessen E, Van Snellenberg J et al. . Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tziortzi AC, Searle GE, Tzimopoulou S et al. . Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. [DOI] [PubMed] [Google Scholar]
- 35. Diaz J, Levesque D, Lammers CH et al. . Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience. 1995;65:731–745. [DOI] [PubMed] [Google Scholar]
- 36. Meador-Woodruff JH, Damask SP, Watson SJ Jr. Differential expression of autoreceptors in the ascending dopamine systems of the human brain. Proc Natl Acad Sci U S A. 1994;91:8297–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nissbrandt H, Ekman A, Eriksson E, Heilig M. Dopamine D3 receptor antisense influences dopamine synthesis in rat brain. Neuroreport. 1995;6:573–576. [DOI] [PubMed] [Google Scholar]
- 38. Rivet JM, Audinot V, Gobert A, Peglion JL, Millan MJ. Modulation of mesolimbic dopamine release by the selective dopamine D3 receptor antagonist, (+)-S 14297. Eur J Pharmacol. 1994;265:175–177. [DOI] [PubMed] [Google Scholar]
- 39. Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. [DOI] [PubMed] [Google Scholar]
- 40. Koeltzow TE, Xu M, Cooper DC et al. . Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci. 1998;18:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tepper JM, Sun BC, Martin LP, Creese I. Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo. J Neurosci. 1997;17:2519–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suridjan I, Rusjan P, Addington J, Wilson AA, Houle S, Mizrahi R. Dopamine D2 and D3 binding in people at clinical high risk for schizophrenia, antipsychotic-naive patients and healthy controls while performing a cognitive task. J Psychiatry Neurosci. 2013;38:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller TJ, McGlashan TH, Rosen JL et al. . Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 45. Pruessner JC, Dedovic K, Khalili-Mahani N et al. . Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. [DOI] [PubMed] [Google Scholar]
- 46. Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lederbogen F, Kirsch P, Haddad L et al. . City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. [DOI] [PubMed] [Google Scholar]
- 48. Akdeniz C, Tost H, Streit F et al. . Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. [DOI] [PubMed] [Google Scholar]
- 49. Ginovart N, Galineau L, Willeit M et al. . Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. [DOI] [PubMed] [Google Scholar]
- 50. Martinez D, Slifstein M, Broft A et al. . Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. [DOI] [PubMed] [Google Scholar]
- 51. Rusjan P, Mamo D, Ginovart N et al. . An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. [DOI] [PubMed] [Google Scholar]
- 52. Innis RB, Cunningham VJ, Delforge J et al. . Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 53. Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. [DOI] [PubMed] [Google Scholar]
- 54. Ginovart N, Willeit M, Rusjan P et al. . Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–871. [DOI] [PubMed] [Google Scholar]
- 55. Willeit M, Ginovart N, Graff A et al. . First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. [DOI] [PubMed] [Google Scholar]
- 56. Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. [DOI] [PubMed] [Google Scholar]
- 57. Boileau I, Payer D, Houle S et al. . Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gallezot JD, Beaver JD, Gunn RN et al. . Affinity and selectivity of [¹¹C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. [DOI] [PubMed] [Google Scholar]
- 59. Gurevich EV, Joyce JN. Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology. 1999;20:60–80. [DOI] [PubMed] [Google Scholar]
- 60. Hernaus D, Collip D, Kasanova Z et al. . No evidence for attenuated stress-induced extrastriatal dopamine signaling in psychotic disorder. Transl Psychiatry. 2015;5:e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J Neurosci. 2004;24:2825–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Suridjan I, Boileau I, Bagby M et al. . Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res. 2012;46:890–897. [DOI] [PubMed] [Google Scholar]
- 63. Booij L, Welfeld K, Leyton M et al. . Dopamine cross-sensitization between psychostimulant drugs and stress in healthy male volunteers. Transl Psychiatry. 2016;6(2):e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Egerton A, Mehta MA, Montgomery AJ et al. . The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee DE, Gallezot JD, Zheng MQ et al. . Test-retest reproducibility of [11C]-(+)-propyl-hexahydro-naphtho-oxazin positron emission tomography using the bolus plus constant infusion paradigm. Mol Imaging. 2013;12:77–82. [PMC free article] [PubMed] [Google Scholar]
- 66. Gallezot JD, Zheng MQ, Lim K et al. . Parametric Imaging and Test-Retest Variability of ¹¹C-(+)-PHNO Binding to D2/D3 Dopamine Receptors in Humans on the High-Resolution Research Tomograph PET Scanner. J Nucl Med. 2014;55:960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.