Abstract
Objective
Progress toward understanding brain mechanisms in psychosis is hampered by failures to account for within-group heterogeneity that exists across neuropsychological domains. We recently identified distinct cognitive subgroups that might assist in identifying more biologically meaningful subtypes of psychosis. In the present study, we examined whether underlying structural brain abnormalities differentiate these cognitively derived subgroups.
Method
1.5T T1 weighted structural scans were acquired for 168 healthy controls and 220 patients with schizophrenia/schizoaffective disorder. Based on previous work, 47 patients were categorized as being cognitively compromised (impaired premorbid and current IQ), 100 as cognitively deteriorated (normal premorbid IQ, impaired current IQ), and 73 as putatively cognitively preserved (premorbid and current IQ within 1 SD of controls). Global, subcortical and cortical volume, thickness, and surface area measures were compared among groups.
Results
Whole cortex, subcortical, and regional volume and thickness reductions were evident in all subgroups compared to controls, with the largest effect sizes in the compromised group. This subgroup also showed abnormalities in regions not seen in the other patient groups, including smaller left superior and middle frontal areas, left anterior and inferior temporal areas and right lateral medial and inferior frontal, occipital lobe and superior temporal areas.
Conclusions
This pattern of more prominent brain structural abnormalities in the group with the most marked cognitive impairments—both currently and putatively prior to illness onset, is consistent with the concept of schizophrenia as a progressive neurodevelopmental disorder. In this group, neurodevelopmental and neurodegenerative factors may be important for cognitive function.
Keywords: neuropsychological subgroups, brain volume, cortical thickness, cortical surface area, total brain volume, intracranial volume
Schizophrenia is a heterogeneous disorder that varies in its cognitive presentation. Past literature indicates a broad spectrum of cognitive functioning, ranging from intact ability or mild deficits, to severe and profound impairments.1,2 Work using different statistical clustering techniques shows that schizophrenia patients can be subgrouped into 2-to-4 more homogeneous clusters on the basis of neuropsychological data tapping predominantly “fluid” cognitive processes.3–7 These clusters can be distinguished in terms of the severity of their cognitive impairments and their psychosocial outcomes; with the more severely impaired patients tending to demonstrate evidence of poorer functioning relative to less-impaired groups.3,8
Among individuals with schizophrenia with moderate-to-severe cognitive deficits in fluid intelligence, there is variability in the extent to which crystallized intelligence is also affected.4,7–10 Crystallized intelligence is generally measured by performance on “hold tests” including vocabulary or word reading, that tap into abilities that require intact functioning during development for adequate performance. As these hold tests are thought to be immune to age-related decline, their use in psychiatric disorders is considered to index the extent of intellectual functioning (premorbid IQ) prior to illness onset.11,12 For some patients, impaired current cognition in the context of intact premorbid IQ, suggests a putatively normal early developmental cognitive path followed by a decline in intellectual functioning possibly associated with pathology at illness onset. For others, severe deficits in current cognitive functioning in the context of low premorbid IQ implies early limitations to cognitive capacity that may be consistent with neurodevelopmental insults and/or early and ongoing cognitive degeneration.6,7
Recently, Woodward and Heckers9 reported that cognitively impaired patients with psychosis (bipolar disorder and schizophrenia) had reduced total brain volumes (TBV) as well as regionally specific fronto-temporal and subcortical gray matter loss relative to healthy individuals. However, cognitively impaired patients with compromised premorbid IQ showed evidence of brain hypoplasia in the form of reduced intracranial volume (ICV). Conversely, cognitively impaired patients with intact premorbid IQ, showed evidence of neural atrophy as demonstrated by reduced TBV but normal ICV. Czepielewski et al13 largely replicated these findings in a smaller cohort of individuals diagnosed with schizophrenia only, demonstrating that patients with impaired current and premorbid cognitive functioning had reduced ICV and reductions in TBV, global cortical thickness, global gray matter and regional insula volumes. Patients with impaired current but intact premorbid cognitive impairments however, had only reduced TBV alongside reduced global cortical thickness and gray matter volume relative to controls. Weinberg et al5 also recently reported extensive volumetric abnormalities in whole brain, total gray and white matter as well as in several cortical regions in a similarly categorized psychosis subgroup with average premorbid but below average current IQ. However, due to low statistical power these authors were unable to analyze a subgroup akin to that identified as having low premorbid and current intellectual function in previous studies.
Although cross-sectional in nature, these studies assumed that in the context of impaired current cognitive functioning, estimates of impaired premorbid IQ represented a “compromised” cognitive phenotype, while estimates of intact premorbid IQ represented a potentially “declining” cognitive phenotype. These studies therefore suggest that both neurodevelopmental and neurodegenerative processes may be of importance to understanding variability in brain–cognition relationships in schizophrenia.14
However, all of these studies comprised small-medium sized samples for cognitive clustering, making it unclear whether the above brain structural findings are reproducible with larger samples. These studies also only examined global brain thickness and/or volumetric estimates, or regional brain volumes using voxel-wise or mean-regional volume approaches in relation to cognitive subgroups. Yet knowledge of brain–cognition relationships may further benefit from understandings of regional thickness differences between subgroups, as well as the unique contribution of surface area. The latter may have a neurodevelopmental basis as it has been shown to scale with the degree of cortical folding, a process occurring during mid-gestation and early postnatal brain development.15–17 Both thickness and surface area are component measures of cortical volume thought to be negatively related,18 genetically independent19,20 and have differing neurodevelopmental trajectories and relationships to IQ.21,22 It remains unclear however, whether thickness, surface area, and volume show similar or different relationships with cognitive variability in schizophrenia/schizoaffective disorder, particularly in the context of premorbid IQ; to date, there is a paucity of literature examining discrete brain structural indices in cognitive subgroups of psychosis with putatively differing cognitive courses.5,6,8,9,13
In our recent study,8 we used a data-driven approach to subgroup patients with schizophrenia and schizoaffective disorder into 3 groups based on estimated premorbid and current IQ. One group showed evidence of average premorbid and current cognitive performance, suggesting the presence of a “preserved” cognitive course unlikely to be impacted by neurodevelopmental or neurodegenerative abnormalities. A second group of patients with average premorbid IQ but current IQ below the control mean, showed evidence of a decline in cognitive functioning; these patients appeared to have a “deteriorated” cognitive course, with progressive degeneration assumed to originate at or after illness onset. Finally, a third group with low premorbid and low current IQ were considered to show evidence of a “compromised” cognitive course with abnormalities assumed to originate years before and continue after illness onset. Functional outcomes and symptomatology in the compromised subgroup were worse than that of preserved patients, suggesting group differentiation consistent with differences in cognitive profiles in and of themselves. These findings suggest that the pathways leading to cognitive outcomes in each of the subgroups may be different. However, the presence of other potentially distinguishing factors, including brain structure, was not examined.
Here, we aimed to build on work in this cohort by determining whether underlying structural brain abnormalities differentiate these cognitively derived subgroups. Our objectives were 2-fold; first, we aimed to replicate the findings by Woodward and Heckers9 and Czepielewski et al.13 in the current larger cohort. We predicted that smaller ICV would be apparent in the compromised subgroup as evidence of hypoplasia, whereas smaller TBV and normal ICV would be apparent in the deteriorated subgroup as evidence of atrophy. Second, we aimed to extend previous findings by examining whether the cognitive subgroups could be differentiated in terms of global and regional brain measures of volume, thickness and surface area. We predicted that both cognitively impaired subgroups would show brain structural abnormalities relative to controls, but that these would be more extensive in the compromised subgroup. Whether surface area differences would be evident only in the compromised patients with greater presumed neurodevelopmental influences remained exploratory.
Methods
Participants
Neuroimaging data from 220 patients with schizophrenia/schizoaffective disorder and 168 healthy controls was obtained from the Australian Schizophrenia Research Bank (ASRB). All participants provided informed consent for the analysis of their stored data. Study procedures were approved by the Melbourne Health Human Research Ethics Committee. Details of participant characterization are given in the supplementary material.
Cognitive subgroups were previously determined by applying clustering analysis to a larger dataset (n = 534) of patients from the ASRB (see Wells et al8 for details). Briefly, tree and K-means clustering algorithms were used to determine the optimal number of clusters to retain from the data using standardized scores from the following tests; Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) immediate memory and attention index scores and the Wechsler Adult Intelligence Test–III Letter-Number Sequencing test. This strategy resulted in a 3-group solution, defined as putatively preserved, deteriorated and compromised subgroups of people with schizophrenia/schizoaffective disorder.
MRI Image Acquisition and Processing
T1-weighted (MPRAGE) structural scans were acquired using Siemens Avanto 1.5 tesla scanners. T1-weighted images comprised 176 sagittal slices/brain of 1 mm thickness without gap; field of view = 250 × 250 mm2; repetition time/echo time = 1980/4.3 ms; data matrix size = 256 × 256; voxel dimensions = 1.0 × 1.0 × 1.0 mm3. The same acquisition sequence was acquired at all ASRB sites. Image processing was conducted using the Freesurfer software package (version 5.1.0, http://surfer.nmr.mgh.harvard.edu/), comprising a volume and a surface-based stream.23,24 The former was used to extract mean volume estimates for ICV (estimate based on the talairach transform), TBV (brain segmentation volume without ventricles) and subcortical and cortical areas across the whole brain. The latter was used to extract cortical thickness and surface area measurements by reconstructing a 3-dimensional cortical surface model. Details of the preprocessing procedure are provided in the supplementary material.
Statistical Analysis
Demographic data were analyzed with one-way analyses of variance or chi-square tests using the Statistical Package for the Social Sciences (SPSS v22). Mean global and regional volume, thickness, and surface area values were extracted from Freesurfer and imported into SPSS, where the null hypothesis of equality across the 4 groups (controls, deteriorated, putatively preserved, and compromised) in each of these brain measures was tested using general linear models controlling for scanner site, gender, age, and ICV (for global volume except absolute TBV, regional volume, and surface area analyses only). Bivariate correlations were also conducted in the whole patient sample to ascertain relationships between brain measures and negative symptoms, given group differences in negative symptom severity. As no correlations survived false discovery rate (FDR; P < .05) correction for multiple testing, negative symptoms were not included as a covariate in the models comparing the 3 subgroups. The FDR was also used to correct for multiple comparisons for the following analyses separately: (1) global brain estimates: total left, right and whole cortex volume, thickness and surface area, total left and right cortical white matter volume, total cortical and subcortical gray matter volume, ICV, absolute TBV, and TBV adjusted for ICV (12 comparisons); (2) subcortical volume across the left and right putamen, pallidum, hippocampus, amygdala, thalamus, caudate, accumbens area, and cerebellum (16 comparisons); (3) regional cortical volume; (4) regional cortical thickness; and (5) regional cortical surface area. Analyses 3–5 were conducted on all 34 regions delineated by the Desikan–Killiany brain atlas; the left and right hemispheres were corrected separately using an FDR rate of P < .05 (34 comparisons per hemisphere per measure). Whenever the null hypothesis of equality across the 4 groups was rejected at a significance that survived FDR correction, pair-wise post hoc tests (6 comparisons) were performed and corrected using an FDR rate of 5% to assess where group differences lay. Effect sizes are reported as Cohen’s d.
Results
The size of the subgroups in the ASRB subset of patients with available neuroimaging data were n = 73 patients putatively preserved; n = 100 deteriorated, n = 47 compromised. Demographic/clinical proportions and cognitive performance patterns on the clustering variables largely adhered to that seen in the larger sample (supplementary figure 1). There were no differences in antipsychotic medication use between subgroups, but there were more deteriorated patients taking anxiolytics and lithium than other subgroups. Table 1 presents the descriptive statistics for the sample.
Table 1.
Demographic Characteristics of the Sample
| CIQ (n = 47) | DIQ (n = 100) | PIQ (n = 73) | HC (n = 168) | Comparisons | ||
|---|---|---|---|---|---|---|
| Gender (% male) | 74 | 69 | 74 | 48 | χ2(3) = 23.06, P < .001 | |
| Diagnostic distribution (% SZ) | 82 | 86 | 81 | — | χ2(2) = 0.53, P = .77 | |
| Medications (% using) | ||||||
| Anticholinergic | 13 | 8 | 1 | χ2(2) = 6.25, P = .04 | ||
| Anticonvulsant | 11 | 18 | 12 | χ2(2) = 1.83, P = .40 | ||
| Antidepressant | 32 | 35 | 33 | χ2(2) = 0.17, P = .92 | ||
| Atypical antipsychotics | 85 | 81 | 84 | χ2(2) = 0.43, P = .81 | ||
| Typical antipsychotics | 13 | 7 | 11 | χ2(2) = 1.50, P = .48 | ||
| Anxiolytic | 9 | 20 | 3 | χ2(2) = 12.69, P = .002 | ||
| Lithium | 2 | 8 | 0 | χ2(2) = 7.47, P = .02 | ||
| M (SD) | M (SD) | M (SD) | M (SD) | Comparisons | Post hoc comparisons a | |
| Age | 37.30 (8.45) | 36.05 (9.33) | 40.06 (10.97) | 39.74 (13.74) | F(3, 384) = 2.88, P = .036 | PIQ > DIQ; DIQ < HC |
| Illness duration | 13.59 (9.85) | 13.31 (7.71) | 16.25 (10.54) | - | F(2, 217) = 2.64, P = .074 | PIQ > DIQ |
| Positive symptoms—current | 2.29 (2.93) | 1.88 (2.56) | 1.30 (2.38) | F(2, 189) = 2.08, P = .13 | - | |
| Negative symptoms | 35.00 (20.53) | 24.55 (17.18) | 22.66 (14.87) | F(2, 205) = 7.89, P < .001 | CIQ > DIQ and PIQ | |
| GAF | 44.68 (11.79) | 55.13 (11.33) | 56.39 (12.50) | 84.48 (9.46) | F(3, 354) = 242.48, P < .001 | CIQ < DIQ = PIQ < HC |
Note: CIQ, compromised patients; DIQ, deteriorated patients; PIQ, putatively preserved patients.
aSignificant at P < .05.
Global Brain Estimates
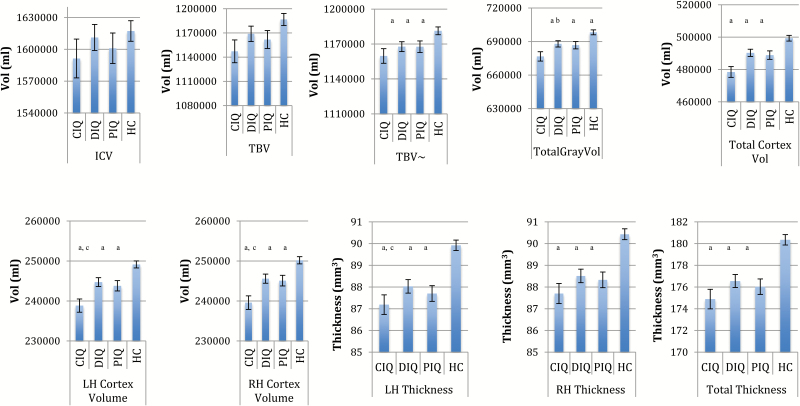
Figure 1 and supplementary table 1 present comparisons of ICV, TBV, and ICV adjusted for TBV, as well as global brain structural volume and thickness estimates for regions surviving FDR correction.
Fig. 1.
Mean differences in global brain measures that survived false discovery rate (FDR) correction across healthy controls (HC), compromised patients (CIQ), deteriorated patients (DIQ), and preserved patients (PIQ). LH, left hemisphere; RH, right hemisphere; Vol, volume; ~TBV co-varying for ICV; error bars represent standard errors. Global volume analyses adjusted for ICV, age, gender, and site. Thickness analyses adjusted for age, gender, and site. aDifferent to HC (P < .05 FDR corrected). bCIQ different to DIQ only (P < .05 FDR corrected). cCIQ different to DIQ and PIQ (P < .05 FDR corrected).
Subgroups Vs Controls.
Although in the compromised subgroup ICV was qualitatively (albeit negligibly) the smallest of all the subgroups compared to controls (d = −.21 vs d = −.05 and d = −.03), this effect was not statistically significant. In fact, there were no significant group differences in ICV, absolute TBV, bilateral cortical white matter volume, or surface area in any of the cognitive subgroups compared to controls. However, statistically significant volumetric reductions were evident in left, right, and total cortical thickness and volume as well as total gray matter volume and in TBV after correction for ICV compared to controls. Compromised patients showed the greatest patient–control effect size differences of all subgroups (effect sizes ranges: compromised d = −.70 to −.92; vs deteriorated d = −.36 to −.62 and putatively preserved d = −.40 to −.72).
Subgroup Comparisons.
Relative to both deteriorated and putatively preserved patients, the compromised subgroup had statistically significant reductions in left (d = −.52; d = −.44, respectively), right (d = −.52; d = −.48, respectively) and total cortical volumes (d = −.53; d = −.46, respectively). This group also showed statistically significant reductions in total gray matter (d = −.40) compared to deteriorated patients.
Subcortical Volume
Table 2 presents comparisons of subcortical and cortical regions surviving FDR correction.
Table 2.
Group Comparisons of Subcortical and Regional Cortical Volumes for Regions Surviving FDR Correction
| Region | ANCOVA a | Subgroup | LH | RH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M b | SD | Comparison to HC c | D d | M b | SD | Comparison to HC c | D d | |||
| Subcortical | ||||||||||
| Putamen | LH: F(3, 377) = 8.62, P < .001; RH: F(3, 377) = 6.77, P ≤ .001 | CIQ | 5596.05 | 504.16 | >HC | .43 | 5313.80 | 473.37 | =HC | .38 |
| DIQ | 5684.85 | 495.20 | >HC | .61 | 5404.99 | 486.30 | >HC | .56 | ||
| PIQ | 5598.80 | 494.47 | >HC | .44 | 5296.83 | 485.50 | =HC | .34 | ||
| HC | 5378.68 | 504.79 | — | 5131.10 | 495.59 | — | ||||
| Pallidum | LH: F(3, 377) = 12.39, P < .001; RH: F(3, 377) = 7.095, P < .001 | CIQ | 1783.80 | 193.03 | =HC | .36 | 1624.20 | 183.31 | >HC | .43 |
| DIQ | 1856.06 | 189.60 | >HC | .74 | 1631.41 | 180.00 | >HC | .47 | ||
| PIQ | 1818.51 | 189.25 | >HC | .54 | 1641.47 | 179.46 | >HC | .53 | ||
| HC | 1714.56 | 193.23 | — | 1545.49 | 183.52 | — | ||||
| Hippocampus | LH: F(3, 377) = 11.02, P < .001; RH: F(3, 377) = 8.18, P < .001) | CIQ | 4002.47 | 361.13 | <HC and DIQ | −.86e | 4124.79 | 370.79 | <HC and DIQ | −.77e |
| DIQ | 4163.72 | 354.70 | <HC | −.58 | 4300.02 | 364.20 | <HC | −.30 | ||
| PIQ | 4120.41 | 354.15 | <HC | −.54 | 4245.81 | 363.63 | <HC | −.45 | ||
| HC | 4313.05 | 361.45 | — | 4409.29 | 371.17 | — | ||||
| Cortical | ||||||||||
| Fusiform | LH: F(3, 378) = 3.26, P = .02; RH: F(3, 378) = 4.86, P = .002 | CIQ | 10071.56 | 1322.46 | =HC | −.42e | 9572.05 | 1268.96 | <HC | −.52e |
| DIQ | 10551.33 | 1300.50 | =HC | −.06 | 9929.30 | 1247.9 | =HC | −.24 | ||
| PIQ | 10199.49 | 1297.00 | =HC | −.32 | 9687.59 | 1245.47 | <HC | −.43 | ||
| HC | 10625.40 | 1324.51 | — | 10227.88 | 1270.86 | — | ||||
| Inferior parietal | LH: F(3, 378) = 6.24, P < .001 | CIQ | 13080.66 | 1847.10 | <HC | −.51 | 16014.79 | 2193.64 | — | |
| DIQ | 13374.66 | 1816.5 | <HC | −.05 | 16407.21 | 2157.73 | — | |||
| PIQ | 13075.90 | 1812.96 | <HC | −.51 | 16222.73 | 2153.10 | — | |||
| HC | 14019.13 | 1849.91 | — | 16717.04 | 2197.11 | — | ||||
| Inferior temporal | LH: F(3, 378) = 3.62, P = .01; RH: F(3, 378) = 3.43, P = .02 | CIQ | 11242.91 | 1699.28 | <HC | −.52e | 10849.87 | 1667.50 | =HC | −.44e |
| DIQ | 11804.80 | 1671.0 | =HC | −.19 | 11312.06 | 1579.20 | =HC | −.16 | ||
| PIQ | 11651.84 | 1667.86 | =HC | −.28 | 11014.54 | 1576.14 | <HC | −.35 | ||
| HC | 12124.28 | 1701.91 | — | 11569.84 | 1608.34 | — | ||||
| Lateral occipital | RH: F(3, 378) = 4.2, P = .006 | CIQ | 11692.94 | 1530.63 | — | 11632.60 | 1531.25 | <HC and DIQ | −.52e | |
| DIQ | 12099.35 | 1505.20 | — | 12400.87 | 1505.90 | =HC | −.02 | |||
| PIQ | 12191.40 | 1502.36 | — | 12008.64 | 1502.95 | =HC | −.23 | |||
| HC | 12487.08 | 1532.91 | — | 12432.30 | 1533.56 | — | ||||
| Lateral orbitofrontal | LH: F(3, 378) = 7.48, P < .001; RH: F(3, 378) = 3.93, P = .009 | CIQ | 7601.63 | 795.15 | <HC and DIQ and PIQ | −.79e | 7643.29 | 851.04 | <HC | −.55e |
| DIQ | 7981.65 | 782.00 | <HC | −.30 | 7887.34 | 836.90 | =HC | −.26 | ||
| PIQ | 8117.45 | 784.70 | =HC | −.18 | 7928.27 | 835.30 | =HC | −.21 | ||
| HC | 8216.70 | 769.39 | — | 8108.94 | 852.38 | — | ||||
| Medial orbitofrontal | RH: F(3, 378) = 5.25, P = .001 | CIQ | 5488.09 | 709.93 | — | 5219.30 | 585.47 | <HC | −.66e | |
| DIQ | 5733.61 | 698.2 | — | 5488.51 | 575.80 | =HC | −.21 | |||
| PIQ | 5659.09 | 696.86 | — | 5498.74 | 574.66 | =HC | −.19 | |||
| HC | 5846.06 | 711.12 | — | 5608.69 | 586.31 | — | ||||
| Middle temporal | LH: F(3, 378) = 5.81, P = .001; RH: F(3, 378) = 6.33, P < .001 | CIQ | 11441.47 | 1525.77 | <HC | −.39 | 12547.31 | 1536.52 | <HC | −.52 |
| DIQ | 11467.48 | 1500.50 | <HC | −.38 | 12742.12 | 1511.10 | <HC | −.40 | ||
| PIQ | 11249.40 | 1497.57 | <HC | −.52 | 12600.20 | 1508.16 | <HC | −.49 | ||
| HC | 12035.68 | 1528.11 | — | 13344.91 | 1538.87 | — | ||||
| Parahippocampal | LH: F(3, 378) = 4.00, P = .008 | CIQ | 2069.54 | 320.17 | <HC and DIQ and PIQ | −.57e | 1969.82 | 312.98 | — | |
| DIQ | 2235.98 | 314.90 | =HC | −.05 | 2101.15 | 307.80 | — | |||
| PIQ | 2216.11 | 314.27 | =HC | −.11 | 2037.46 | 307.18 | — | |||
| HC | 2251.83 | 320.63 | — | 2108.74 | 313.50 | — | ||||
| Pars opercularis | RH: F(3, 378) = 3.44, P = .02 | CIQ | 4903.83 | 890.43 | — | −.41 | 4103.53 | 714.04 | =HC | −.41e |
| DIQ | 5075.91 | 875.70 | — | −.22 | 4158.74 | 702.20 | =HC | −.34 | ||
| PIQ | 5160.64 | 873.98 | — | −.13 | 4208.90 | 700.88 | =HC | −.27 | ||
| HC | 5273.59 | 891.78 | — | 4398.32 | 715.13 | — | ||||
| Pars orbitalis | LH: F(3, 378) = 4.70, P = .003; RH: F(3, 378) = 4.80, P = .003 | CIQ | 2217.42 | 304.96 | <HC | −.51e | 2734.77 | 413.95 | <HC | −.46e |
| DIQ | 2276.26 | 299.90 | <HC | −.32 | 2779.57 | 407.10 | <HC | −.36 | ||
| PIQ | 2254.05 | 299.33 | <HC | −.39 | 2758.69 | 406.25 | <HC | −.41 | ||
| HC | 2371.95 | 305.47 | — | 2926.06 | 414.59 | — | ||||
| Pars triangularis | RH: F(3, 378) = 5.56, P = .001 | CIQ | 3604.94 | 606.29 | — | 4073.36 | 776.79 | <HC and DIQ and PIQ | −.68e | |
| DIQ | 3734.62 | 596.30 | — | 4463.13 | 763.90 | =HC | −.18 | |||
| PIQ | 3679.73 | 595.07 | — | 4422.19 | 762.37 | =HC | −.23 | |||
| HC | 3867.34 | 607.31 | — | 4603.04 | 777.99 | — | ||||
| Precentral | RH: F(3, 378) = 4.61, P = .004 | CIQ | 13091.18 | 1441.99 | — | 13097.08 | 1451.04 | <HC | −.54e | |
| DIQ | 13568.81 | 1418.1 | — | 13383.43 | 1427.00 | <HC | −.35 | |||
| PIQ | 13685.61 | 1415.33 | — | 13681.55 | 1424.22 | =HC | −.14 | |||
| HC | 13776.86 | 1444.26 | — | 13886.62 | 1453.33 | — | ||||
| Rostral middle frontal | LH: F(3, 378) = 5.10, P = .002; RH: F(3, 378) = 3.96, P = .008 | CIQ | 15922.51 | 2075.34 | <HC | −.61e | 16567.98 | 2077.95 | <HC | −.55e |
| DIQ | 16637.01 | 2010.90 | =HC | −.27 | 17307.51 | 2043.50 | =HC | −.20 | ||
| PIQ | 16496.78 | 2036.97 | =HC | −.33 | 17175.91 | 2039.52 | =HC | −.26 | ||
| HC | 17183.04 | 2078.52 | — | 17721.57 | 2081.12 | — | ||||
| Superior frontal | LH: F(3, 378) = 5.01, P = .002; RH: F(3, 378) = 4.85, P = .003 | CIQ | 22766.52 | 2459.90 | <HC and DIQ | −.63e | 22033.16 | 2563.75 | <HC and DIQ | −.59e |
| DIQ | 23967.29 | 2419.20 | =HC | −.14 | 23262.33 | 2521.30 | =HC | −.11 | ||
| PIQ | 23606.27 | 2424.51 | =HC | −.29 | 22728.39 | 2516.40 | <HC | −.32 | ||
| HC | 24305.33 | 2463.70 | — | 23545.91 | 2567.76 | — | ||||
| Superior temporal | LH: F(3, 392) = 3.26, P = .009; RH: F(3, 378) = 3.54, P = .02 | CIQ | 12149.52 | 1457.95 | =HC | −.46e | 11762.27 | 1409.80 | <HC | −.48e |
| DIQ | 12563.03 | 1433.80 | =HC | −.18 | 12099.70 | 1386.50 | =HC | −.25 | ||
| PIQ | 12261.24 | 1431.05 | <HC | −.39 | 12021.85 | 1383.74 | =HC | −.30 | ||
| HC | 12822.84 | 1460.20 | — | 12444.80 | 1411.99 | — | ||||
| Supra marginal | RH: F(3, 378) = 5.12, P = .02 | CIQ | 11606.79 | 1691.13 | — | 10446.57 | 1515.01 | <HC | −.53e | |
| DIQ | 11760.31 | 1663.10 | — | 10721.11 | 1489.90 | <HC | −.35 | |||
| PIQ | 11619.21 | 1659.92 | — | 10682.73 | 1487.07 | <HC | −.38 | |||
| HC | 11856.57 | 1693.74 | — | 11254.56 | 1517.36 | — | ||||
| Frontal pole | RH: F(3, 378) = 6.97, P < .001 | CIQ | 800.39 | 163.92 | — | 1051.86 | 201.18 | <HC | −.61e | |
| DIQ | 840.99 | 161.20 | — | 1107.16 | 197.80 | <HC | −.34 | |||
| PIQ | 825.64 | 160.89 | — | 1072.84 | 197.44 | <HC | −.51 | |||
| HC | 845.13 | 164.20 | — | 1174.36 | 201.53 | — | ||||
| Temporal pole | LH: F(3, 378) = 5.52, P = .004 | CIQ | 2473.33 | 403.88 | <HC and DIQ and PIQ | −.72e | 2348.56 | 383.53 | <HC | −.53e |
| DIQ | 2673.99 | 397.20 | =HC | −.22 | 2436.54 | 377.10 | =HC | −.31 | ||
| PIQ | 2644.84 | 396.43 | =HC | −.30 | 2426.84 | 376.44 | <HC | −.33 | ||
| HC | 2736.53 | 404.48 | — | 2553.89 | 384.13 | — | ||||
Note: CIQ, compromised patients; DIQ, deteriorated schizophrenia patients; PIQ, putatively preserved schizophrenia patients; LH, left hemisphere; RH, right hemisphere.
aAll Ps thresholded at a false discovery rate of 5%.
bAll values are adjusted for age, gender, site, and ICV.
cIf a comparison is not reported, it did not survive post hoc comparison correction. =HC (healthy controls) implies no significant difference to HCs; <HC implies significant reductions relative to HCs.
d d = Cohen’s d effect sizes for patient–control comparisons.
eRepresents qualitatively larger control–patient effect sizes in compromised patients.
Subgroups Vs Controls.
All subgroups showed subcortical volumetric abnormalities, with statistically significant bilateral increases in the putamen and pallidum, and reductions in the hippocampus. Larger patient–control effect sizes for hippocampal reductions were evident in the compromised group (left d = −.86 and right d = −.77 vs all ds < −.58 for deteriorated and putatively preserved patients).
Subgroup Comparisons.
Compared to deteriorated patients, the compromised group had statistically significant reduced volumes of the hippocampus bilaterally (d = −.45 and d = −.47, respectively).
Regional Cortical Volume
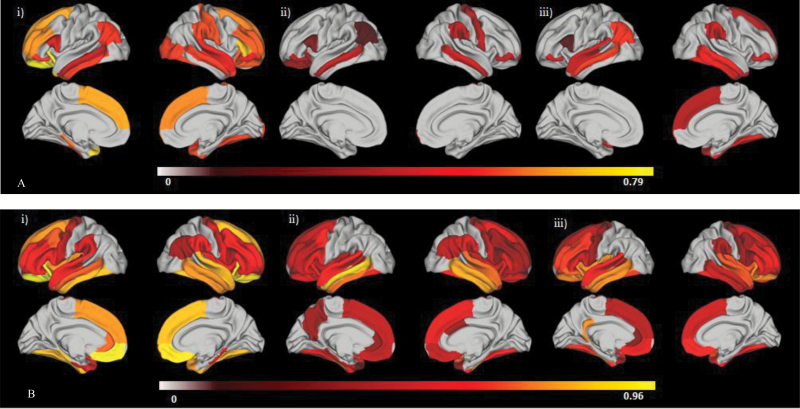
Figure 2A shows the effect size maps for comparisons of cortical volume in regions surviving FDR correction. Table 2 quantifies the regions that significantly differed in cortical volume between groups.
Fig. 2.
Patient–control effect size differences for regions surviving false discovery rate (FDR) correction. (A) Regional cortical volume; (B) regional cortical thickness. (i) Compromised cognitive subgroup (CIQ); (ii) deteriorated cognitive subgroup (DIQ); (iii) preserved cognitive subgroup (PIQ). Note that the left of each panel = left hemisphere, right = right hemisphere, top = lateral view, bottom = medial view. FDR correction at P < .05 was applied to between-groups comparisons for each modality for left and right hemispheres separately (34 comparison per hemisphere per modality). Post hoc pairwise comparisons of each region surviving the between-groups correction were also corrected. Regions that did not survive correction were assigned a value of 0. Color bar represents Cohen’s d values, with all subgroups showing reductions in volume or thickness compared to controls.
Subgroups Vs Controls.
All subgroups showed statistically significant reductions in left inferior parietal cortex, right supramarginal gyrus and frontal pole and in the middle temporal gyrus and pars orbitalis bilaterally. Both cognitively impaired subgroups showed significantly smaller volumes of the right precentral gyrus and left lateral orbitofrontal cortex not seen in the putatively preserved patients. The compromised subgroup, however, was the only group to show significant volumetric reductions relative to controls bilaterally in the rostral middle frontal region and in the left temporal pole, left inferior temporal, superior frontal, and parahippocampal gyri and the right lateral occipital and superior temporal gyri, right lateral and medial orbitofrontal cortices and right pars triangularis. The putatively Preserved group showed specific significant reductions in the left superior temporal gyrus and right inferior temporal gyrus that were not seen in the other subgroups. Larger effect size differences were seen in the compromised (d = −.39 to −.79) vs deteriorated (d = −.02 to −.40) and putatively preserved (d = −.11 to −.52) subgroups in more than half of the regions surviving correction.
Subgroup Comparisons.
Relative to both deteriorated and putatively preserved subgroups, Compromised patients showed significant reductions in the left lateral orbitofrontal cortex (d = −.48; d = −.65, respectively), parahippocampal gyrus (d = −.52; d = −.46, respectively) and temporal pole (d = −.50; d = −.43, respectively), as well as the right pars triangularis (d = −.51; d = −.45, respectively). Significant reductions in the right lateral occipital gyrus (d = −.51) and bilateral superior frontal region (left d = −.49; right d = −.48) were also seen in compromised patients relative to deteriorated patients.
Regional Cortical Thickness and Surface Area
Figure 2B shows the effect size maps for comparisons of cortical thickness for regions surviving FDR correction. Table 3 quantifies the regions that significantly differed in cortical thickness between groups.
Table 3.
Regional Cortical Thickness Group Comparisons for Regions Surviving FDR Correction
| LH | RH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | ANCOVA a | Subgroup | M b | SD | Comparison to HC c | d d | M b | SD | Comparison to HC c | d d |
| Banks of superior temporal sulcus | LH: F(3, 378) = 5.79, P = .001; RH: F(3, 378) = 3.00, P = .03 | CIQ | 2.56 | .14 | =HC | −.15 | 2.65 | .14 | =HC | 0 |
| DIQ | 2.50 | .20 | <HC | −.47 | 2.61 | .20 | =HC | −.24 | ||
| PIQ | 2.51 | .17 | <HC | −.46 | 2.60 | .17 | =HC | −.33 | ||
| HC | 2.58 | .13 | — | 2.65 | .13 | — | ||||
| Caudal anterior cingulate | RH: F(3, 378) = 3.40, P = .02 | CIQ | 2.70 | .21 | — | 2.62 | .21 | =HC | −.17 | |
| DIQ | 2.66 | .20 | — | 2.58 | .20 | <HC | −.34 | |||
| PIQ | 2.64 | .26 | — | 2.60 | .26 | =HC | −.23 | |||
| HC | 2.68 | .26 | — | 2.66 | .26 | =HC | ||||
| Caudal middle frontal | LH: F(3, 378) = 9.15, P < .001; RH: F(3, 378) = 5.77, P = .001 | CIQ | 2.57 | .14 | <HC | −.67e | 2.57 | .14 | <HC | −.59e |
| DIQ | 2.59 | .10 | <HC | −.60 | 2.61 | .10 | <HC | −.35 | ||
| PIQ | 2.59 | .09 | <HC | −.62 | 2.60 | .17 | <HC | −.33 | ||
| HC | 2.66 | .13 | — | 2.65 | .13 | — | ||||
| Entorhinal | LH: F(3, 378) = 5.39, P = .001 | CIQ | 3.26 | .34 | <HC | −.46e | 3.43 | .34 | — | |
| DIQ | 3.29 | .30 | <HC | −.40 | 3.43 | .40 | — | |||
| PIQ | 3.32 | .34 | =HC | −.30 | 3.45 | .34 | — | |||
| HC | 3.43 | .39 | — | 3.54 | .39 | — | ||||
| Fusiform | LH: F(3, 378) = 12.97, P < .001; RH: F(3, 378) = 11.60, P < .001 | CIQ | 2.60 | .14 | <HC | −.81e | 2.60 | .14 | <HC | −.81e |
| DIQ | 2.65 | .10 | <HC | −.52 | 2.64 | .10 | <HC | −.60 | ||
| PIQ | 2.63 | .17 | <HC | −.62 | 2.62 | .17 | <HC | −.59 | ||
| HC | 2.71 | .13 | — | 2.71 | .13 | — | ||||
| Inferior parietal | LH: F(3, 378) = 2.99, P = .03; RH: F(3, 378) = 4.06, P = .007 | CIQ | 2.58 | .14 | =HC | −.30 | 2.61 | .14 | <HC | −.37 |
| DIQ | 2.59 | .10 | =HC | −.26 | 2.61 | .10 | <HC | −.43 | ||
| PIQ | 2.59 | .17 | =HC | −.20 | 2.62 | .17 | =HC | −.26 | ||
| HC | 2.62 | .13 | — | 2.66 | .13 | — | ||||
| Inferior temporal | LH: F(3, 378) = 12.88, P < .001; RH: F(3, 378) = 10.45, P < .001 | CIQ | 2.82 | .14 | <HC | −.81e | 2.84 | .14 | <HC | −.74 |
| DIQ | 2.85 | .20 | <HC | −.69 | 2.85 | .10 | <HC | −.78 | ||
| PIQ | 2.82 | .17 | <HC | −.73 | 2.87 | .17 | <HC | −.46 | ||
| HC | 2.93 | .13 | — | 2.94 | .13 | — | ||||
| Isthmus cingulate | LH: F(3, 378) = 5.79, P = .001 | CIQ | 2.53 | .21 | =HC | −.23 | 2.45 | .21 | — | |
| DIQ | 2.52 | .20 | =HC | −.30 | 2.43 | .20 | — | |||
| PIQ | 2.46 | .17 | <HC | −.73 | 2.44 | .17 | — | |||
| HC | 2.57 | .13 | — | 2.49 | .13 | — | ||||
| Lateral orbitofrontal | LH: F(3, 378) = 17.28, P < .001; RH: F(3, 378) = 15.51, P = .001 | CIQ | 2.60 | .14 | <HC | −1.11e | 2.64 | .14 | <HC | −.89e |
| DIQ | 2.65 | .20 | <HC | −.59 | 2.67 | .20 | <HC | −.53 | ||
| PIQ | 2.64 | .17 | <HC | −.73 | 2.65 | .17 | <HC | −.73 | ||
| HC | 2.75 | .13 | — | 2.76 | .13 | — | ||||
| Lingual | RH: F(3, 378) = 3.14, P = .03 | CIQ | 1.98 | .14 | — | 2.02 | .14 | =HC | −.37 | |
| DIQ | 1.99 | .10 | — | 2.05 | .10 | =HC | −.17 | |||
| PIQ | 1.98 | .09 | — | 2.03 | .09 | =HC | −.66 | |||
| HC | 2.01 | .13 | — | 2.07 | .13 | =HC | ||||
| Medial orbitofrontal | LH: F(3, 378) = 9.52, P < .001; RH: F(3, 378) = 11.50, P < .001 | CIQ | 2.53 | .14 | <HC | −.96e | 2.50 | .14 | <HC | −.89e |
| DIQ | 2.59 | .20 | <HC | −.42 | 2.55 | .20 | <HC | −.42 | ||
| PIQ | 2.58 | .17 | <HC | −.53 | 2.53 | .17 | <HC | −.59 | ||
| HC | 2.66 | .13 | — | 2.62 | .13 | — | ||||
| Middle temporal | LH: F(3, 378) = 13.14, P < .001 | CIQ | 2.92 | .14 | <HC | −.67 | 2.95 | .14 | <HC | −.74 |
| DIQ | 2.91 | .10 | <HC | −.86 | 2.96 | .10 | <HC | −.77 | ||
| PIQ | 2.90 | .17 | <HC | −.73 | 2.98 | .17 | <HC | −.46 | ||
| HC | 3.01 | .13 | — | 3.05 | .13 | — | ||||
| Parahippocampal | LH: F(3, 378) = 7.71, P < .001 RH: F(3, 378) = 11.21, P < .001 | CIQ | 2.46 | .34 | <HC and DIQ and PIQ | −.83e | 2.53 | .27 | <HC | −.49e |
| DIQ | 2.61 | .30 | <HC | −.36 | 2.59 | .30 | =HC | −.25 | ||
| PIQ | 2.64 | .34 | =HC | −.23 | 2.60 | .26 | =HC | −.23 | ||
| HC | 2.71 | .26 | — | 2.66 | .26 | — | ||||
| Pars opercularis | LH: F(3, 378) = 6.42, P < .001; RH: F(3, 378) = 13.80, P < .001 | CIQ | 2.58 | .14 | <HC | −.59e | 2.59 | .14 | <HC | −.34e |
| DIQ | 2.60 | .10 | <HC | −.52 | 2.61 | .10 | <HC | −.25 | ||
| PIQ | 2.59 | .17 | <HC | −.46 | 2.60 | .17 | <HC | −.27 | ||
| HC | 2.66 | .13 | — | 2.70 | .13 | — | ||||
| Pars orbitalis | LH: F(3, 378) = 13.81, P < .001 RH: F(3, 378) = 7.90, P < .001 | CIQ | 2.74 | .21 | <HC | −.59 | 2.78 | .21 | <HC | −.51e |
| DIQ | 2.77 | .20 | <HC | −.47 | 2.81 | .20 | <HC | −.39 | ||
| PIQ | 2.73 | .17 | <HC | −.68 | 2.80 | .17 | <HC | −.46 | ||
| HC | 2.88 | .26 | — | 2.90 | .26 | — | ||||
| Pars triangularis | LH: F(3, 378) = 8.72, P < .001 RH: F(3, 378) = 8.76, P < .001 | CIQ | 2.53 | .14 | <HC | −.59e | 2.55 | .14 | <HC | −.59 |
| DIQ | 2.52 | .20 | <HC | −.53 | 2.57 | .10 | <HC | −.52 | ||
| PIQ | 2.53 | .17 | <HC | −.53 | 2.54 | .17 | <HC | −.59 | ||
| HC | 2.61 | .13 | — | 2.63 | .13 | — | ||||
| Posterior cingulate | LH: F(3, 378) = 3.41, P = .02 | CIQ | 2.57 | .14 | =HC | −.37e | 2.53 | .14 | — | |
| DIQ | 2.57 | .20 | =HC | −.30 | 2.53 | .10 | — | |||
| PIQ | 2.57 | .17 | =HC | −.33 | 2.54 | .17 | — | |||
| HC | 2.62 | .13 | — | 2.56 | .13 | — | ||||
| Precentral | LH: F(3, 378) = 4.28, P = .005; RH: F(3, 378) = 4.88, P = .002 | CIQ | 2.46 | .14 | <HC | −.44e | 2.45 | .14 | <HC | −.52e |
| DIQ | 2.47 | .10 | <HC | −.43 | 2.47 | .10 | <HC | −.43 | ||
| PIQ | 2.47 | .17 | <HC | −.33 | 2.47 | .17 | <HC | −.33 | ||
| HC | 2.52 | .13 | — | 2.52 | .13 | — | ||||
| Precuneus | LH: F(3, 378) = 3.67, P = .01 | CIQ | 2.41 | .14 | =HC | −.44e | 2.41 | .14 | — | |
| DIQ | 2.43 | .10 | <HC | −.34 | 2.43 | .10 | — | |||
| PIQ | 2.46 | .09 | =HC | −.09 | 2.45 | .09 | — | |||
| HC | 2.47 | .13 | — | 2.46 | .13 | — | ||||
| Rostral anterior cingulate | LH: F(3, 378) = 6.65, P < .001 | CIQ | 2.83 | .21 | <HC and DIQ and PIQ | −.68e | 2.96 | .21 | — | |
| DIQ | 2.92 | .20 | <HC | −.30 | 2.97 | .20 | — | |||
| PIQ | 2.92 | .26 | <HC | −.27 | 3.01 | .17 | — | |||
| HC | 2.99 | .26 | — | 3.02 | .26 | — | ||||
| Rostral middle frontal | LH: F(3, 378) = 7.36, P < .001; RH: F(3, 378) = 7.42, P < .001 | CIQ | 2.47 | .14 | <HC | −.59 | 2.48 | .14 | <HC | −.59e |
| DIQ | 2.50 | .10 | <HC | −.43 | 2.52 | .10 | <HC | −.34 | ||
| PIQ | 2.48 | .09 | <HC | −.63 | 2.50 | .09 | <HC | −.54 | ||
| HC | 2.55 | .13 | — | 2.56 | .13 | — | ||||
| Superior frontal | LH: F(3, 378) = 5.86, P < .001; RH: F(3, 378) = 9.44, P < .001 | CIQ | 2.77 | .14 | <HC | −.74e | 2.76 | .14 | <HC | −.81e |
| DIQ | 2.82 | .10 | <HC | −.43 | 2.81 | .10 | <HC | −.52 | ||
| PIQ | 2.81 | .17 | <HC | −.40 | 2.80 | .17 | <HC | −.46 | ||
| HC | 2.87 | .13 | — | 2.87 | .13 | — | ||||
| Superior temporal | LH: F(3, 378) = 8.97, P < .001; RH: F(3, 378) = 14.82, P < .001 | CIQ | 2.77 | .14 | <HC | −.59 | 2.79 | .14 | <HC | −.81e |
| DIQ | 2.78 | .10 | <HC | −.60 | 2.82 | .10 | <HC | −.69 | ||
| PIQ | 2.76 | .17 | <HC | −.59 | 2.80 | .17 | <HC | −.66 | ||
| HC | 2.85 | .13 | — | 2.90 | .13 | — | ||||
| Supramarginal | LH: F(3, 378) = 3.65, P = .01 RH: F(3, 378) = 7.06 P <.001 | CIQ | 2.61 | .14 | <HC | −.52e | 2.61 | .14 | <HC | −.59e |
| DIQ | 2.64 | .10 | =HC | −.34 | 2.63 | .10 | <HC | −.52 | ||
| PIQ | 2.65 | .17 | =HC | −.20 | 2.63 | .17 | <HC | −.40 | ||
| HC | 2.68 | .13 | — | 2.69 | .13 | — | ||||
| Frontal pole | RH: F(3, 378) = 4.56, P = .004 | CIQ | 2.90 | .27 | — | 2.84 | .27 | <HC | −.42 | |
| DIQ | 2.96 | .30 | — | 2.87 | .30 | =HC | −.28 | |||
| PIQ | 2.88 | .26 | — | 2.83 | .26 | <HC | −.46 | |||
| HC | 2.96 | .26 | — | 2.95 | .26 | |||||
| Temporal pole | LH: F(3, 378) = 5.28, P = .001; RH: F(3, 378) = 7.85, P < .001 | CIQ | 3.55 | .34 | <HC | −.52 | 3.66e | .34 | <HC and DIQ | −.73e |
| DIQ | 3.68 | .30 | <HC | −.17 | 3.79 | .30 | <HC | −.32 | ||
| PIQ | 3.61 | .34 | <HC | −.36 | 3.74 | .34 | <HC | −.46 | ||
| HC | 3.74 | .39 | — | 3.88 | .26 | — | ||||
| Transverse temporal | RH: F(3, 378) = 4.33, P = .005 | CIQ | 2.41 | .21 | — | −.13 | 2.43 | .21 | =HC | −.25 |
| DIQ | 2.38 | .20 | — | −.26 | 2.42 | .20 | <HC | −.30 | ||
| PIQ | 2.38 | .17 | — | −.27 | 2.39 | .17 | <HC | .46 | ||
| HC | 2.44 | .26 | — | 2.49 | .26 | — | ||||
| Insula | LH: F(3, 378) = 12.28, P < .001; RH: F(3, 378) = 8.86, P < .001 | CIQ | 3.03 | .14 | <HC | −.74 | 3.00 | .21 | <HC | −.57 |
| DIQ | 3.06 | .20 | <HC | −.42 | 3.02 | .20 | <HC | −.47 | ||
| PIQ | 3.02 | .17 | <HC | −.73 | 3.00 | .17 | <HC | −.66 | ||
| HC | 3.13 | .13 | — | 3.10 | .13 | — | ||||
Note: CIQ, compromised patients; DIQ, deteriorated schizophrenia patients; PIQ, putatively preserved schizophrenia patients; LH, left hemisphere; RH, right hemisphere.
aAll Ps thresholded at a false discovery rate of 5%.
bAll values are adjusted for age, gender, site.
cIf a comparison is not reported, it did not survive post hoc comparison correction. =HC (healthy controls) implies no significant difference to HCs; <HC implies significant reductions relative to HCs.
d d = Cohen’s d effect sizes for patient–control comparisons.
eRepresents qualitatively larger control–patient effect sizes in compromised patients.
Subgroups Vs Controls.
No surface area abnormalities were evident in any of the subgroups. A significantly thinner cortex was evident for all subgroups in the left rostral anterior cingulate and right supramarginal gyrus, as well as regions of the lateral and medial orbitofrontal cortex, inferior frontal, caudal and rostral middle frontal, superior frontal, precentral, inferior temporal, middle temporal and fusiform gyri, and the superior temporal, temporal pole and insula bilaterally. Significant thickness reductions in the right parahippocampal and left supramarginal gyri were evident in compromised patients only. On the other hand, thickness reductions in the right caudal anterior cingulate and left precuneus was evident only in the deteriorated patients, while reductions in the left isthmus of the cingulate was seen only in the putatively preserved patients and not in other groups. Both compromised and deteriorated patients had significantly thinner right inferior parietal cortex, left parahippocampal gyrus and left entorhinal cortex that was not evident in the putatively preserved group. The deteriorated and putatively preserved groups showed significant reductions in the left bank of the superior temporal sulcus and the right transverse temporal cortex not seen in the compromised patients. Greater effect size differences were seen in the compromised patients (range of d = 0.1 to −1.11) vs deteriorated (range of d = −.24 to −.77) and putatively preserved patients (range of d = −.11 to −.52) in half of the regions surviving correction.
Subgroup Comparisons.
Relative to deteriorated and putatively preserved patients, compromised patients had significantly thinner cortex in the left rostral anterior cingulate (d = −.44; d = −.36, respectively) and the left parahippocampal gyrus (d = −.47; d = −.83, respectively). Significantly thinner cortex was also seen in the right temporal pole in the compromised group relative to deteriorated patients (d = −.41).
Discussion
In this study, we sought to determine the relationship between cognitive functioning and brain structure in schizophrenia/schizoaffective disorder, by examining whether structural abnormalities could differentiate 3 cognitive subgroups manifesting putatively differing cognitive trajectories of the disorder.6,8 While reduced ICV in compromised patients was found, it was of a small magnitude, did not reach statistical significance and failed to support our hypothesis. Rather, we found evidence for reduced TBV (adjusting for ICV) in all subgroups compared with controls. It has been argued that ICV and TBV are markers of neurodevelopment and neurodegeneration, respectively.9 This is due to the similar rate of increase in both measures prior to the teenage years, where, after reaching a critical point, there is a divergence in trajectory as represented by a decline in TBV in the context of relative stability in ICV over time.25 Recent work9,13 shows that ICV reductions are present in psychosis patients with cognitive impairments presumed to originate prior to illness onset (indicating hypoplasia), whereas TBV reductions are evident in patients with cognitive impairments presumed to originate after illness onset (indicating atrophy). Our results did not clearly support these findings and our index of abnormal brain development (ICV) did not map onto our index of abnormal cognitive development (patients with premorbid IQ deficits). Instead, all of our cognitive subgroups appeared to be susceptible to some brain atrophy. Thus, hypoplasia, and subsequently, the notion of a static encephalopathy in compromised patients do not appear to be well-supported here. The discrepancy between our results and previous work is not likely to be explained by differences in the measures used to assess ICV and TBV, given that we re-ran our analyses using voxel-based morphometry generated values calculated with the same method as Woodward and Heckers, and the findings did not change (supplementary table 2). However, there are other differences between ours and the previous studies that may account for these discrepant results. For example, disparate cognitive tests and clustering methods may have yielded different boundaries for defining cognitive subgroups; the studies of both Woodward and Heckers9 and Czepielewski et al13 used composite scores from cognitive batteries tapping several broad cognitive domains to group patients into cognitively impaired or unimpaired samples based on whether premorbid IQ or the discrepancy between premorbid and current cognition was above or below the 10th percentile of the control distribution. In contrast, our subgroups were generated via a data-driven statistical clustering method based on performance on 3 separate memory and attention-indexing cognitive tests as well as premorbid IQ score, which resulted in 3 groups initially. As a result, the proportion of patients classified into the putatively preserved, deteriorated, and compromised subgroups in our cohort was less evenly distributed than that of the other samples, respectively, Woodward and Heckers reported distributions of n = 41, 52, 38 and Czepielewski et al reported distributions of n = 25, 31, 36. In contrast our distributions were n = 73, 100, and 47. While the proportion of putatively preserved patients was similar across studies (33% vs 31% and 27%) and therefore seemingly representative of the schizophrenia/schizoaffective population, our classification resulted in a lesser number of patients being classified as compromised (21% vs 29% and 39%). This suggests that our cohort was either a cognitively higher functioning cohort in general, or a cohort whose current cognitive impairments were not tapped to the same extent as the other studies.
Given the lack of statistical evidence for ICV reductions in any of the subgroups in our data, it is perhaps not surprising that there was also an absence of differences in surface area between groups. Surface area is influenced by the number of ontogenetic cortical columns orientated perpendicular to the brain’s surface; which are established during early fetal development through the migration of neurons from the ventricular zone to their columnar location.26,27 Surface area therefore has early neurodevelopmental relevance, which does not appear to be of substantial influence on our data. Rather, maturational and/or adult neurodegenerative processes may be more significant contributors to the neural tissue underpinnings of all cognitive subgroups represented in our sample.
Indeed, cortical thickness and volume in schizophrenia are known to be susceptible to accelerated aging and are associated with cognitive decline in neurodegenerative diseases.22,28–31 In all of our subgroups, we saw global and regional volume and thickness abnormalities. While the volume abnormalities in the deteriorated group were less widespread than anticipated on the basis of a recently published study,5 as expected, the pattern of both volume and thickness reductions was qualitatively and quantitatively most pronounced in compromised patients in neural tissue across all 4 lobes, particularly in the orbital, inferior (pars triangularis) and superior frontal, temporal (parahippocampus, temporal pole) and occipital regions (volume) as well as the left parahippocampus and rostral anterior cingulate (thickness). The compromised group also had smaller volumes and thickness in a number of regions not evident in the other groups; including in the left superior and middle frontal areas, left anterior and inferior temporal areas and right lateral medial and inferior frontal, occipital and superior temporal areas (volume), as well as the right parahippocampal and left supramarginal gyri (thickness).
The pattern of more prominent structural abnormalities in the subgroup with the most marked cognitive impairments—both currently and prior to illness onset, is consistent with the concept of schizophrenia as a progressive neurodevelopmental disorder.32 It also fits with the concept and implications of variability in cognitive reserve; which describes the protective effect that higher premorbid intellect (reserve) has against age/illness-related degeneration of neural and cognitive processes. Proxies of cognitive reserve include performance on crystallized intelligence tests such as the WTAR, which tap into intellectual functions theoretically immune to age/illness-related decline. Lower performance on such tests, and thus, lower cognitive reserve, is thought to confer greater liability for cognitive and brain degeneration because the extent to which compensatory mechanisms (associated with pre-existing cognitive processes) can be enlisted to cope with age/illness-related pathology is reduced.33,34 In healthy adults, reduced cognitive reserve has been linked to exaggerated brain structural abnormalities, as well as reduced protection against the detrimental effects of these abnormalities on cognitive performance.35 Since below-average premorbid IQ in the compromised group represents a marker of poor premorbid cognitive reserve capacity, it is not surprising that this group demonstrated the most pronounced brain abnormalities in several regions including the hippocampal complex; an area of reduced neurogenesis36 and that is known to be at increased risk of illness and age-related decline and degeneration in healthy, psychiatric, and neurological disorder groups.37–39
Critically, an indirect association between cognitive impairment and brain structural abnormalities was evident in our data, as both cognitively impaired patient groups did show specific volume and thickness reductions in circumscribed areas that were not seen in the putatively preserved patients. However, our preserved patients also had structural deficits in several other regions including in the superior frontal gyrus, superior inferior temporal gyri and inferior parietal lobule that were unexpected, since previous studies5,9,13 reported reduced TBV and/or total gray and cortex volume in preserved patients but not sizeable localized gray matter volume reductions. The inconsistencies in results may relate to differences in the mean age of the preserved samples across studies; our putatively preserved group was somewhat older than the previous studies, and its illness duration was almost double that reported by Woodward and Heckers. Thus, a potentially greater impact of age and/or schizophrenia-related tissue decline in the putatively preserved patients in our data may partially explain the present findings. Given that age was significantly associated with brain structural deficits (most global and local thickness, surface area, and volume estimates) in the current study (data not shown), this remains a strong possibility.
Another important consideration relates to whether the preserved patients assessed here are in fact, truly “preserved.” This may not necessarily be the case, since recent work shows that the association between intellectual functioning and the risk for schizophrenia is strongly predicted by the extent to which patients deviate from their familial cognitive aptitude, rather than their observed cognitive achievement.40 Further, evidence indicating that neuropsychologically “normal” schizophrenia patients perform worse on cognitive tests than their unaffected monozygotic twins suggests that such patients deviate from what would be expected of their performance based upon genetic predisposition.41 When framed in this context it is plausible that the structural brain abnormalities observed in the putatively preserved group here, represent the neural underpinnings of subtle cognitive impairments or a pathology-related cognitive decline that is not adequately captured by the current experimental design. Further work is required to explore this notion further. Nonetheless, it may explain the thinning and volume reductions evident in both cognitively impaired and unimpaired patients.
Our findings should be considered in the context of a number of limitations. Firstly, given limited medication data available in the ASRB (including dosing information), we were unable to adequately assess the effects of medication in the sample. However, previous work in this cohort has shown that antipsychotic medication is not correlated to cortical volumes.31 Secondly, exclusion criteria in the ASRB precluded recruitment of patients with estimated premorbid IQs of less than 75. It is possible that even more pronounced differences would have been evident, including in ICV, had more severely premorbidly intellectually impaired patients been included in the analysis. Finally, distinctions between compromised and deteriorated patients are dependent on the assumption that differences between premorbid estimates and current cognition estimates represent actual cognitive decline over time. Despite evidence verifying the WTAR as a representative measure of premorbid IQ,42 the cross-sectional nature of our study limits the extent to which this assumption can be established.
Despite this, our study is the largest of its kind to examine structural brain–cognition relationships through cognitive subgroups in schizophrenia/schizoaffective disorder, in the context of putative neurodevelopmental and neurodegenerative influences. Although our findings suggest that cortical volume and thickness reductions are present in all cognitive subgroups, the overall pattern of findings does appear to have some relevance in distinguishing them; with compromised patients demonstrating greater abnormalities in specific regions and potentially representing the manifestation of a greater impact of neurodegenerative processes. As no correlations were evident between negative symptom severity and any imaging measure, these brain structural differences are unlikely to be a simple reflection of differences in the severity of illness. Further work is needed to determine the extent to which these results replicate using similar cognitive batteries and in similar sized samples.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
Dr Van Rheenen was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1088785). Dr Cropley was supported by an NHMRC Early Career Fellowship (628880), a Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (21660), and a University of Melbourne Faculty of Medicine, Dentistry, and Health Sciences Research Fellowship. Dr Zalesky was supported by an NHMRC Fellowship (1047648). Dr Bousman was supported by a University of Melbourne Research Fellowship and a NARSAD Young Investigator Award (20526). Dr Pereira was supported by the One-in-Five Association and the AMP Foundation. Prof Shannon Weickert was supported by an NHMRC Principal Research Fellowship (1117079), the NSW Ministry of Health, Office of Health and Medical Research, the University of New South Wales, and Neuroscience Research Australia. Dr Pantelis was supported by an NHMRC Senior Principal Research Fellowship (628386 and 1105825) and by a NARSAD Distinguished Investigator Award.
Supplementary Material
Acknowledgments
Data for this study were provided by the Australian Schizophrenia Research Bank (ASRB), which is supported by the NHMRC (enabling grant 386500), the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, and the Schizophrenia Research Institute. The authors thank the chief investigators and manager of the ASRB: Carr V, Schall U, Scott R, Jablensky A, Mowry B, Michie P, Catts S, Henskens F, Pantelis C, and Loughland C. None of the funding sources played any role in the study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. Prof Sundram has received consulting fees, advisory board fees, research support, speakers honoraria, or travel support from AstraZeneca, the Australian National Health and Medical Research Council, the Australian Department of Immigration and Border Protection, Bristol-Myers Squibb, Eli Lilly, the Flack Trust, GlaxoSmithKline, Lundbeck, the One-in-Five Association, Otsuka, Pfizer, Roche, and the United Nations High Commissioner for Refugees. Prof Shannon Weickert is on an advisory board for and has received advisory board fees from Lundbeck, Australia Pty Ltd and in collaboration with Astellas Pharma, Inc., Japan. Over the last 4 years, Prof Pantelis has been on advisory boards for AstraZeneca, Janssen-Cilag, Lundbeck, and Servier; and he has received honoraria for talks presented at educational meetings organized by AstraZeneca, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer, and Shire. Dr Van Rheenen receives grant funding unrelated to the current paper from the Jack Brockhoff Foundation, University of Melbourne, Barbara Dicker Brain Sciences Foundation, Rebecca L Cooper Foundation, and the Society of Mental Health Research (SMHR Australia). The other authors report no financial relationships with commercial interests.
References
- 1. Palmer BW, Heaton RK, Paulsen JS et al. . Is it possible to be schizophrenic yet neuropsychologically normal?Neuropsychology. 1997;11:437–446. [DOI] [PubMed] [Google Scholar]
- 2. Reichenberg A, Harvey PD, Bowie CR et al. . Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewandowski KE, Sperry SH, Cohen BM, Ongür D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med. 2014;44:3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reser MP, Allott KA, Killackey E, Farhall J, Cotton SM. Exploring cognitive heterogeneity in first-episode psychosis: what cluster analysis can reveal. Psychiatry Res. 2015;229:819–827. [DOI] [PubMed] [Google Scholar]
- 5. Weinberg D, Lenroot R, Jacomb I et al. . Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2015;73:1251–1259. [DOI] [PubMed] [Google Scholar]
- 6. Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. [DOI] [PubMed] [Google Scholar]
- 7. Van Rheenen T, Lewandowski K, Tan E et al. . Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med. 2017;47:1848–1864. [DOI] [PubMed] [Google Scholar]
- 8. Wells R, Swaminathan V, Sundram S et al. . The impact of premorbid and current intellect in schizophrenia: cognitive, symptom, and functional outcomes. NPJ Schizophr. 2015;1:15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodward ND, Heckers S. Brain structure in neuropsychologically defined subgroups of schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2015;41:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson HE, Pantelis C, Carruthers K, Speller J, Baxendale S, Barnes TR. Cognitive functioning and symptomatology in chronic schizophrenia. Psychol Med. 1990;20:357–365. [DOI] [PubMed] [Google Scholar]
- 11. Nelson HE, O’Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. [DOI] [PubMed] [Google Scholar]
- 12. Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. [DOI] [PubMed] [Google Scholar]
- 13. Czepielewski LS, Wang L, Gama CS, Barch DM. The relationship of intellectual functioning and cognitive performance to brain structure in schizophrenia. Schizophr Bull. 2017;43:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantelis C, Yücel M, Wood SJ et al. . Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. [DOI] [PubMed] [Google Scholar]
- 15. Striedter GF, Srinivasan S, Monuki ES. Cortical folding: when, where, how, and why?Annu Rev Neurosci. 2015;38:291–307. [DOI] [PubMed] [Google Scholar]
- 16. Budday S, Steinmann P, Kuhl E. Physical biology of human brain development. Front Cell Neurosci. 2015;9:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mota B, Herculano-Houzel S. Brain structure. Cortical folding scales universally with surface area and thickness, not number of neurons. Science. 2015;349:74–77. [DOI] [PubMed] [Google Scholar]
- 18. Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 2014;34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winkler AM, Kochunov P, Blangero J et al. . Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panizzon MS, Fennema-Notestine C, Eyler LT et al. . Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal changes in white matter tract integrity across the adult lifespan and its relation to cortical thinning. PLoS One. 2016;11:e0156770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubota M, van Haren NE, Haijma SV et al. . Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72:803–812. [DOI] [PubMed] [Google Scholar]
- 23. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 24. Fischl B, Salat DH, Busa E et al. . Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 25. Courchesne E, Chisum HJ, Townsend J et al. . Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. [DOI] [PubMed] [Google Scholar]
- 26. Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. [DOI] [PubMed] [Google Scholar]
- 27. Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 (Pt 4):701–722. [DOI] [PubMed] [Google Scholar]
- 28. Velayudhan L, Proitsi P, Westman E et al. ; dNeuroMed Consortium Entorhinal cortex thickness predicts cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2013;33:755–766. [DOI] [PubMed] [Google Scholar]
- 29. Pereira JB, Svenningsson P, Weintraub D et al. . Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology. 2014;82:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am J Psychiatry. 2016;173:607–616. [DOI] [PubMed] [Google Scholar]
- 31. Cropley VL, Klauser P, Lenroot R et al. . Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. In Press. [DOI] [PubMed] [Google Scholar]
- 32. Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. [DOI] [PubMed] [Google Scholar]
- 33. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferreira D, Bartrés-Faz D, Nygren L et al. . Different reserve proxies confer overlapping and unique endurance to cortical thinning in healthy middle-aged adults. Behav Brain Res. 2016;311:375–383. [DOI] [PubMed] [Google Scholar]
- 36. Allen KM, Fung SJ, Shannon Weickert C. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust N Z J Psychiatry. 2016;50:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sumowski JF, Rocca MA, Leavitt VM et al. . Searching for the neural basis of reserve against memory decline: intellectual enrichment linked to larger hippocampal volume in multiple sclerosis. Eur J Neurol. 2016;23:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–274. [DOI] [PubMed] [Google Scholar]
- 39. Velakoulis D, Wood SJ, Wong MT et al. . Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 40. Kendler KS, Ohlsson H, Mezuk B, Sundquist JO, Sundquist K. Observed cognitive performance and deviation from familial cognitive aptitude at age 16 years and ages 18 to 20 years and risk for schizophrenia and bipolar illness in a Swedish national sample. JAMA Psychiatry. 2016;73:465–471. [DOI] [PubMed] [Google Scholar]
- 41. Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990;47:1066–1072. [DOI] [PubMed] [Google Scholar]
- 42. Dykiert D, Deary IJ. Retrospective validation of WTAR and NART scores as estimators of prior cognitive ability using the Lothian Birth Cohort 1936. Psychol Assess. 2013;25:1361–1366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.