Abstract
Background
Antiretroviral therapy (ART) for persons with HIV infection prevents tuberculosis (TB) disease. Additionally, sequential ART after initiation of TB treatment improves outcomes. We examined ART use, retention in care, and viral suppression (VS) before, during, and 3 years following TB treatment for an inner-city cohort in the United States.
Methods
Retrospective cohort study among persons treated for culture-confirmed TB between 2008 and 2015 at an inner-city hospital.
Results
Among 274 persons with culture-confirmed TB, 96 (35%) had HIV co-infection, including 23 (24%) new HIV diagnoses and 73 (76%) previous diagnoses. Among those with known HIV prior to TB, the median time of known HIV was 6 years, and only 10 (14%) were on ART at the time of TB diagnosis. The median CD4 at TB diagnosis was 87 cells/uL. Seventy-four (81%) patients received ART during treatment for TB, and 47 (52%) has VS at the end of TB treatment. Only 32% of patients had continuous VS 3 years after completing TB treatment. There were 3 TB recurrences and 3 deaths post–TB treatment; none of these patients had retention or VS after TB treatment.
Conclusions
Among persons with active TB co-infected with HIV, we found that the majority had known HIV and were not on ART prior to TB diagnosis, and retention in care and VS post–TB treatment were very low. Strengthening the HIV care continuum is needed to improve HIV outcomes and further reduce rates of active TB/HIV co-infection in our and similar settings.
Keywords: continuous retention, HIV, tuberculosis, viral suppression
HIV infection is a major contributor to the tuberculosis (TB) epidemic [1]. HIV-associated immune dysfunction increases the risk of TB disease, is associated with worse TB treatment outcomes, and increases the risk of TB relapse after initial cure [1, 2]. Antiretroviral therapy (ART)–mediated immunological recovery is associated with decreased risk of TB, and sequential ART after initiation of TB treatment has been shown to improve outcomes [1–7]. However, suboptimal rates of ART use during TB treatment have been reported [8, 9].
Additionally, continuous ART use and viral suppression (VS) are required to maintain immune function against Mycobacterium tuberculosis and decrease risk of recurrent disease after TB treatment [1]. Thus, scale-up of ART for people living with TB should also focus on retention and VS after completion of TB treatment [8]. To our knowledge, there are no published data on long-term retention in care and VS among patients co-infected with TB/HIV.
We sought to describe ART use, retention in care, and VS before, during, and up to 3 years following TB treatment among patients diagnosed with or admitted for complications of TB at Grady Memorial Hospital (GMH), an inner-city safety net hospital in Atlanta. Additionally, we sought to compare practice patterns of ART use in this cohort to the recommendations provided by the American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America and the US Department of Health and Human Services (DHHS). These guidelines recommend initiating ARTs within 2 weeks of TB therapy for patients with CD4 <50 cells/uL and 8–12 weeks for patients with CD4 ≥ 50 cells/uL in the absence of meningeal disease [10, 11]. Through a better understanding of the HIV care continuum during and following TB treatment, we aim to identify target areas for future public health interventions aimed at improving care and outcomes among this population.
METHODS
This is a retrospective cohort study of adults (aged ≥18 years) diagnosed with or admitted for complications of culture-confirmed pulmonary and/or extrapulmonary TB/HIV co-infection at Grady Memorial Hospital (GMH) in Atlanta, Georgia, between January 2008 and October 2015. Following hospital discharge, all patients were referred to a county health TB clinic, where they received TB treatment via directly observed therapy (DOT). Additionally, ART was available at all county health clinics from a co-located Ryan White program and prescribed per the discretion of the treating physician. Upon completion of TB treatment, patients with CD4 <200 cells/uL are usually referred to the Grady Infectious Diseases Program (IDP) while those with CD4 ≥200 cells/uL have the option of remaining in care at a Ryan White county health clinic. TB care and medications are provided at no cost to the patient, and ART is provided through the AIDS Drug Assistance Program (ADAP) for patients without insurance. Incentives such as housing, transportation, and food vouchers were offered during TB treatment as needed. Upon completion of treatment for TB, patients without insurance could receive care through Ryan White and ADAP programs, with a possible copayment. Incentives offered during TB treatment were no longer available. The study was approved by the Emory University and Georgia Department of Public Health Institutional Review Boards and the GMH Research Oversight Committee.
Data Collection
A review of inpatient, county health clinics, Georgia State Electronic Notifiable Surveillance System (SENDSS), and IDP records was performed. SENDSS and clinic records were last checked on April 25, 2017. Patients without encounters or HIV viral loads (VLs) after review of these records for any of the 3 years following completion of TB treatment were matched with the Georgia Department of Public Health Enhanced HIV/AIDS Surveillance Database (eHARS) to obtain all available CD4 counts and VLs (Supplementary Table 1). Georgia state law mandates that all laboratories report any HIV-related results to eHARS [12, 13]. Case report forms were used for data abstraction, and data were entered into a REDCap online database [14].
Definitions
Disseminated tuberculosis disease was defined as having a blood culture positive for M. tuberculosis and/or the presence of miliary tuberculosis based on radiology report. Time to ART was the interval between starting TB therapy and initiating ART. ART with DOT was defined as ART given by a TB outreach worker together with TB medications 5 days per week. Recurrent TB was defined as culture-positive TB within 2 years of cure caused by strains of the same genotype by laboratory testing performed by the Centers for Disease Control and Prevention [15].
To accommodate for challenges in practice, guideline-adherent ART was defined as starting ART ≤4 weeks after initiation of TB treatment for patients with CD4 <50 cells/uL (2 weeks later than recommended) and ≤12 weeks for those with CD4 ≥50 cells/uL (while guidelines recommend initiating ART within 8–12 weeks). Patients without meningeal disease and with survival >14 days after TB diagnosis were defined as being eligible for guideline-adherent ART. Rates of guideline-adherent ART were compared between those with TB diagnosis before and after March 2012, when DHHS guidelines changed to recommend early ART during TB treatment, reflecting seminal studies published in late 2011 [3–5, 11]. VS at the end of TB treatment was defined as last VL prior to completion of TB treatment ≤200 copies/mL. Two patients with TB recurrence diagnosed with a second TB episode during the study period and off ART were evaluated twice as they were at risk for initiating ART and being linked to care with each TB episode.
Retention in care and VS were determined yearly for up to 3 patient-years following completion of TB treatment. Each year was defined as a discrete period, and patients without retention/VS during year 1 and years 1 and/or 2 were eligible for retention/VS during years 2 and 3, respectively. However, continuous retention/VS during years 2 and 3 was dependent on retention/VS during year 1 and years 1 and 2, respectively. Follow-up after completion of TB treatment for ≥6, 18, and 30 months was required for evaluation of retention/VS for years 1, 2, and 3, respectively. Patients alive and without TB recurrence at the end of follow-up were included in the analysis of associations with continuous retention/VS.
We used the same definitions of retention/VS of Colasanti et al.’s report of patients receiving HIV care at the Grady IDP clinic [16]. Retention in care was defined as attendance of 2 provider visits ≥90 days apart within a patient-year [16, 17]. eHARS laboratory data were used as a proxy for provider visits when matching was required [13, 16]. VS per year was defined as the last VL for each patient-year being ≤200 copies/mL, and VS was not dependent on retention [16]. Patients without VL before completion of TB treatment and for any patient-year following TB treatment were considered to have VL >200 copies/mL for that period [16].
Data Analyses
Data analyses were performed in R version 3.3.3. For univariate comparisons, differences in nominal variables were tested using either a Fisher’s exact or χ2 test, and for continuous variables, either a Mann-Whitney or 2-sample t test was used, as appropriate. A two-sided P value <.05 was considered significant.
RESULTS
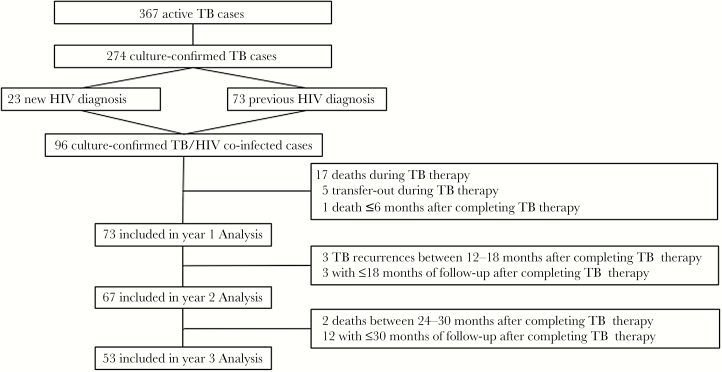
Thirty-five percent of all active TB cases identified in DeKalb and Fulton counties (the 2 counties served by GMH) during the study period were diagnosed at GMH. Among 367 patients admitted for active TB at GMH during the study period, 274 had culture-confirmed disease (Figure 1). All 274 culture-confirmed patients were tested for HIV or had a previous known diagnosis of HIV. Overall, 96 (35%) patients had HIV co-infection and were included in the analysis. Among these 96 co-infected patients, 84 (87%) were diagnosed with TB at GMH. The 12 patients diagnosed with TB elsewhere were admitted to GMH for a TB-related issue a median (interquartile range [IQR]) of 5 (4–23) days after initiating TB treatment. Fifty-six (58%) were diagnosed with TB before March 2012, and 40 (52%) were diagnosed with TB after March 2012, when DHHS guidelines were modified to recommend early ART during TB treatment [11].
Figure 1.
Study flow diagram. Abbreviation: TB, tuberculosis.
Seventy-three (76%) patients had known HIV prior to TB diagnosis, with a median interval (IQR) between HIV and TB diagnosis of 6 (2–12) years (Table 1). Among patients with known HIV infection, 10 (14%) were on ART at the time of TB diagnosis, 5 (7%) had suppressed VL, and 25 (34%) were previously on ART (Figure 2). The median CD4 count (IQR) was 87 (31–213) cells/uL, and 38 (40%) patients had a CD4 count <50 cells/uL. The median CD4 count was higher among patients with known HIV infection and on ART at the time of TB diagnosis, compared with those with known HIV infection at the time of TB diagnosis and off ART and those newly diagnosed with HIV (217 vs 95 vs 38 cells/uL, respectively; P < .01) (Supplementary Table 2).
Table 1.
Baseline Patient Cohort Characteristics
| Characteristic | Total (n = 96)a |
|---|---|
| Age, median (IQR), y | 45.9 (39.2–50.1) |
| Male | 68 (71%) |
| Black | 81 (84%) |
| Index BMI, median (IQR) | 20.7 (19.2–24.1) |
| US born | 69 (72%) |
| Non-English speaker | 9 (9%) |
| History of homelessness | 48 (50%) |
| History of incarceration | 38 (40%) |
| Unemployed | 67 (70%) |
| Single | 67 (70%) |
| Insurance | |
| No insurance/Ryan White | 38 (40%) |
| Medicare/Medicaid | 23 (24%) |
| Private | 1 (1%) |
| Missing | 34 (35%) |
| Medical history | |
| Psychiatric disease | 23 (24%) |
| Previous active TB | 9 (9%) |
| History of LTBI | 18 (19%) |
| Drug use | |
| Tobacco use | 57 (59%) |
| Alcohol use | 49 (51%) |
| Illicit drug use | 37 (39%) |
| HIV presentation | |
| New HIV diagnosis | 23 (24%) |
| Known HIV prior to TB diagnosis | 73 (76%) |
| Years of known HIV, median (IQR) | 6 (2–12) |
| On ART at TB diagnosis (n = 73)b | 10 (14%) |
| Previously on ART (n = 73) | 25 (34%) |
| OI at time of TB diagnosisc | 35 (36%) |
| HIV risk factord | |
| MSM | 14 (15%) |
| Heterosexual | 64 (67%) |
| Injection drug use | 7 (7%) |
| Baseline CD4, median (IQR) | 87 (31–213) |
| CD4 < 200 | 71 (74%) |
| CD4 < 50 | 38 (40%) |
| TB presentation | |
| Isoniazid resistance | 32 (34%) |
| Multidrug resistancee | 1 (1%) |
| Extrapulmonary involvementf | 48 (50%) |
| Extrapulmonary only | 5 (5%) |
| CNS | 8 (8%) |
| Disseminated | 20 (21%) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CNS, central nervous system; IQR, interquartile range; LTBI, latent tuberculosis infection; MSM, men who have sex with men; OI, opportunistic infection; TB, tuberculosis.
aNinety-four unique patients.
bSuppressed HIV viral load at baseline (n = 5).
cOropharyngeal and/or esophageal candidiasis (n = 23), Pneumocystis pneumonia (n = 13), Cryptococcus (n = 5), CNS.
Toxoplasmosis (n = 2); Kaposi sarcoma, Cryptosporidiosis, and CMV retinitis (n = 1 each). Some patients had >1 OI at presentation.
dSome patients had >1 HIV risk factor.
eResistance to isoniazid and rifampin.
fLymphatic (n = 18), pleural (n = 5), genitourinary (n = 2), pericardial, soft tissue, and splenic (n = 1 each). Some patients have >1 site of extrapulmonary involvement.
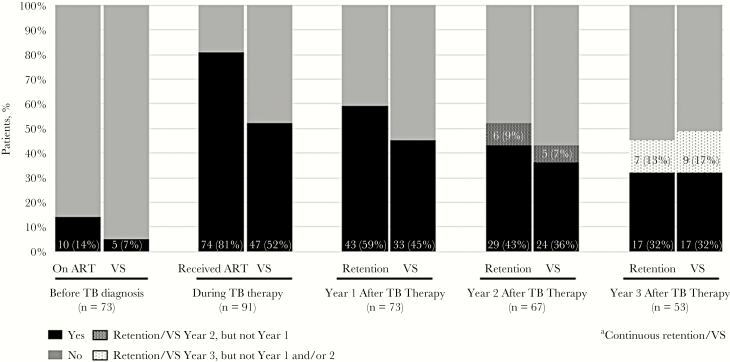
Figure 2.
Antiretroviral therapy use, retention in care, and viral suppression before, during, and 3 years after tuberculosis therapy. Abbreviations: ART, antiretroviral therapy; TB, tuberculosis; VS, viral suppression.
Most patients were male (68%), black (84%), US-born (72%), and had a history of homelessness (50%) (Table 1). At the time of TB diagnosis, 70% were unemployed and 31% were uninsured. Twenty-four percent had a history of psychiatric illness, and 39% reported illicit drug use. Overall, 48 (50%) had extrapulmonary involvement, including 5 (5%) with no pulmonary disease and 8 (8%) with meningeal disease.
ART Use During TB Treatment
Among 91 patients who were alive >14 days after TB diagnosis, 81% received ART during TB treatment, and 52% had VS at the end of TB treatment (Table 2). Ten (11%) patients received ART under DOT, of which 8 (80%) had VS during TB therapy. Among these 10 patients, 9 had a history of homelessness, 9 were unemployed, and 8 had known HIV prior to TB diagnosis and were not on ART. ART use during TB treatment increased from 74% among those diagnosed with TB before March 2012 to 92% among those diagnosed after March 2012 (P = .03).
Table 2.
ART Use During TB Therapy and End of TB Treatment Outcomes
| Characteristic | CD4 < 50 (n = 38) |
CD4 ≥ 50 (n = 58) |
Total (n = 96)a |
|---|---|---|---|
| Received ART during TB therapy (n = 91)b | 29 (81%) | 45 (82%) | 74 (81%) |
| TB diagnosis < March 2012 | 15 (71%) | 24 (75%) | 39 (74%) |
| TB diagnosis ≥ March 2012 | 14 (93%) | 21 (91%) | 35 (92%) |
| ART given as DOT (n = 91)b | 5 (14%) | 5 (9%) | 10 (11%) |
| Viral suppression at end of TB therapy (n = 91) b | 17 (47%) | 30 (55%) | 47 (52%) |
| Time to ART, median (IQR), dc | 59 (27–103) | 81 (46–143) | 76 (34–125) |
| Median time on ART prior to end of TB treatment (IQR), mo | 6.9 (5.0–9.2) | 6.8 (5.6–9.2) | 6.9 (5.1–9.2) |
| Guideline adherent ART (n = 76)d,e,f | 8 (24%) | 16 (37%) | 24 (32%) |
| TB diagnosis < March 2012 | 3 (14%) | 8 (33%) | 11 (24%) |
| TB diagnosis ≥ March 2012 | 5 (42%) | 8 (42%) | 13 (42%) |
| Core ART agent (n = 74)g | |||
| INSTI | 1 (3%) | 4 (9%) | 5 (7%) |
| NNRTI | 19 (66%) | 28 (62%) | 47 (64%) |
| PI | 9 (31%) | 12 (27%) | 21 (28%) |
| Otherh | 0 | 1 (2%) | 1 (1%) |
| ART backbone (n = 74)g | |||
| TDF/FTC | 25 (86%) | 41 (91%) | 66 (89%) |
| ABC/3TC | 1 (4%) | 1 (2%) | 2 (3%) |
| Otheri | 3 (10%) | 3 (7%) | 6 (8%) |
| OI during TB therapyj | 9 (25%) | 11 (20%) | 20 (21%) |
| Median length of TB treatment (IQR) (n = 71), mol | 9.6 (7.8–11.4) | 10 (9.1–10.6) | 9.9 (8.8–11.3) |
| Transferred out | 3 (8%) | 2 (3%) | 5 (5%) |
| Final outcomes (n = 91)m | |||
| Cured | 24 (68%) | 44 (78%) | 68 (75%) |
| Lost to follow-up | 1 (3%) | 2 (4%) | 3 (3%) |
| Recurrence | 2 (6%) | 1 (2%) | 3 (3%) |
| Deathn | 8 (23%) | 9 (16%) | 17 (19%) |
Abbreviations: ABC/3TC, abacavir/lamivudine; ART, antiretroviral therapy; DOT, directly observed therapy; INSTI, Integrase strand inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; OI, opportunistic infection; PI, protease inhibitor; TB, tuberculosis; TDF/FTC, tenofovir/emtricitabine.
aNinety-four unique patients (CD4 < 50 n = 36, CD4 ≥ 50 n = 58).
bAmong patients who survived >14 days after TB diagnosis.
cAmong patients started on ART during TB therapy.
dDefined as starting ≤4 weeks for those with CD4 >50 without meningeal disease and ≤12 weeks for those with CD4 ≥50 without meningeal disease. Excluded patients who survived ≤14 days after TB diagnosis and who were already on ART at time of TB diagnosis.
eCD4 < 50 (n = 33).
fCD4 ≥ 50 (n = 43).
gAmong those who received ART during TB therapy (n = 74).
hINSTI and PI combined (n = 1).
iZidovudine/lamivudine (n = 2); zidovudine/tenofovir/emtricitabine (n = 2); zidovudine/abacavir/lamivudine (n = 1); stavudine/didanosine (n = 1).
jOropharyngeal and/or esophageal candidiasis (n = 17), Pneumocystis pneumonia (n = 5), Cryptococcus (n = 2), toxoplasmosis (n = 1).
lAmong those who completed TB therapy.
mExcludes patients transferred out.
nDeath ≤14 days after TB diagnosis (n = 5).
Among 74 patients who received ART during TB treatment, 27 (36%) did not have VS at completion of treatment, including 5 who did not have VL measured between starting ART and completing TB treatment. The median interval (IQR) between last VL obtained during TB treatment and completion of TB treatment was 38 (3–84) days. Patients with VS at completion of TB treatment had a shorter time to ART initiation after the beginning of TB treatment (median days, 62 vs 92; P = .06) and a longer interval between starting ART and completing TB treatment (median months, 7.0 vs 6.2; P = .09) compared with those without VS at the end of TB treatment.
Patients off ART and without meningeal disease who survived >14 days after TB diagnosis were evaluated for guideline-adherent ART (n = 76). Twenty-four (32%) received guideline-adherent ART, 11 (24%) among those diagnosed with TB before March 2012 and 13 (42%) among those diagnosed after March 2012 (P = .13). The increase in guideline-adherent ART rates when comparing patients diagnosed with TB before/after March 2012 was not significant when stratified by CD4 below/above 50 cells/uL. Patients receiving guideline-adherent ART had a lower median number of days to ART (30 vs 117) and were more likely to have VS at the end of TB treatment (47% vs 16%) compared with those not receiving guideline-adherent ART (P < .01 for all) (Supplementary Table 3).
Viral Suppression and Retention in Care After TB Treatment
Excluding 5 patients who were transferred out of Georgia during TB treatment, 68 (75%) of 91 patients were cured, 3 (3%) were lost to follow-up, 3 (3%) had TB recurrence, and 17 (19%) died during TB treatment, leaving 74 patients alive at completion of TB treatment. The numbers of patients eligible for analyses of retention and VS for first, second, and third years after end of TB treatment were 73, 67, and 53, respectively (Figure 1). Three patients died during follow-up, 1 <6 months and 2 between 24 and 30 months after the end of TB treatment. Three TB recurrences occurred between 12 and 18 months after the end of TB treatment. Due to a later date of TB treatment completion, 3 patients had only ≤18 months and 12 patients had ≤30 months of follow-up (Figure 1).
The rates of retention in HIV care at years 1, 2, and 3 were 59%, 52%, and 45%, respectively (Figure 2). Patients retained at year 1 had a relative risk of 3.2 (95% CI, 1.5–6.7) and 3.8 (95% CI, 1.5–9.6) for retention at years 2 and 3, respectively. The rates of VS at years 1, 2, and 3 were 45%, 43%, and 49%, respectively. Patients with VS at year 1 had relative risks of 5.2 (95% CI, 2.2–12.1) and 3.1 (95% CI, 1.6–5.9) for VS at years 2 and 3, respectively. Seventeen (32%) patients had either continuous retention or VS for all 3 years of follow-up, and 15 (28%) had both continuous retention and VS for all 3 years of follow-up (Figure 2).
Among 68 patients alive at end of follow-up and without TB recurrence, 27 (40%) had continuous retention and 24 (35%) had continuous VS. On bivariate analyses, race, ART use during TB treatment, and VS at the end of TB therapy were the only characteristics associated with continuous retention at the end of follow-up (Table 3). Employment at the time of TB diagnosis and VS at the end of TB treatment were the only characteristics associated with continuous VS at the end of follow-up.
Table 3.
Bivariate Analysis for Continuous Retention and Viral Suppression at End of Follow-up
| Variable | Total (n = 68)a,b | |||||
|---|---|---|---|---|---|---|
| Continuous Retention | Continuous Viral Suppressionc,d | |||||
| n/N | % | P | n/N | % | P | |
| Overall | 27/68 | 40 | 24/68 | 35 | ||
| Age ≥ 45 y | .84 | .94 | ||||
| Yes | 13/37 | 37 | 13/35 | 37 | ||
| No | 14/33 | 42 | 11/33 | 33 | ||
| Sex | .43 | 1 | ||||
| Male | 21/48 | 44 | 17/48 | 35 | ||
| Female | 6/20 | 30 | 7/20 | 35 | ||
| Race | .02 | .17 | ||||
| Black | 19/57 | 33 | 18/57 | 32 | ||
| Not black | 8/11 | 73 | 6/11 | 55 | ||
| US born | .28 | .31 | ||||
| Yes | 17/49 | 35 | 15/49 | 31 | ||
| No | 10/19 | 53 | 9/19 | 47 | ||
| English speaker | .24 | .43 | ||||
| Yes | 22/60 | 37 | 20/60 | 33 | ||
| No | 5/8 | 63 | 4/8 | 50 | ||
| History of homelessness | .62 | .20 | ||||
| Yes | 12/34 | 35 | 9/34 | 26 | ||
| No | 15/34 | 44 | 15/34 | 44 | ||
| History of incarceration | .99 | .21 | ||||
| Yes | 12/31 | 39 | 8/31 | 26 | ||
| No | 15/37 | 41 | 16/37 | 43 | ||
| Employed | .05 | ≤.01 | ||||
| Yes | 8/12 | 67 | 9/12 | 75 | ||
| No | 19/56 | 34 | 15/56 | 27 | ||
| Insurance | 1 | .99 | ||||
| No insurance/ Ryan White | 11/28 | 40 | 10/28 | 36 | ||
| Other | 16/40 | 40 | 14/40 | 35 | ||
| Psychiatric disease | .76 | 1 | ||||
| Yes | 9/20 | 45 | 7/20 | 35 | ||
| No | 18/48 | 38 | 17/48 | 35 | ||
| Tobacco | .61 | .10 | ||||
| Yes | 16/44 | 36 | 12/44 | 27 | ||
| No | 11/24 | 46 | 12/24 | 50 | ||
| Alcohol | .92 | .77 | ||||
| Yes | 14/37 | 38 | 12/37 | 32 | ||
| No | 13/31 | 42 | 12/31 | 39 | ||
| Illicit drug use | 1 | .21 | ||||
| Yes | 11/28 | 40 | 7/28 | 25 | ||
| No | 16/40 | 40 | 17/40 | 42 | ||
| Known HIV prior to TB diagnosis | .76 | .89 | ||||
| Yes | 22/53 | 41 | 18/53 | 34 | ||
| No | 5/15 | 33 | 6/14 | 40 | ||
| On ART prior to TB diagnosis | .99 | .99 | ||||
| Yes | 4/9 | 44 | 3/9 | 33 | ||
| No | 23/59 | 39 | 21/59 | 36 | ||
| Baseline CD4 < 50 | .59 | .99 | ||||
| Yes | 8/24 | 33 | 8/24 | 33 | ||
| No | 19/44 | 43 | 16/44 | 36 | ||
| ART with DOT | .40 | |||||
| Yes | 1/7 | 14 | 1/7 | 14 | ||
| No | 26/61 | 43 | 23/61 | 38 | ||
| TB treatment duration ≥9 mo | .57 | .10 | ||||
| Yes | 17/46 | 37 | 13/46 | 28 | ||
| No | 10/22 | 45 | 11/22 | 50 | ||
| Date TB diagnosise | .42 | .64 | ||||
| <March 2012 | 13/38 | 34 | 12/38 | 32 | ||
| ≥March 2012 | 14/30 | 47 | 12/30 | 40 | ||
| ART during TB therapy | .03 | .08 | ||||
| Yes | 26/57 | 46 | 23/57 | 40 | ||
| No | 1/11 | 9 | 1/11 | 9 | ||
| VS end of TB therapy | .04 | ≤.01 | ||||
| Yes | 20/39 | 51 | 20/39 | 51 | ||
| No | 7/29 | 24 | 4/29 | 14 | ||
| On ART ≥6 mo prior to end of TB treatmentf | .88 | .99 | ||||
| Yes | 19/40 | 48 | 16/40 | 40 | ||
| No | 10/17 | 59 | 7/17 | 41 | ||
Abbreviations: ART, antiretroviral therapy; DOT, directly observed therapy; TB, tuberculosis; VS, viral suppression.
aSixty-eight patients had ≥1 year of follow-up, 65 patients had ≥2 years of follow-up, and 53 patients had 3 years of follow-up.
bSixty-seven unique subjects, no change in statistical significance when restricted to unique subjects.
cSubjects with missing viral load assumed to have a viral load >200 copies/mL.
dAmong 43 subjects without continuous viral suppression, 31 (72%) had ≥1 viral load >200 copies/mL and 12 (28%) did not have any viral load during follow-up. No change in statistical significance when missing viral load was treated as unknown.
eNot significant when year of TB diagnosis was analyzed as a continuous variable.
fAmong patients who received ART during TB treatment (n = 57).
Of 3 patients who had recurrent TB, all were started on ART during treatment of their initial active TB episode. However, only 1 had VS during TB treatment and none had VS the first year after the end of TB treatment. All recurrent cases had the same M. tuberculosis strain as identified in their initial infection. The 3 deaths that occurred after TB treatment completion were secondary to nonopportunistic infections (all from community-acquired pneumonia). While these 3 patients achieved VS during TB treatment, none were retained in care or had VS after completion of TB treatment.
DISCUSSION
Among an inner-city TB cohort with high rates of HIV coinfection, we found that most co-infected patients had a known HIV diagnosis for many years, but a minority were in HIV care and receiving ART prior to their TB diagnosis. Additionally, rates of HIV VS during TB treatment were low, as was continuous retention in care and VS during long-term follow-up post–TB treatment. While these results are likely due to a challenging patient population, as characterized by high rates of homelessness, unemployment, and substance abuse, they also highlight areas of intervention to improve the TB/HIV care continuum that could significantly impact progression to active TB disease, TB recurrence, and deaths among HIV/TB co-infected patients [1–7, 18–20].
Our TB cohort had a remarkably high prevalence of HIV that was much higher than the global average of 10% [21], the overall HIV coinfection rate for 2016 in the United States (5.6%) [22], and some sub-Saharan countries such as Angola, Democratic Republic of Congo, and Nigeria, with estimated co-infection rates between 7.8%–16% [21]. The median presenting CD4 count (87 cells/uL) among co-infected patients was extremely low, indicating poor engagement in HIV care. Our cohort presenting CD4 count was lower than those reported among co-infected patients in South Africa and the Democratic Republic of Congo (approximately 160 cells/uL) [6, 9]. ART use is associated with decreased risk of TB, and therefore some TB cases in our cohort likely could have been prevented if patients with known HIV were in care [7, 18, 19, 23]. Additionally, patients with new HIV diagnosis presented with significantly lower CD4 count compared with those with known HIV, indicating likely missed opportunities for HIV testing and enrollment in care. Untreated HIV can hamper efforts to eliminate TB in the United States by fueling TB outbreaks, as seen in the late 1980s in New York City and more recently in Atlanta [24, 25].
It was a reassuring finding that most patients received ART sometime during TB treatment and that use increased from 74% to 92% for those diagnosed with TB after March 2012. This high ART usage rate is encouraging, and the rapid and significant increase after the release of updated recommendations highlights the impact of guidelines on clinical practice [11]. However, although ART use was high, most patients were started later than recommended, as demonstrated by early/guideline-adherent ART rates of 24% and 42% before and after March 2012. We could not ascertain reasons for delay, but this should be a target for quality improvement as early ART appears to have long-term survival benefits in those with low CD4 [20]. As in other reports, integration of HIV and TB care was not sufficient to achieve 100% ART use and eliminate delays in initiation of ART [8, 9, 26]. Of significant concern, only 52% had VS at completion of TB treatment. This is a lower rate as compared with observational studies and clinical trials enrolling patients with TB/HIV co-infection, which have found that 70%–90% of patients achieve VS at 6 months after ART initiation [3–5, 27–29]. Late ART initiation in our cohort, leading to less time on ART before completion of TB treatment, may have contributed to the low rate of VS. In addition, some patients (n = 5) did not have a VL measured between initiation of ART and completion of TB treatment. However, our rate of VS at the end of TB therapy was similar to the rate of VS at 12 months in a cohort of newly diagnosed patients with HIV during a hospitalization at Grady Memorial Hospital between 2011 and 2012 (48%), suggesting that local patient population and health system issues may play an important role [30]. In our county health clinics, ART is given as DOT with TB medications by outreach workers in exceptional cases at clinician request. Eighty percent of patients receiving ART as DOT had VS at completion of TB treatment. Thus, co-administration of treatment for TB and HIV under DOT could be explored in settings where DOT is already in place. While this strategy is likely to improve ART use and VS during treatment, it is unclear if it would lead to increased long-term retention in HIV care and VS [31].
To our knowledge, there are no published data on long-term retention in HIV care and VS among TB/HIV co-infected patients in the United States. At the end of follow-up, continuous retention and VS rates were 40% and 35%, respectively. Among those with 3 years of follow-up, only 32% had continuous retention and/or VS. These VS rates are low compared with a cohort from Ethiopia, with VS rates of 86% among co-infected patients with a median of 2.5 years of follow-up [29]. The low rates in our cohort are in keeping with the overall HIV patient population in our setting, with reported rates of continuous retention and VS 3 years after enrollment of 49% and 39%, respectively [16]. Most patients in this cohort were referred to IDP upon completion of TB therapy, as Ryan White county health clinics do not usually provide care for patients with CD4 <200. Generally, a written referral is provided, and patients are given directions to the clinic. Upon completion of TB therapy, some patients have follow-up in a TB clinic within 6 months, but daily contact with the health care system through the DOT outreach worker ceases and incentives are no longer available. A better mechanism for transition of care between TB clinics and IDP is needed, and a formal mechanism of transition with use of a linkage to care coordinator is being explored in our setting. All 3 episodes of TB recurrence occurred among patients without VS in the first year after TB treatment, a reminder of the importance of continuous VS after successful TB treatment [1, 2, 32, 33]. Additionally, all 3 deaths after completion of TB treatment were due to infections and occurred among patients who attained VS during TB treatment but lost VS after completion of TB treatment and were also potentially preventable by continuous VS [18].
Our data suggest that patients employed at the time of TB diagnosis and with VS at the end of TB treatment are more likely to have continuous VS. Future studies with increased sample size should aim to better identify high-risk patients for loss to HIV care and TB recurrence and address measures to link patients to HIV care upon completion of TB treatment.
Retention in HIV care and VS are major challenges for vulnerable populations living in poverty in the southern United States [34]. Studies of interventions to improve retention in care and VS in these patients have had limited efficacy, and a recent study using patient navigators and contingency management failed to improve viral suppression 6 months after the intervention ended [35]. While the durability of financial incentives for HIV care is unclear, the cost of treatment of 1 TB case has been estimated to be US $34 600, and risk of TB recurrence could justify their use [36, 37]. Innovative strategies tailored to local populations are needed to improve the HIV care continuum.
Our study has several limitations, including retrospective design and small sample size. VS at completion of TB treatment and for each following year was defined as having the last VL for each period suppressed, missing episodes of intermittent VS. There was a large proportion of patients with missing VLs after the end of TB treatment, but there was no difference in associations and statistical significance when missing VLs were treated as unknown. We relied on surveillance data to capture patients without follow-up at Ryan White county health clinics and IDP, potentially missing episodes of retention and VS. However, Georgia state law mandates that all laboratories report any HIV-related results to eHARS [12, 13]. In addition, we could not capture data for patients who moved out of the state of Georgia.
In summary, we found very low rates of HIV VS before TB diagnosis and during TB treatment, with declining rates of VS after the end of TB treatment. New strategies are needed to retain vulnerable populations in HIV care and are being explored in clinical trials such as HPTN 078 (NCT0266321). Improving the HIV care continuum will be critical to reducing new tuberculosis cases and recurrences.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are indebted to Ms Sadie Sellers at the Fulton County Board of Health Department, Drs Rose Marie F. Sales and Pascale Wortley at the Georgia Department of Public Health, and the nurses providing care for patients living with TB in Georgia for their assistance with data collection.
Financial support. The study was funded in part by the National Institutes of Health National Institute of Allergy and Infectious Diseases (K23AI103044 to R. R. K.), and the Atlanta Clinical and Translational Science Institute (UL1TR000454).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24:351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol 2018; 16:80–90. [DOI] [PubMed] [Google Scholar]
- 3. Abdool Karim SS, Naidoo K, Grobler A et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanc FX, Sok T, Laureillard D et al. ; CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Havlir DV, Kendall MA, Ive P et al. ; AIDS Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan R, Caldwell J, Middelkoop K et al. Impact of ART on TB case fatality stratified by CD4 count for HIV-positive TB patients in Cape Town, South Africa (2009-2011). J Acquir Immune Defic Syndr 2014; 66:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golub JE, Chaisson RE, Martinson NA. Additive effects of isoniazid preventive therapy and HAART. AIDS 2009; 23:1446–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lessells RJ, Swaminathan S, Godfrey-Faussett P. HIV treatment cascade in tuberculosis patients. Curr Opin HIV AIDS 2015; 10:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel MR, Nana M, Yotebieng M et al. Delayed antiretroviral therapy despite integrated treatment for tuberculosis and HIV infection. Int J Tuberc Lung Dis 2014; 18:694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nahid P, Dorman SE, Alipanah N et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents Available at: https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003093.pdf. Accessed 1 December 2017.
- 12. Georgia Department of Public Health. HIV/AIDS epidemiology section HIV surveillance summary, Georgia 2015 Available at: https://dph.georgia.gov/reporting-forms-data-requests. Accessed 1 December 2017.
- 13. Gray KM, Cohen SM, Hu X et al. Jurisdiction level differences in HIV diagnosis, retention in care, and viral suppression in the United States. J Acquir Immune Defic Syndr 2014; 65:129–32. [DOI] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Tuberculosis genotyping—United States, 2004–2010. MMWR Morb Mortal Wkly Rep 2012; 61:723–5. [PubMed] [Google Scholar]
- 16. Colasanti J, Kelly J, Pennisi E et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2016; 62:648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mugavero MJ, Westfall AO, Zinski A et al. ; Retention in Care (RIC) Study Group Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr 2012; 61:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lundgren JD, Babiker AG, Gordin F et al. ; INSIGHT START Study Group Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danel C, Moh R, Gabillard D et al. ; TEMPRANO ANRS 12136 Study Group A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 20. Marcy O, Laureillard D, Madec Y et al. ; CAMELIA (ANRS 1295-CIPRA KH001) Study Team Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis 2014; 59:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Global Tuberculosis Report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 22. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2015. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 23. Lawn SD, Myer L, Edwards D et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009; 23:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powell KM, VanderEnde DS, Holland DP et al. Outbreak of drug-resistant Mycobacterium tuberculosis among homeless people in Atlanta, Georgia, 2008–2015. Public Health Rep 2017; 132:231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brudney K, Dobkin J. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis 1991; 144:745–9. [DOI] [PubMed] [Google Scholar]
- 26. Gupta AK, Singh GP, Goel S et al. Efficacy of a new model for delivering integrated TB and HIV services for people living with HIV/AIDS in Delhi—case for a paradigm shift in national HIV/TB cross-referral strategy. AIDS Care 2014; 26:137–41. [DOI] [PubMed] [Google Scholar]
- 27. Zishiri V, Charalambous S, Shah MR et al. Implementing a large-scale systematic tuberculosis screening program in correctional facilities in South Africa. Open Forum Infect Dis 2015; 2:ofu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soeters HM, Napravnik S, Patel MR et al. The effect of tuberculosis treatment on virologic and CD4+ cell count response to combination antiretroviral therapy: a systematic review. AIDS 2014; 28:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reepalu A, Balcha TT, Sturegård E et al. Long-term outcome of antiretroviral treatment in patients with and without concomitant tuberculosis receiving health center-based care-results from a prospective cohort study. Open Forum Infect Dis 2017; 4:ofx219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colasanti J, Goswami ND, Khoubian JJ et al. The perilous road from HIV diagnosis in the hospital to viral suppression in the outpatient clinic. AIDS Res Hum Retroviruses 2016; 32:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hart JE, Jeon CY, Ivers LC et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr 2010; 54:167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Houben RM, Glynn JR, Mboma S et al. The impact of HIV and ART on recurrent tuberculosis in a sub-Saharan setting. AIDS 2012; 26:2233–9. [DOI] [PubMed] [Google Scholar]
- 33. Lahey T, Mackenzie T, Arbeit RD et al. Recurrent tuberculosis risk among HIV-infected adults in Tanzania with prior active tuberculosis. Clin Infect Dis 2013; 56:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reif SS, Whetten K, Wilson ER et al. HIV/AIDS in the Southern USA: a disproportionate epidemic. AIDS Care 2014; 26:351–9. [DOI] [PubMed] [Google Scholar]
- 35. Metsch LR, Feaster DJ, Gooden L et al. Effect of patient navigation with or without financial incentives on viral suppression among hospitalized patients with HIV infection and substance use: a randomized clinical trial. JAMA 2016; 316:156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bassett IV, Wilson D, Taaffe J, Freedberg KA. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS 2015; 10:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh P, Pascopella L, Barry PM, Flood JM. A systematic synthesis of direct costs to treat and manage tuberculosis disease applied to California, 2015. BMC Res Notes 2017; 10:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.