Abstract
Background
Hippocampal abnormalities have been largely reported in patients with schizophrenia and bipolar disorder, and are considered to be involved in the pathophysiology of the psychosis. The hippocampus consists of several subfields but it remains unclear their involvement in the early stages of psychosis.
Aim
The aim of this study was to investigate volumetric alterations in hippocampal subfields in patients at the first-episode psychosis (FEP).
Methods
Magnetic resonance imaging (MRI) data were collected in 134 subjects (58 FEP patients; 76 healthy controls [HC]). A novel automated hippocampal segmentation algorithm was used to segment the hippocampal subfields, based on an atlas constructed from ultra-high resolution imaging on ex vivo hippocampal tissue. The general linear model was used to investigate volume differences between FEP patients and HC, with age, gender and total intracranial volume as covariates.
Results
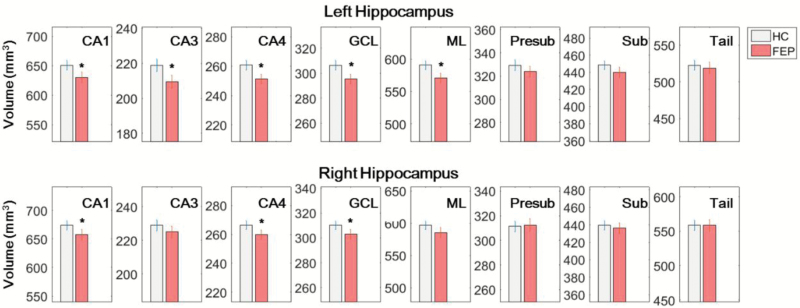
We found significantly lower volumes of bilateral CA1, CA4, and granule cell layer (GCL), and of left CA3, and left molecular layer (ML) in FEP patients compared to HC. Only the volumes of the left hippocampus and its subfields were significantly lower in FEP than HC at the False Discovery Rate (FDR) of 0.1. No correlation was found between hippocampal subfield volume and duration of illness, age of onset, duration of medication, and Positive and Negative Syndrome Scale (PANSS).
Conclusion
We report abnormally low volumes of left hippocampal subfields in patients with FEP, sustaining its role as a putative neural marker of psychosis onset.
Keywords: hippocampus, schizophrenia, psychotic bipolar disorder, volumetry
Introduction
Psychotic spectrum disorders have a combined lifetime prevalence of approximately 3%.1 In particular, schizophrenia is the most common with a lifetime morbid risk of 1%.2 It is characterized by delusions and hallucinations, speech and thought disorganization, flatted affection, and impaired cognitive functioning.3 Hippocampal alterations have been consistently reported in schizophrenia.4
Hippocampal volume decrease was associated with increased blood perfusion,5 reduced activation during novelty and memory tasks,6,7 symptom severity,8,9 and social functioning10 and antipsychotic medication.8,11 Given that the hippocampus owns crucial relevance in memory, emotion processing and other essential cognitive functions4,6,12 these alterations may contribute to the pathology and cognitive impairment of psychosis spectrum.
While previous findings indicated that abnormalities of the whole hippocampus might be related to psychoses, few could localize which part of the hippocampus underwent the greatest impairment. Because the hippocampus is not a uniform structure,13 and consists of several subfields with distinct functions, such as the Cornu Ammonis regions (CA1-4), the dentate gyrus (DG), the molecular layers (MLs) and the subiculum.14
Previous findings in psychotic disorders (eg, schizophrenia) demonstrated smaller hippocampal subfield volumes in CA2/3, CA4/DG subiculum, and CA113,15,16 which may indicate the involvement of specific subfields during the early development of psychosis, especially CA1.16
Postmortem studies in schizophrenia reported hippocampal volume reduction, with reduced cell size in left CA1 and CA2 and right CA3.17 Moreover, non-pyramidal cell size and density in CA2 were decreased in a mixed sample of schizophrenia and bipolar disorder patient.18
Although the total number of hippocampal neurons seems to be normal,19 the size of pyramidal neurons in CA120 and non-pyramidal cell layer volume in CA2/319 were found to be decreased.
Tamminga4 suggested that in patients suffering from schizophrenia, molecular changes can be caused by a reduction in glutamate transmission in the DG to CA3, affecting in particular mossy efferent fibers. Since the DG is the major afferent of the fibers from the entorhinal cortex, it might be the first step in the processing of information leading to the composition of episodic memories.21 This alteration in DG glutamatergic output and in CA3 homeostasis might underlie 2 crucial processes in memory, named pattern separation and pattern completion. This model may explain the “psychotic associations” leading to a failure in discrimination and association between present and past tracks in memory.4
However, in vivo studies were still lacking to confirm whether these subfields were affected already at the onset of psychosis or after the progressive period of the disease, and whether the alteration of their volumes was caused by the antipsychotic medications. Thus, investigating patients at the first episode of psychosis is particularly informative since potential confounding factors associated with chronicity and long-term exposure to antipsychotic medications are minimized.
Recently, a novel automatic algorithm that provides reliable segmentation of hippocampus has been developed on in vitro tissues and validated in vivo images.22 This state of the art method makes it possible for us to investigate all the subfields within the hippocampus from a large sample. In the present study, we investigated the volume changes of hippocampal subfields in patients with the first-episode psychosis (FEP). We hypothesized FEP had lower volumes of hippocampal subfields compared to healthy subjects.
Materials and Methods
Participants
The patient sample consisted of 58 subjects with FEP with an ICD-10 diagnosis: (1) within the non-affective psychosis spectrum (NAP = 49, age = 29.3 ± 9.3, gender = 30 male, 61%; table 1) [schizophrenia (F20) (n = 17), schizotypal disorder (F21) (n = 2), schizoaffective disorders (F25) (n = 7), delusional disorders (F22) (n = 10), brief psychotic disorder (F23) (n = 11), unspecified psychosis not due to a substance or known physiological condition (F29) (n = 2)], or (2) within the affective psychosis (AP = 9; age = 34.4 ± 9.2, gender = 4 male; 44% male) [manic episode, severe with psychotic symptoms (F30.2) (n = 4), major depressive disorder, single episode, severe with psychotic features (F32.3) (n = 5)] recruited from the multicenter GET UP project (Genetics Endophenotypes and Treatment: Understanding early Psychosis).23,24 The GET UP project has been funded by the Italian Ministry of Health as part of a National Health Care Research Program (Ricerca Sanitaria Finalizzata) coordinated by the Academic Hospital of Verona (Azienda Ospedaliera Universitaria Integrata Verona).
Table 1.
Mean Demographic and Clinical Characteristics of the Subjects
| Control Subjects (n = 76) | First-Episode Psychotic Patients (n = 58) | F/X2 | P Value | |
|---|---|---|---|---|
| Age | 30.89 ± 8.80 | 30.01 ± 9.43 | 1.510 | .089 |
| Gender | ||||
| Male | 32 (42%) | 34 (58%) | 0.883 | .199 |
| Female | 44 (58%) | 24 (42%) | ||
| Education | 20.336 | <.001 | ||
| 0 (unknown) | 0 | 1 | ||
| 1 (5 y) | 0 | 1 | ||
| 2 (8 y) | 2 | 19 | ||
| 3 (13 y) | 28 | 29 | ||
| 4 (>14 y) | 21 | 8 | ||
| Age of onset | — | 28.00 ± 9.46 | ||
| PANSS positive | — | 14.37 ± 6.14 | ||
| PANSS negative | — | 16.39 ± 7.18 | ||
| PANSS psychopathology | — | 35.14 ± 10.04 | ||
| PANSS total | — | 65.90 ± 19.20 | ||
| Duration of untreated psychosis (days) | — | 25.40 ± 27.40 | ||
Note: PANSS, Positive And Negative Symptoms Scale.
Seventy-six healthy controls (HC) were also studied. Participants had no DSM-IV Axis I disorder, determined using a brief modified version of the Structured Clinical Interview for DSM-IV—Non-Patient Version, no history of psychiatric disorder among first-degree relatives, no history of alcohol or substance misuse and no current major medical illness.
After clinical stabilization, patients were assessed by independent evaluators to measure current psychotic symptoms (Positive and Negative Syndrome Scale; PANSS).
Inclusion and Exclusion Criteria
Inclusion criteria were based on the screening method adopted in the WHO 10-country study,24 and included:
Age 18–54 years.
Residence in the catchment area of participating CMHCs (community mental health centers).
Presence of (1) at least one of the following symptoms: hallucinations, delusions, qualitative speech disorder, qualitative psychomotor disorder, bizarre or grossly inappropriate behavior; or (2) at least 2 of the following symptoms: loss of interest, initiative and drive, social withdrawal, episodic severe excitement, purposeless destructiveness, overwhelming fear, marked self-neglect, as measured by the Screening Schedule for Psychosis.24
First lifetime contact with participating to CMHCs, prompted by the symptoms enumerated in the point above.
Exclusion Criteria Included
Pre-existing anti-psychotic medication (>3 mo) prescribed by any psychiatric or other medical agencies for a mental disorder identical or similar to the current one.
Mental disorders due to a general medical condition.
Moderate to severe mental retardation as determined by clinical functional assessment.
Ethics Committee Approval
This study was conducted in accordance with globally accepted standards of good clinical practice, in agreement with the Declaration of Helsinki, and in keeping with local regulations.
Formal ethics approval for conducting the trial has been sought and obtained by the Coordinating Center’s Ethics Committee (Comitato Etico per la Sperimentazione Clinica, Azienda Ospedaliera di Verona, which approved the study protocol, the information and informed consent sheets) and by the Ethics Committee of each participating unit.23
Image Acquisition
All participants underwent magnetic resonance imaging (MRI) scanning on the same 3.0 T Siemens Allegra MRI scanner (Siemens Ag) at the Section of Neuroradiology of the Verona Hospital. A standard head coil was used for radio frequency transmission and reception of the MRI signal, and restraining foam pads were used to minimize head motion. T1-weighted images were acquired using an axial 3-dimensional magnetization prepared rapid gradient echo (3D MPRAGE) sequence with the following parameters: matrix size 256 × 256; slice number, 160; voxel size, 1 × 1 × 1 mm3; TR 2300 ms; TE 3.93 ms; flip angle 12°.
Image Processing
Subcortical segmentation was performed with the FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu). The procedure included intensity normalization, motion correction, automated topology corrections and automatic segmentations of cortical and subcortical regions.25,26
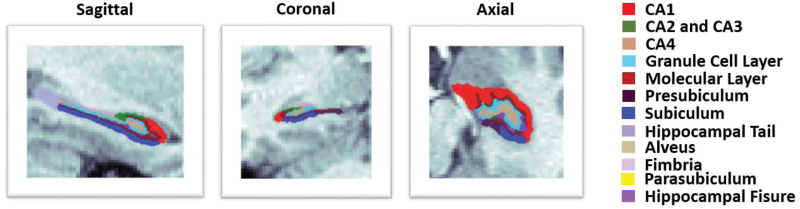
A novel automated hippocampal segmentation algorithm published with FreeSurfer 6.0 was used to label the hippocampal subfields. The subfield atlas was derived from high resolution ex vivo MRI data of post mortem medial temporal tissue at 7-T (figure 1).22 The algorithm could reliably identify CA regions and granule cell layer (GCL) within the dentate gyrus (DG) using images with 1 mm3 resolution or higher acquired on a regular 3T scanner, and was demonstrated to be more accurate than the previous algorithm, especially on the CA1.15,22,27,28 The ML in this algorithm refers to a band consists the stratum radiatum, lacunosum moleculare, hippocampal sulcus and ML of the dentate gyrus.22 In the present study, we considered 8 hippocampal subfields: CA1, CA2 and CA3 (together referred as CA2/3), CA4, GCL, ML, presubiculum, subiculum and the hippocampal tail (the posterior end of the hippocampus).
Fig. 1.
Representative subfield labels of the left hippocampus of a healthy subject. Sagittal, coronal and axial planes are shown.
We implemented a 2-step quality control (QC) procedure that was similar to the ENIGMA protocol (http://enigma.ini.usc.edu/). In step 1, any apparent outlier (5 SDs) of any hippocampal subfield was excluded. In step 2, each labeled hippocampus image was visually inspected by the authors (B.C. and V.B.) to exclude segmentations with poor registration or with wrong assignment of the subfields. After this QC procedure, we included a total of 134 subjects as reported in the previous section.
Statistical Analyses
Statistical analyses were conducted using SPSS 23 (IBM Corp). For each hippocampal subfield, we used 1-way ANOVA to investigate the effect of diagnosis. Diagnosis group (H.C. and F.E.P.) was included as an independent variable, and whole hippocampal volume and subfield volumes were included as a dependent variable, with gender and the intracranial volume (ICV) as covariates. In a pilot study, we did not find any main effect of age and education on the hippocampal subfield volumes, so we did not include them as covariates. The correlation analysis was performed between the hippocampal subfield volumes and duration of illness, age of onset, duration of medication, and PANSS. A P value of .05 was considered as significant. The false discovery rates (FDRs) of 0.1 and 0.05 were used to control the false discoveries due to multiple comparisons.
Results
We found significantly lower volumes of CA1 (left side: F1,129 = 6.26, P = .014; right side: F1,129 = 4.20, P = .041), left CA2/3 (F1,129 = 6.52, P = .011), CA4 (left side: F1,129 = 7.39, P = .007; right side: F1,129 = 3.97, P = .046, GCL (left side: F1,129 = 7.38, P = .007; right side: F1,129 = 4.09, P = .043), and left ML (F1,129 = 7.38, P = .007) in FEP patients compared to HC (table 2; figure 2). Only the whole left hippocampal volume and left CA1, CA3, CA4, GCL, and ML volumes were significantly lower in FEP at the FDR of 0.1, and no subfield was significant at the FDR of 0.05. We did not find any significant correlation between the hippocampal subfield volumes and the duration of illness, age of onset, duration of medication, and PANSS.
Table 2.
Statistical Comparisons of Hippocampal Subfield Volumes (mm3) in First-Episode Psychosis (FEP) Patients and Healthy Controls (HC)
| Left Hippocampus | HC | FEP | F1,129 | P |
|---|---|---|---|---|
| CA1 | 654.043 ± 7.234 | 626.305 ± 8.296 | 6.265 | .014 |
| CA2/3 | 220.184 ± 3.161 | 207.823 ± 3.625 | 6.518 | .011 |
| CA4 | 261.687 ± 2.835 | 249.883 ± 3.252 | 7.386 | .007 |
| GCL | 307.698 ± 3.310 | 293.754 ± 3.796 | 7.562 | .007 |
| ML | 592.611 ± 5.885 | 568.126 ± 6.75 | 7.375 | .007 |
| Presubiculum | 330.249 ± 3.893 | 322.917 ± 4.465 | 1.511 | .223 |
| Subiculum | 449.693 ± 4.729 | 438.288 ± 5.423 | 2.479 | .114 |
| Tail | 523.622 ± 6.748 | 517.726 ± 7.738 | 0.325 | .581 |
| Right Hippocampus | HC | FEP | F1,129 | P |
| CA1 | 676.457 ± 7.036 | 654.397 ± 8.069 | 4.189 | .041 |
| CA2/3 | 229.945 ± 2.939 | 223.801 ± 3.370 | 1.862 | .168 |
| CA4 | 266.997 ± 2.617 | 259.012 ± 3.002 | 3.965 | .046 |
| GCL | 311.023 ± 2.995 | 301.751 ± 3.434 | 4.085 | .043 |
| ML | 598.516 ± 5.713 | 583.531 ± 6.552 | 2.932 | .085 |
| Presubiculum | 312.506 ± 3.828 | 310.933 ± 4.390 | 0.072 | .774 |
| Subiculum | 440.639 ± 4.872 | 434.747 ± 5.588 | 0.623 | .415 |
| Tail | 559.986 ± 6.969 | 556.925 ± 7.992 | 0.082 | .775 |
Note: ML, molecular layer; GCL, granule cell layer.
Fig. 2.
Left and right hippocampal volumes (mm3) in first-episode psychosis (FEP) patients and healthy controls (HC). Volumes of bilateral CA1, CA4, and granule cell layer (GCL), and of left CA3, and left molecular layer (ML) were significantly smaller in FEP patients compared to HC.
Discussion
In this study we demonstrated that hippocampal subfield volumes in left CA1, CA3, CA4, GCL, and ML, and right CA1 and CA4 and GCL were abnormally lower in patients at FEP compared to healthy subjects, although these abnormalities were only significant in the left hippocampus at FDR of 0.1. These findings indicate early shrinkage of hippocampal subfields, especially CA regions in the psychosis spectrum.
Great efforts have been exerted towards the in vivo automatic segmentation of hippocampal subfields, recently, due to the interest in further understanding the clinical and cognitive roles of hippocampal subfields in psychosis.13,15,29–33 Some studies focused on the surface components30,33,34 or the long-axis,35 while some others used atlases developed from high-resolution MRI images.13,28,29 However, it is only until recent that a novel segmentation method was developed based on ultra-high resolution MRI images of ex vivo hippocampal tissue,22 which provides a detailed atlas and reliable segmentations of subfields, especially CA1.15,16,28,36
The bilateral low volumes of CA1 in FEP was in line with previous studies in early-course16,30 and chronic schizophrenia patients.9,29 As recently reported by a longitudinal study by Ho,16 in subjects who developed psychosis hippocampal volume loss might arise from CA1, and consequently spread to others subfields as the course and worsening of the illness progress.16,30 Several studies suggested possible pathophysiological mechanisms related this finding, relying on observation that CA1 subfield was found to be particularly vulnerable to hypoxia25,26 and to hyperexcitation of glutamate receptors (NMDA). However, given that hippocampal subfields are interconnected, such as the tri-synaptic circuitry between GCL, CA2/3, and CA1, pathology in a specific subfield would ultimately impact the neighboring connections. Specifically, it has been hypothesized that glutamatergic hyperactivity in CA1 might be caused by a lack of inhibition of glutamatergic CA3 input and leading to a dopaminergic hyperfunction.27 Indeed, CA1 hypermetabolism/hyperexcitation may conduct to excitotoxic damage, with secondary CA1 and CA2/3 volume loss.27
Within the hippocampus, areas CA1 and CA2/3 are the principal pyramidal cell fields14 and recent studies have suggested important differences in the function in spatial and contextual memory.37,38 It has been suggested that these regions play an important role in encoding and associating memory traces, and alterations in such patterns would eventually result in psychosis phenomenon.4 Thus, abnormalities in CA2/3 may underlie specific psychotic psychopathologic features, that cannot be corrected because of inability to perform pattern separation, conjunctive encoding, and/or pattern completion.39
CA4 is considered an important anatomic site of intersection for innervation pathways connecting the hippocampus with several other cerebral sites. Indeed, CA4 volume loss, as seen here, might affect connectivity to other hippocampal and brain regions in patients with major psychosis. For example, Fatemi et al40 observed significant decreases in Reelin levels, a secretory glycoprotein responsible for lamination of brain, in CA4 area of schizophrenia and mood disorder patients.
The GCL takes part of the trisynaptic circuitry and is involved in neurogenesis and synaptic plasticity during brain development and adulthood.41 GCL volume loss was observed over time as significant effects of the illness, and could be related with stress, environmental factors, substance abuse and fundamental cell abnormalities. Thus, multiple genes associated with neuronal development,41,42 including schizophrenia susceptibility genes,43 eg, neuregulin-1,44 and dysbindin,45 are expressed in the GCL.46 The low GCL volume in FEP observed in our study may indicate some of the neuropathological changes underlying schizophrenia, as decreased cell proliferation47 and memory impairment.48
Finally we found a shrinkage in the left hippocampal subfields. Currently, despite the emerging of new approaches and techniques in the MRI fields, there are relatively few studies related to hippocampal subfields in FEP and, specifically, investigating symmetry. Consistent with our results, Velakoulis et al49 found reduction in left hippocampal volumes from the onset of both schizophrenic and affective psychosis. These results are in line with the continuum of psychosis spectrum; however, although hippocampal involvement is reported both in affective and non-affective psychoses,13,49,50 the asymmetry in volume reduction is still controversial, also because different ways of hippocampal segmentation may introduce further variability. In particular, the left hippocampal volumes have been reported to be smaller at the onset of the psychosis,49 while bilaterally in psychosis spectrum chronic patients.50,51 Thus, the hippocampus has been reported to be decreased in patients with bipolar disorder with psychotic features but not in those without psychosis.52
In this study we have examined hippocampal alterations across the psychotic spectrum. Indeed, according with several lines of evidence, affective and non-affective psychosis share clinical,53 morphological,54 and genetic vulnerabilities and risk factors,55,56 leading to a growing consensus to read these 2 entities along a continuum.57 Investigating the first episode appears particularly important because it allows to examine psychosis phenomenon as a dimensional continuum, in light of the Research Domain Criteria (RDoC),58 and not as categorical (and chronic) diagnoses. Anyway, in this work we did not investigate from where the volumetric difference in hippocampal subfields between psychotic and healthy subjects stems, but it is certainly worthy of consideration for future studies.
A major limitation should be considered in our study. Although at the first episode, patients had received some medication exposure during stabilization. We thus were not able to fully exclude the impact of psychotropic drugs on the hippocampus in FEP, although no associations between duration of medication and any hippocampal volumes were found. The novel segmentation method of hippocampal subfields estimated the ML volume with the total hippocampal volume and the volumes of other subfields, when T1 images were used alone. Although the novel automatic segmentation method has been proved to capture hippocampal subfield abnormalities in several clinical populations, it is not fully compared with manually labeled atlas of hippocampal subfields. Thus, our results still need to be interpreted with caution.
Conclusions
We found abnormal lower hippocampal subfield volumes in FEP compared to healthy subjects, especially in the left CA regions, being a potential neural marker for psychosis onset. Future longitudinal MRI study coupled with neuropsychological investigations should explore the possible changes of hippocampal subfields over time in FEP to elucidate their impact on cognition along the spectrum of psychosis.
Funding
This study was supported by grants from the Italian Ministry of Health (Ricerca Sanitaria Finalizzata 2004, Giunta Regionale del Veneto to M.R.; Ricerca Sanitaria Finalizzata 2005, Giunta Regionale del Veneto to A.L.S.; GR-2010-2316745 to P.B., GR-2010-2319022 to M.B., GR H61J08000200001 to M.R.) and by the Fondazione Cariverona (Promoting research to improve quality of care; Sotto-obiettivo A9 “Disabilità cognitiva e comportamentale nelle demenze e nelle psicosi” to P.B. and M.R.) and by Ministry of Health, Italy—Ricerca Sanitaria Finalizzata, GET UP Project Code H61J08000200001.
Acknowledgments
There is no conflict of interest concerning the authors in conducting this study and preparing the manuscript.
References
- 1. Maggioni E, Bellani M, Altamura AC, Brambilla P. Neuroanatomical voxel-based profile of schizophrenia and bipolar disorder. Epidemiol Psychiatr Sci. 2016;25:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Craddock N, O’Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultze-lutter F, Ruhrmann S, Picker H, von Reventlow HG, Brockhaus-dumke A, Klosterkotter J. Basic symptoms in early psychotic and depressive disorders. Br J Psychiatry. 2007;191:s31–s37. [DOI] [PubMed] [Google Scholar]
- 4. Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. [DOI] [PubMed] [Google Scholar]
- 5. Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. [DOI] [PubMed] [Google Scholar]
- 6. Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss AP, Zalesak M, DeWitt I, Goff D, Kunkel L, Heckers S. Impaired hippocampal function during the detection of novel words in schizophrenia. Biol Psychiatry. 2004;55:668–675. [DOI] [PubMed] [Google Scholar]
- 8. Panenka WJ, Khorram B, Barr AM et al. A longitudinal study on the effects of typical versus atypical antipsychotic drugs on hippocampal volume in schizophrenia. Schizophr Res. 2007;94:288–292. [DOI] [PubMed] [Google Scholar]
- 9. Zierhut KC, Graßmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136:804–814. [DOI] [PubMed] [Google Scholar]
- 10. Brambilla P, Perlini C, Rajagopalan P et al. Schizophrenia severity, social functioning and hippocampal neuroanatomy: three-dimensional mapping study. Br J Psychiatry. 2013;202:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;40:1409–1422. [DOI] [PubMed] [Google Scholar]
- 12. Sala M, Perez J, Soloff P et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. [DOI] [PubMed] [Google Scholar]
- 13. Haukvik UK, Westlye LT, Mørch-Johnsen L et al. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77:581–588. [DOI] [PubMed] [Google Scholar]
- 14. Duvernoy HM. The Human Hippocampus. Berlin, Heidelberg: Springer Berlin Heidelberg;1998. [Google Scholar]
- 15. Ho NF, Iglesias JE, Sum MY et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho NF, Holt DJ, Cheung M et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaidel DW, Esiri MM, Harrison PJ. Size, shape, and orientation of neurons in the left and right hippocampus: investigation of normal asymmetries and alterations in schizophrenia. Am J Psychiatry. 1997;154:812–818. [DOI] [PubMed] [Google Scholar]
- 18. Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. [DOI] [PubMed] [Google Scholar]
- 19. Konradi C, Zimmerman EI, Yang CK et al. Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry. 2011;68:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Schulz SC, Lee S, Reutiman TJ, Fatemi SH. Hippocampal CA1 pyramidal cell size is reduced in bipolar disorder. Cell Mol Neurobiol. 2007;27:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 2007;163:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iglesias JE, Augustinack JC, Nguyen K et al. ; Alzheimer’s Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruggeri M, Bonetto C, Lasalvia A et al. ; GET UP GROUP. A multi-element psychosocial intervention for early psychosis (GET UP PIANO TRIAL) conducted in a catchment area of 10 million inhabitants: study protocol for a pragmatic cluster randomized controlled trial. Trials. 2012;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruggeri M, Bonetto C, Lasalvia A et al. ; GET UP Group. Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: results from the cluster-randomized controlled GET UP PIANO Trial in a catchment area of 10 million inhabitants. Schizophr Bull. 2015;41:1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. Neuroimage. 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 26. Fischl B, Salat DH, Busa E et al. Whole brain segmentation. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 27. Bilder RM, Bogerts B, Ashtari M et al. Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophr Res. 1995;17:47–58. [DOI] [PubMed] [Google Scholar]
- 28. Van Leemput K, Bakkour A, Benner T et al. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kühn S, Musso F, Mobascher A, Warbrick T, Winterer G, Gallinat J. Hippocampal subfields predict positive symptoms in schizophrenia: first evidence from brain morphometry. Transl Psychiatry. 2012;2:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schobel SA, Chaudhury NH, Khan UA et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voineskos AN, Winterburn JL, Felsky D et al. Hippocampal (subfield) volume and shape in relation to cognitive performance across the adult lifespan. Hum Brain Mapp. 2015;36:3020–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao B, Passos IC, Mwangi B et al. Hippocampal subfield volumes in mood disorders. Mol Psychiatry. 2017;22(9): 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joshi SH, Espinoza RT, Pirnia T et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cong S, Rizkalla M, Du EY et al. ; for the Alzheimer’s Disease Neuroimaging Initiative. Building a surface atlas of hippocampal subfields from MRI scans using FreeSurfer, FIRST and SPHARM. Conf Proc (Midwest Symp Circuits Syst). 2014;2014:813–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17:230–240. [DOI] [PubMed] [Google Scholar]
- 36. Wisse LE, Biessels GJ, Geerlings MI. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front Aging Neurosci. 2014;6:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem. 2008;15:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear-conditioning. Hippocampus. 2004;14:301–310. [DOI] [PubMed] [Google Scholar]
- 39. Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. [DOI] [PubMed] [Google Scholar]
- 40. Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. [DOI] [PubMed] [Google Scholar]
- 41. van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altar CA, Jurata LW, Charles V et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. [DOI] [PubMed] [Google Scholar]
- 43. Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. [DOI] [PubMed] [Google Scholar]
- 44. Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. [DOI] [PubMed] [Google Scholar]
- 45. Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE. Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res. 2008;98:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rioux L, Arnold SE. The expression of retinoic acid receptor alpha is increased in the granule cells of the dentate gyrus in schizophrenia. Psychiatry Res. 2005;133:13–21. [DOI] [PubMed] [Google Scholar]
- 47. Meyer KD, Morris JA. Disc1 regulates granule cell migration in the developing hippocampus. Hum Mol Genet. 2009;18:3286–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Velakoulis D, Pantelis C, McGorry PD et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. [DOI] [PubMed] [Google Scholar]
- 50. Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. [DOI] [PubMed] [Google Scholar]
- 51. Mathew I, Gardin TM, Tandon N et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. [DOI] [PubMed] [Google Scholar]
- 52. Strasser HC, Lilyestrom J, Ashby ER et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57:633–639. [DOI] [PubMed] [Google Scholar]
- 53. Simonsen C, Sundet K, Vaskinn A et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rimol LM, Hartberg CB, Nesvåg R et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. [DOI] [PubMed] [Google Scholar]
- 55. Andreassen OA, Thompson WK, Schork AJ et al. Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9:e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lichtenstein P, Yip BH, Björk C et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Craddock N, O’Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. 2009;35:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]