Abstract
Although a number of studies examined recollection and familiarity memory in schizophrenia, most of studies have focused on nonsocial episodic memory. Little is known about how schizophrenia patients remember social information in everyday life and whether social episodic memory changes over the course of illness. This study aims to examine episodic memory for dynamic social interaction with multimodal social stimuli in schizophrenia across phase of illness. Within each phase of illness, probands and demographically matched controls participated: 51 probands at clinical high risk (CHR) for psychosis and 36 controls, 80 first-episode schizophrenia patients and 49 controls, and 50 chronic schizophrenia patients and 39 controls. The participants completed the Social Remember-Know Paradigm that assessed overall social episodic memory, social recollection and familiarity memory, and social context memory, in addition to social cognitive measures and measures on community functioning. Probands showed impairment for recollection but not in familiarity memory and this pattern was similar across phase of illness. In contrast, impaired social context memory was observed in the first-episode and chronic schizophrenia samples, but not in CHR samples. Social context memory was associated with community functioning only in the chronic sample. These findings suggest that an impaired recollection could be a vulnerability marker for schizophrenia whereas impaired social context memory could be a disease-related marker. Further, a pattern of impaired recollection with intact familiarity memory for social stimuli suggests that schizophrenia patients may have a different pattern of impaired episodic memory for social vs nonsocial stimuli.
Keywords: social episodic memory, recollection, familiarity, social context, schizophrenia, phase of illness
Introduction
The way we remember others is crucial for social functioning. When we encounter someone on the street, we easily recall vivid details of a previous encounter with that person, but sometimes only a vague feeling that he/she looks familiar without recollecting specific details. The extent to which we remember details of previous encounters influences future interactions. Despite extensive work on memory in schizophrenia, little is known about how schizophrenia patients remember dynamic social interactions in everyday life across the phases of illness.
The subjective experience of memory can be divided into recollection and familiarity.1,2 Recollection involves episodic memory, and is accompanied by contextual information or other details that were part of the encoding event. Familiarity involves a feeling that a stimulus is familiar without conscious recollection of the event in which the stimulus was previously encountered. Recollection and familiarity memory are functionally separable,3 although it remains unclear whether they are supported by 2 distinct neural systems (eg, hippocampus for recollection; perirhinal cortex for familiarity),4–6 or represent different levels of memory strength depending on a single neural system.7,8
A number of studies have examined recollection and familiarity memory in schizophrenia. A meta-analytic review9 found medium to large effect sizes between patients and controls for recollection, and small to medium effect sizes for familiarity. In other words, schizophrenia patients show impairment on both types of memory, but the levels of impairment was greater for recollection memory than familiarity memory. This meta-analysis highlights a critical limitation in the literature: most studies have focused on nonsocial stimuli and were conducted with chronic schizophrenia patients.
Little is known about episodic memory for social information in schizophrenia. Recent evidence indicates that social information processing is particularly disrupted in schizophrenia.10 For example, healthy controls showed better memory for social stimuli than nonsocial stimuli and this beneficial effect of social information was not present in schizophrenia.11 Schizophrenia patients show disproportionate impairment on social cognitive tasks relative to nonsocial cognitive tasks.12,13 Thus, schizophrenia patients may show different patterns of recollection and familiarity for social vs nonsocial information. However, only 2 studies examined recollection and familiarity memory for social information in schizophrenia, and they showed discrepant findings. One study found that patients showed larger impairment in familiarity than recollection,14 and the other study found the opposite.15 Further, both studies employed relatively simple stimuli (ie, static face stimuli) that do not capture the richness of social stimuli people encounter in everyday life.
Moreover, while overall memory impairment is shown to be present across phase of illness,16,17 few studies have examined how recollection and familiarity memory change over the course of illness. With nonsocial stimuli, one study showed that both individuals who were at high risk for schizophrenia and patients with recent psychotic episodes presented impaired recollection but intact familiarity memory compared to healthy controls.18 Thus, it remains to be determined whether recollection and familiarity memory for social stimuli is associated with a similar pattern of impairment across phase of illness.
This study examined how schizophrenia patients remember dynamic social interaction and whether memory for dynamic social interaction changes across phase of illness. To do so, with a cross-sectional design, we recruited 3 clinical samples at different phases of illness (ie, patients in first-episode and chronic phase and participants who are at clinical high risk [CHR] for psychosis, referred to as probands hereinafter) and demographically matched controls. We employed a novel Social Remember-Know (R-K) paradigm using dynamic, multi-modal social stimuli, and examined the following research questions: (1) do probands show impaired social recognition memory and, if so, do deficits occur in both recollection and familiarity memory and are deficits comparable across phase of illness? (2) Do probands show impairment in identifying social context for specific social interactions, and are deficits comparable across phase of illness? and (3) Is performance on the Social R-K paradigm related to performance on social cognitive tasks and daily functioning?
Methods
Participants
Participants were recruited through the University of California Los Angeles (UCLA) Center for Neurocognition and Emotion in Schizophrenia. Within each phase of illness, probands and controls were demographically matched: 51 probands at CHR for psychosis and 36 controls, 80 first-episode schizophrenia patients and 49 controls, and 50 chronic schizophrenia patients and 39 controls. Psychiatric diagnoses were established with the Structural Interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV) (SCID)19 and SCID-II.20 Participants were 18 and 35 years of age for the CHR samples and between 18 and 60 years of age for the first-episode and chronic samples. All participants had no significant neurological disorder, head injury, or other physical disorder that could affect brain functioning, no intellectual disability (ie, estimated IQ greater than 70), and sufficient fluency in English to understand the study procedure.
Proband Samples.
Probands in the CHR sample were included if they (1) did not meet DSM-IV criteria for schizophrenia or schizoaffective disorder, and (2) met criteria for 1 of 3 possible definitions of a prodromal syndrome, as assessed by the Structured Interview for Prodromal Symptoms (SIPS).21 The first one, Genetic Risk and Deterioration, is defined as having a family history of schizophrenia (ie, first-degree relative), or meeting criteria for schizotypal personality disorder, in addition to a decline of at least 30% of general functioning in the past 12 months, as assessed by the Global Assessment of Functioning.22 The second one, the attenuated positive symptom state, is defined as having 1 or more of the following symptoms, rated as at least moderate but less than psychotic in intensity on the SIPS and beginning or worsening to the current level of intensity in the past 12 months: unusual thought content/delusional ideas, suspiciousness/persecutory ideas, grandiosity, perceptual abnormalities/distortions, and/or conceptual disorganization. The third one, the Brief Intermittent Psychotic Symptoms state, is defined as having 1 or more of the following symptoms, rated as at least moderate in severity, present intermittently for periods of hours or days but less than 1 month: hallucination (auditory, visual, tactile, etc.), delusions (thought broadcasting, thought insertion, paranoia, grandeur, etc.), and formal thought disorder (loosening of association, flight of ideas, etc.).
Probands in the first-episode sample were included if they (1) met a diagnosis by DSM-IV of schizophrenia, schizoaffective disorder, mainly depressed type, or schizophreniform disorder, (2) did not have significant alcohol or substance use disorder within the 6 months prior to the first episode, (3) lived within the commuting distance of the UCLA Aftercare Research Program, and (4) did not have an inadequate response to an adequate previous trial of oral or long-acting injectable risperidone. Probands in the chronic sample were included if they (1) were a previous participant at the UCLA Aftercare Research program as a first-episode patient, (2) have a diagnosis of DSM-IV schizophrenia, or schizoaffective disorder, mainly depressed type, and (3) were clinically stable, as indicated by no antipsychotic medication changes in the month prior to testing. The mean duration of illness of probands in the chronic sample was 10.1 years (SD = 6.5 y, range = 4.9–32.2 y).
Control Samples.
The same inclusion criteria were used for controls in the CHR, first-episode and chronic samples. Controls were included if they: (1) did not have a history of any Axis I Psychotic Disorders, (2) did not have a family history of psychotic disorder among first-degree relatives, (3) did not meet criteria for any of the 3 prodromal syndromes defined above, (4) did not have a major depressive disorder that is current, recurrent, or a single episode lasting longer than 1 year, and (5) did not have a diagnosis of bipolar disorder, obsessive-compulsive disorder, attention deficit disorder, posttraumatic stress disorder, or any of schizophrenia-spectrum Axis II disorders (ie, paranoid, schizotypal, schizoid, and/or avoidant personality disorder), and (6) did not have lifetime alcohol/substance dependence or alcohol/substance abuse in the past 6 months.
Clinical symptoms of the probands were assessed using the Brief Psychiatric Rating Scale (BPRS).23 To assess community functioning, we employed the Global Functioning Scale: Role (GFSR)24 and the Global Functioning Scale: Social (GFSS).24 For details, see supplementary material. All participants have provided a written consent form after study procedures were fully explained. For participants who were minor at the time of study participation, parental consent was also obtained. This study was approved by the University of California Los Angeles Institutional Review Board.
Social R-K Paradigm
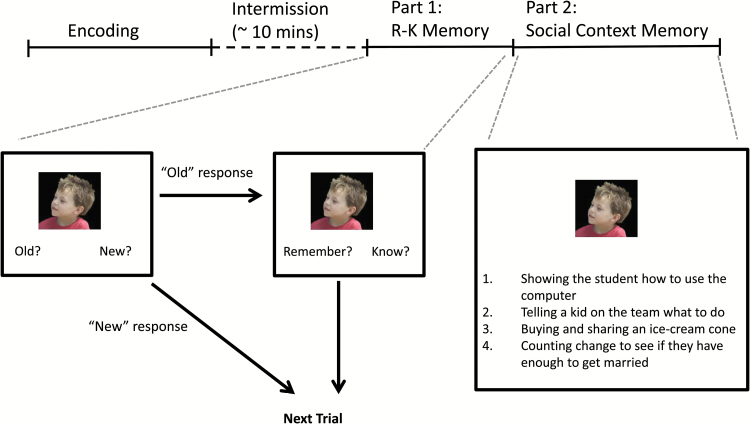
The Social R-K paradigm was modeled after previous work using nonsocial stimuli.25 Specifically, we employed dynamic, multimodal social stimuli (ie, short video clips showing 2 individuals interacting with each other) and applied an incidental memory task. There were 2 phases: encoding and retrieval (figure 1). During the encoding phase, participants watched 24 clips. After a 10-minute break, participants completed 2 parts of the retrieval phase (ie, an R-K task and a social context task).
Fig. 1.
A schematic diagram of the Social Remember-Know Paradigm.
Encoding.
During the encoding phase, participants were instructed to watch and attend to 24 video clips presented in a pseudo-randomized order. Because the task was intended to be an incidental measure of relationship perception resembling everyday natural observation, no explanation was given to participants about social relationships in the clips. The 24 clips, each lasting 20 seconds, were selected out of 36 clips that were originally developed to examine neural activation related to social relationship perception.26 The 24 clips showed an initial 12-second segment during which a single actor was visible (“alone”), followed by a “relational” segment of 8 seconds, in which the first actor interacted with a second actor. Speech was present in both the alone and relational segments for half of the clips, but absent for the other half.
Retrieval Part 1: R-K Memory.
After a 10-minute break, participants were given an R-K memory task to assess recollection vs familiarity-based memory for pictures of people shown in the video clips. The participants were provided with a detailed description of the difference between remember and know responses before starting, and their understanding of the difference between the responses was confirmed by the experimenter.
During the R-K task, 48 faces were shown in a random order. There were 24 faces that appeared in the video clip (“targets”) and 24 photos of faces that were matched for gender and age that did not appear in the clips (“foils”). Targets were selected from the actor of each clip who appeared both alone and in relational segment. Both targets and foils were comparable in viewing angle, portion of the face and visual resolution. For each face, participants were asked to decide whether the face was “Old” (ie, appeared in a clip) or “New” (ie, did not appear in a clip). For each face the participants identified as “Old”, they were then asked to make an additional judgment: “Remember” if they had a specific recollection of the face, or “Know” if they had a general feeling of familiarity about it. This 2-step response format was chosen to reduce the tendency for participants to operationalize K responses as guesses.27 The main dependent measures were d′ for Old vs New judgments to assess overall social memory, and the number of hits and false alarms (FA) for Remember and Know responses to assess recollection and familiarity memory.
Retrieval Part 2: Social Context Memory.
For the second part of the retrieval phase, 24 targets were presented with one sentence describing the context of each of the clips along with 3 lure sentences. Sentenced were created to minimize demands on reading and comprehension. The average sentence length was 6.7 words (SD = 1.8 words) and word complexity was comparable across sentences. To establish comparable levels of difficulty across trials, we created 4 statements in a following way: 1 correct statement, 2 describing a social interaction that occurred in a different video clip, and 1 describing an interaction that did not occur in a video. For each target face, participants were asked to performed a 4-way forced-choice recognition memory task to select which brief statement best describes the interpersonal activities the pictured person (ie, target) was engaged in. The main dependent measure was accuracy.
Social Cognitive Measures and Nonsocial Cognitive Measures
To assess social cognitive function, we employed the Mayer-Salovey-Caruso Emotional Intelligence Test 2.0 (MSCEIT)28 and the Relationships Across Domain (RAD).29 The MSCEIT was selected because it comprehensively assesses the domain of emotional processing, a core social cognitive construct. The RAD was selected because it assesses social perception, the ability to understand social relationships and make inferences about the behavior of others in future interactions. The MATRICS Consensus Cognitive Battery (MCCB)30 was employed to assess nonsocial cognitive ability. For details, see supplementary material.
Statistical Analyses
Using univariate ANOVA for continuous variables and logistic regression for dichotomous variables, demographic characteristics were examined as a function of group (probands, controls), phase (CHR, first-episode and chronic), and group by phase interaction. Any demographic variables that were significantly different between groups or across phase of illness were statistically controlled in further analyses. The same approach was used to examine performance on social cognitive tasks.
Performance on the Social R-K Memory paradigm was examined in 3 steps. First, to examine whether overall social memory differs between groups and across phase of illness, a 2 × 3 ANOVA was conducted using d′, with group (proband and control) and phase (CHR, first-episode and chronic) as between-subject factors. Second, to examine whether social recollection and familiarity memory differ between groups and across phase of illness, a 2 × 2 × 2 × 3 repeated measures ANOVA was conducted using the number of endorsed responses, with memory type (Remember and Know) and response type (hit, FA) as within-subject factors and group and phase of illness as between-subject factors. Third, to examine whether social context memory differs between groups and across phase of illness, a 2 × 3 ANOVA was conducted using accuracy, with group and phase as between-subject factors. For all of the analyses, any significant effects were followed up by post hoc analyses.
Finally, to examine the associations between social memory, other social cognitive functions and community functioning, we conducted correlation analyses (Pearson’s r) among relevant measures. As we had more than 1 measure in each construct, we employed a Bonferroni correction to correct for multiple comparisons for each construct. Specifically, the level of significance for correlation analyses was set at P < .025 (P = .05/2) for social cognitive tasks and P < .025 (P = .05/2) for functional measures to correct for multiple comparisons. For any significant correlation, we examined whether the strength of association differs across phase of illness with the Fisher’s r to z transformation.
Results
Demographic and Clinical Characteristics
Table 1 presents demographic and clinical characteristics of participants. For age, only a phase effect was significant (F1,296 = 184.37, P < .001, = .55). As expected, participants in the CHR phase were younger than participants in the first-episode phase (P < .001) who were younger than participants in the chronic phase (P < .001). For gender, ANOVA revealed no significant effect. For parental education, only a phase effect was significant (F2,292 = 3.33, P < .05, = .02). Participants in the chronic phase had higher parental education than participants in the CHR sample (P < .05). Participants in the first-episode phase showed intermediate level of parental education and did not significantly differ from the other phase groups. For the subsequent analyses for the Social R-K Memory Task, age and parental education were included in the model as covariates. Comparison of performance on social cognitive and nonsocial cognitive tasks is presented in the supplementary material.
Table 1.
Demographic and Clinical Characteristics of Participants
| CHR | First-Episode | Chronic | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Probands (N = 51) | Controls (N = 36) | Probands (N = 80) | Controls (N = 49) | Probands (N = 50) | Controls (N = 39) | ||
| Age | 18.7 (3.5) | 20.1 (3.4) | 22.8 (3.7) | 22.7 (2.3) | 31.2 (7.1) | 32.3 (4.5) | group: F1,296 = 2.16, NS |
| phase: F1,296 = 184.37, P < .001 | |||||||
| group × phase: F1,296 = .96, NS | |||||||
| Gender (% Female) | 29.4 | 38.9 | 28.7 | 40.8 | 30.6 | 40.5 | group: = .91, NS |
| phase: = .02, NS | |||||||
| group × phase: = .04, NS | |||||||
| Parental Edu. | 12.9 (3.5) | 13.7 (2.4) | 13.9 (3.9) | 14.5 (3.1) | 14.5 (3.3) | 14.8 (3.3) | group: F1,296 = 1.85, NS |
| phase: F1,296 = 3.33, P < .05 | |||||||
| group × phase: F1,296 = .07, NS | |||||||
| GRFS | 6.1 (2.4) | 8.6 (0.7) | 3.8 (2.2) | 8.0 (1.4) | 4.6 (2.8) | 8.9 (0.8) | group: F1,217 =v142.79, P < .001 |
| phase: F1,217 = 8.89, P < .001 | |||||||
| group × phase: F1,217 = 3.56, P < .05 | |||||||
| GSFS | 6.2 (1.5) | 9.0 (0.7) | 5.3 (1.9) | 8.3 (1.3) | 6.3 (2.0) | 8.8 (1.3) | group: F1,211 = 139.54, P < .001 |
| phase: F1,211 = 5.43, P < .001 | |||||||
| group × phase: F1,211 = .37, NS | |||||||
| BPRS total | 39.8 (9.1) | 42.1 (12.1) | 41.6 (9.4) | phase: F1,162 = .71, NS | |||
| RAD | 57.1 (7.3) | 60.0 (5.0) | 47.6 (8.4) | 59.9 (5.3) | 48.6 (9.7) | 56.8 (8.2) | group: F1,273 = 59.39, P < .001 |
| phase: F1,273 = 11/80, P < .001 | |||||||
| group × phase: F1,273 = 9.89, P < .001 | |||||||
| MSCEIT | |||||||
| B1 | 109.5 (11.5) | 115.7 (12.5) | 99.8 (17.1) | 112.1 (12.8) | 98.1 (15.1) | 108.2 (15.5) | group: F1,260 = 20.97, P < .001 |
| phase: F1,260 = 6.97, P < .01 | |||||||
| group × phase: F1,260 = 1.23, NS | |||||||
| B2 | 99.5 (12.4) | 106.3 (10.7) | 89.1 (16.5) | 105.9 (11.5) | 93.3 (18.8) | 105.9 (11.3) | group: F1,265 = 37.84, P < .001 |
| phase: F1,265 = 4.61, P < .05 | |||||||
| group × phase: F1,265 = 2.77, NS | |||||||
| B3 | 92.9 (11.7) | 99.7 (10.4) | 82.0 (14.7) | 98.6 (9.2) | 84.2 (14.1) | 100.2 (11.7) | group: F1,264 = 65.18, P < .001 |
| phase: F2,264 = 7.11, P < .01 | |||||||
| group × phase: F2,264 = 3.26, P < .001 | |||||||
| B4 | 83.6 (11.0) | 96.6 (7.5) | 88.5 (9.0) | 100.5 (8.5) | group: F1,193 = 78.18, P < .001 | ||
| phase: F1,193 = 3.01, NS | |||||||
| group × phase: F1,193 = .17, NS | |||||||
| Total | 85.0 (15.4) | 104.9 (10.1) | 88.1 (15.5) | 105.7 (12.8) | group: F1,193 = 80.89, P < .001 | ||
| phase: F1,193 = .18, NS | |||||||
| group × phase: F1,193 =. 38, NS | |||||||
| MCCB | |||||||
| SOP | 44.4 (14.6) | 51.0 (901) | 32.1 (14.3) | 47.7 (8.7) | 36.5 (11.4) | 52.1 (10.8) | group: F1,248 = 47.13, P < .001 |
| phase: F2,248 = 49.42, P < .001 | |||||||
| group × phase: F2,248 = 3.37, P < .05 | |||||||
| AV | 40.7 (9.1) | 46.1 (10.5) | 33.9 (10.9) | 48.3 (9.1) | 41.0 (11.3) | 55.2 (8.7) | group: F1,244 = 81.14, P < .001phase: F2,244 = 1.75, NSgroup × phase: F2,244 = 4.88, P < .01 |
| WM | 45.1 (10.1) | 49.2 (8.8) | 38.7 (11.4) | 50.0 (9.8) | 41.1 (13.6) | 50.1 (13.5) | group: F1,248 = 29.65, P < .001 |
| phase: F2,248 = 1.38, NS | |||||||
| group × phase: F2,248 = 1.35, NS | |||||||
| Vrbl Lrng | 45.6 (9.7) | 46.4 (9.0) | 37.9 (9.5) | 49.2 (9.1) | 41.1 (11.8) | 52.3 (10.7) | group: F1,248 = 31.64, P < .001 |
| phase: F2,248 = 1.35, NS | |||||||
| group × phase: F2,248 = 6.85, P < .01 | |||||||
| Vis Lrng | 42.5 (11.9) | 47.5 (8.1) | 34.6 (10.7) | 45.4 (9.1) | 34.7 (13.5) | 44.1 (13.4) | group: F1,248 = 28.73, P < .001 |
| phase: F2,248 = 4.57, P < .05 | |||||||
| group × phase: F2,248 = 1.47, NS | |||||||
| RPS | 45.7 (10.3) | 46.5 (11.9) | 40.1 (9.5) | 47.6 (9.1) | 44.4 (101) | 47.0 (11.1) | group: F1,248 = 6.90, P < .01 |
| phase: F2,248 = 1.82, NS | |||||||
| group × phase: F2,248 = 2.22, NS | |||||||
| Neurocognitive composite | 41.0 (12.5) | 46.6 (10.1) | 29.7 (12.8) | 47.1 (9.0) | 34.6 (13.7) | 50.0 (13.8) | group: F1,244 = 56.51, P < .001 |
| phase: F2,244 = 4.44, P < .05 | |||||||
| group × phase: F2,244 = 3.92, P < .05 | |||||||
Note: GRFS, the Global Functioning Role Scale; GFSS, the Global Functioning Social Scale; BPRS, the Brief Psychiatric Rating Scale -24 item; RAD, Relationship Across Domains; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test 2.0; MCCB, MATRICS Consensus Cognitive Battery; SOP, Speed of Processing; AV, Attention/Vigilance; WM, Working Memory; Vrbl Lrng, Verbal Learning/Memory; Vis Lrng, Visual Learning/Memory; RPS, reasoning/problem solving. Values are given as mean (SD).
Retrieval Part 1: R-K Memory
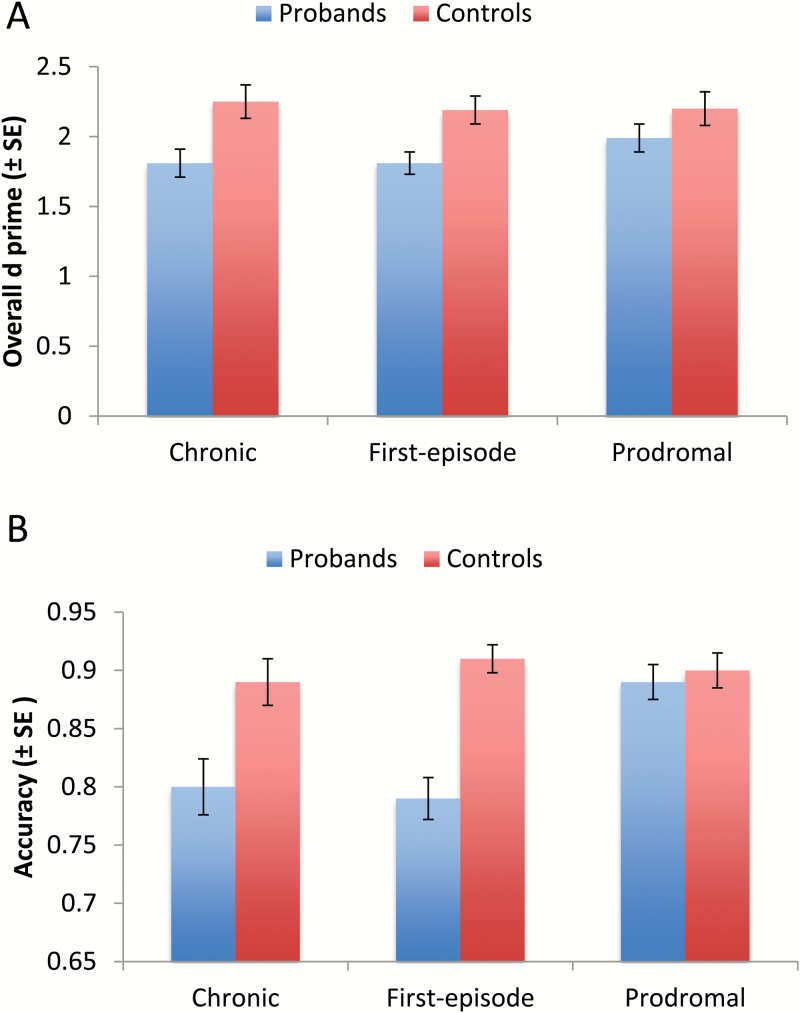
For social episodic memory we first examined overall d′ (figure 2A). ANOVA with phase and group as between-subject factors showed a significant effect of group (F1,296 = 12.73, P < .001, = .04). The phase main effect and phase × group interaction were not significant. Probands showed lower d′ than controls and this pattern did not differ across phase of illness.
Fig. 2.
Performance of overall social episodic memory (A) and social context memory (B).
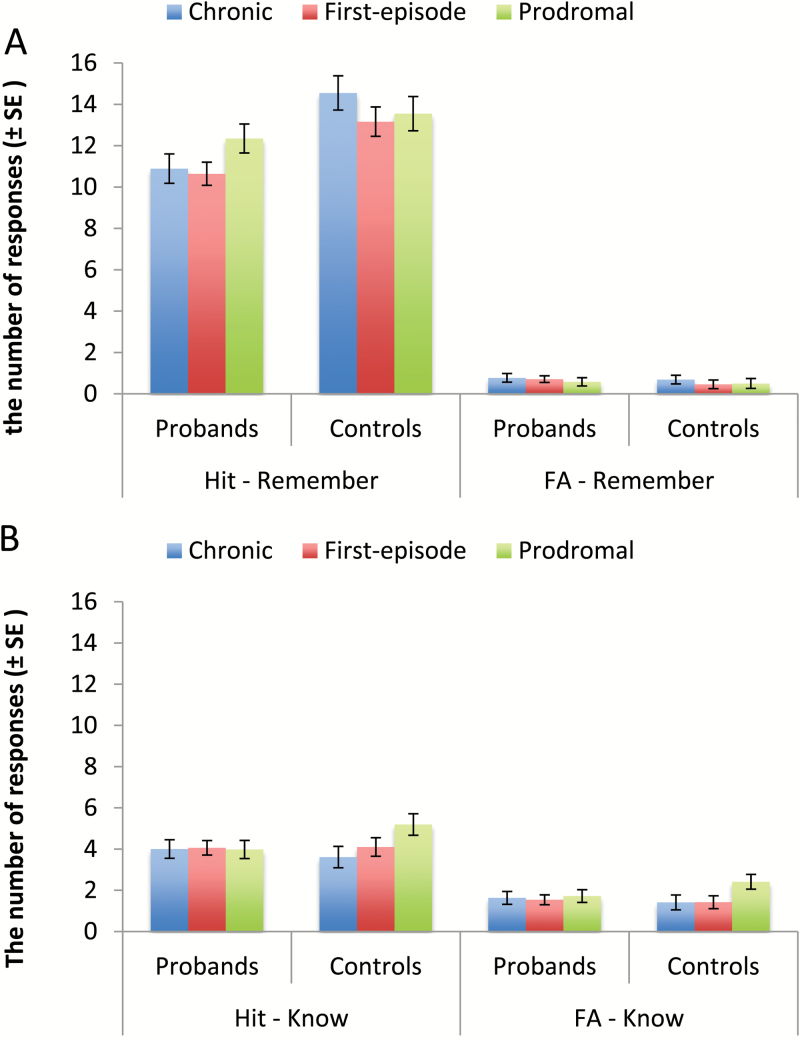
To assess whether there was any group or phase effect for social recollection vs familiarity, we examined the number of hits and FAs for remember and know responses with a 2 × 2 × 2 × 3 repeated measures ANOVA (figures 3A and 3B). We found a significant effect of response type (F1,288 = 32.30, P < .001, = .10) and a significant effect of group (F1,288 = 16,33, P < .001, = .05) and a significant effect of phase (F2,288 = 4.36, P < .05, = .03). Overall, participants made more Remember than Know responses and more hits than FA responses. Probands made fewer overall “old” responses than controls. Participants in the CHR phase made more “old” responses than participants in the first episode (P < .01), who did not differ from those in the chronic phase.
Fig. 3.
Performance of social recollection (A) and familiarity memory (B).
We also found a significant response type × memory type interaction (F1,288 = 8.37, P < .01, = .03) and a response type × group interaction (F1,288 = 20.27, P < .001, = .07). Participants made more hits than FAs, and this difference was larger for Remember than Know responses. Probands made fewer hits than controls (P < .001), but they made a comparable number of FAs. Finally, a 3-way interaction of response type × memory type × group (F1,288 = 7.86, P < .01, = .03) was significant. Post hoc analyses showed that for Remember responses, probands made fewer hits than controls (P < .001), but the 2 groups did not differ on FAs. For Know responses, there were no significant group differences for either hits or FAs.
Finally, we examined whether any significant effects involving social recollection and familiarity memory could be explained by nonsocial memory by including verbal learning or visual learning from the MCCB as additional covariates. When verbal learning was added, all the significant main effects and 2-way interactions described above remained significant, although the main effect of phase and the 3-way interaction (response type, memory type and group) became marginally significant (F1,246 = 2.57, P = .07, = .02; and F1,246 = 3.23, P = .07, = .01, respectively). When visual learning was included, both the main effect of phase and the 3-way interaction of memory type × group × phase became marginally significant (F1,246 = 2.85, P = .059, = .02 and F2,246 = 2.54, P = .08, = .02, respectively). All the other significant effects remained.
Retrieval Part 2: Social Context Memory
We found a significant effect of group (F1,288 = 18.12, P < .001, = .06), a significant effect of phase (F1,288 = 5.58, P < .01, = .04) and a significant group × phase interaction (F1,88 = 3.46, P < .05, = .03; figure 2B). Participants in the CHR phase performed better than the other 2 patient groups (Ps < .01), who did not differ from each other. Although probands overall had lower accuracy than controls, this group effect was not present in the CHR phase (chronic phase, P < .01; first-episode, P < .001; CHR, P = .86). When verbal learning was added as an additional covariate, the group effect and phase effect remained significant (F1,254 = 4.66, P < .05, = .02, and F2,254 = 3.79, P < .05, = .03, respectively), but the group × phase interaction was no longer significant (P = .56). With visual learning as an additional covariate, only the main effect of group was significant (F1,254 = 4.37, P < .05, = .02). Finally, examination of social context memory for target faces that were remembered vs those that were not during the retrieval part 1 is presented in the supplementary material.
Relationships With Social Cognitive Function and Community Functioning
Table 2 shows correlations between indices of the Social R-K Paradigm and performance on social cognitive tasks and community functioning within the proband samples. Within the CHR sample, a higher d′ and a higher number of hits for Remember responses were positively correlated with performance on MSCEIT. A higher number of hits for Know responses was negatively correlated with performance on MSCEIT. A higher accuracy of Part 2, social context memory, was positively associated with performance on RAD and MSCIET. Within the first-episode, a higher accuracy of Part 2 was positively correlated with performance on RAD and MSCEIT. Within the Chronic sample, a higher d′ was positively associated with better performance on MSCEIT and a higher number of hits for Remember responses was positively correlated with better social functioning. A higher accuracy of Part 2 was positively correlated with better performance on RAD and MSCIET and better role and social functioning. Further, the association between Part 2 accuracy and indices of community functioning in the chronic sample significantly different from those in the CHR and first-episode samples (Ps < .05) and remained significant when verbal learning and visual learning were added as covariates.
Table 2.
Associations With Performance on Social Cognitive Tasks and Community Functioning
| Prodromal | First-Episode | Chronic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Part 1 | Part 2 | Part 1 | Part 2 | Part 1 | Part 2 | |||||||
| Hit Response | Accuracy | d′ | Hit Response | Accuracy | d′ | Hit Response | Accuracy | |||||
| d′ | Remember | Know | Remember | Know | Remember | Know | ||||||
| RAD | .19 | .27 | −.27 | .42* | .17 | −.01 | .05 | .32* | .26 | .07 | .16 | .52* |
| MSCEITa | .36* | .39* | −.47* | .40* | .23 | .07 | −.04 | .34* | .34* | .21 | .03 | .52* |
| GFRS | −.01 | .03 | .19 | −.01 | .01 | −.11 | −.15 | .07 | .18 | .18 | −.04 | .51* |
| GFSS | −.01 | .10 | .02 | .04 | .05 | −.05 | −.06 | .04 | .15 | .39* | .10 | .46* |
Note: RAD, Relationship Across Domains; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test 2.0; GRFS, the Global Functioning Role Scale; and GFSS, the Global Functioning Social Scale.
aFor the prodromal phase, average of 3 MSCEIT branches were used. For the first-episode and chronic phase, MSCEIT total score was used.
The level of significance (*) for correlation analyses was set at P < .025 (P = .05/2) for social cognitive tasks and P < .025 (P = .05/2) for functional measures to correct for multiple comparisons.
Discussion
Although previous studies have examined recollection and familiarity memory in schizophrenia, little is known about how schizophrenia patients remember social information in everyday life over the course of illness. To our knowledge, this is the first study to examine episodic memory for social interactions with multimodal, dynamic social stimuli in schizophrenia across phase of illness. In this study, probands showed impaired episodic memory for dynamic social interactions. Further, impairment was present in recollection, but not in familiarity, memory. This pattern of impairment for social information differs from a meta-analytic finding9 that considered primarily studies of nonsocial memory and showed impairments in both types of episodic memory in schizophrenia. Thus, our finding suggests that schizophrenia patients may have a different pattern of episodic memory impairment for social vs nonsocial information. Findings of nonsocial processing impairments in schizophrenia, therefore, do not necessarily inform us about social processing in this disorder.12,13,31,32
By having 3 clinical samples, this study was able to examine whether social episodic memory impairments differ across phase of illness. A pattern of impaired recollection with intact familiarity memory was observed in all 3 clinical samples. This finding suggests that impaired social episodic memory is present even before the onset of psychotic episode and that this pattern does not change much as the illness progresses. This extends our previous findings of nonsocial episodic memory,18,33 in which we found a similar pattern of impairment for nonsocial episodic memory across phase of illness. Recollection memory is supported by the hippocampus.4–6 Interestingly, the hippocampus is also one of key brain structures that have consistently shown to be aberrant in schizophrenia across phase of illness.34–36 Further studies are needed to determine the extent to which aberrant hippocampus is associated with impaired recollection memory across phase of illness in schizophrenia.
In addition to recollection and familiarity memory, this study also examined how well probands remember social context of dynamic social interactions. Impaired social context memory was observed in the first-episode and chronic schizophrenia samples, but not in CHR sample. It is possible that before the onset of psychotic episode, CHR individuals had difficulty re-experiencing details of social interactions (ie, impaired recollection), but were still able to recognize social context when asked specifically. As the illness progresses, however, patients may lose access to social context related to social interaction. These findings suggest that impaired recollection for social interactions may be a vulnerability marker for schizophrenia and impaired social context memory is a disease-related factor.
One may wonder whether intact familiarity memory for dynamic social interaction plays a compensatory function for individuals with schizophrenia in everyday life. However, our findings do not support this possibility. There was no significant association between familiarity memory and indices of community functioning in any of the 3 clinical samples. In contrast, both recollection and social context memory were related to community functioning in the chronic sample. These findings suggest that better community functioning may require better ability to remember details of social interaction and recognize relevant social context, especially for chronic schizophrenia patients. These findings also raise the possibility that psychosocial interventions targeting impaired social context memory could benefit community functioning, at least in those with chronic schizophrenia. Moreover, the association between social context memory and community function in the chronic sample was significantly different from those in the CHR and first-episode samples, suggesting that the association between episodic memory for social interaction and community functioning may change over the course of illness.
This study had some limitations. It employed a cross-section study design. Thus, while having 3 clinical samples across phase of illness enables us to examine change of episodic memory over the course of illness, it was not possible to draw any firm conclusion in a cross-sectional study. Further, as approximately 30% of individuals in the CHR are likely to develop schizophrenia,37 findings from the CHR sample in relation to schizophrenia needs to be interpreted with caution. This study did not include an R-K Paradigm with similarly complex nonsocial stimuli. While we showed that a pattern of findings could not be explained by nonsocial memory assessed with MCCB, it was not possible to directly examine whether the observed pattern in schizophrenia is specific for social stimuli. This study used video stimuli during the encoding phase but still images for the memory task, and it is possible that differences in dynamic vs static stimuli could have affected memory performance. This study also included only performance measures so we do not know whether impaired recollection of social information across clinical groups is based on similar neural abnormalities.
In conclusion, this study examined social episodic memory in schizophrenia across phase of illness using multimodal, dynamic social stimuli. A pattern of impaired recollection but intact familiarity memory for social stimuli suggests that schizophrenia patients have a pattern of impaired episodic memory for social stimuli from what is seen for nonsocial stimuli. Further, the findings of this study also suggest that an impaired recollection could be a vulnerability marker for schizophrenia, whereas impaired social context memory could be a disease-related marker.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the National Institute of Mental Health (P50MH066286 to K.H.N.).
Supplementary Material
Acknowledgments
T.D.C. reports that he is a consultant to the Los Angeles Department of Mental Health and to Boehringer Ingelheim Pharmaceuticals. M.F.G. and K.H.N. are officers within MATRICS Assessment, Inc., the publisher of the MCCB, but do not receive any financial remuneration for their respective roles. M.F.G. has been a consultant to AbbVie, ACADIA, DSP, and Takeda, is on the scientific advisory board of Luc, and has received unrelated research funds from FORUM. K.H.N. has been a consultant to Genentech, Janssen, Otsuka, and Takeda, and has received unrelated research grants from Genentech, Janssen Scientific Affairs and Posit Science. K.L.S. has served as a consultant to Janssen Scientific Affairs, LLC, has been on the speaker’s bureau for Otsuka America Pharmaceutical, Inc., and has received research support from Genentech, Inc., and Janssen Scientific Affairs, LLC through grants to K.H.N. and J.V. J.V. has received funding from Brain Plasticity, Inc., Genentech, Inc., and Janssen Scientific Affairs, LLC, and has served as a consultant to Boehringer-Ingelheim, GmbH, and Brain Plasticity, Inc. All the other authors do not have any conflict of interest.
References
- 1. Jacoby LL. A process dissociation framework - separating automatic from intentional uses of memory. J Mem Lang. 1991;30:513–541. [Google Scholar]
- 2. Mandler G. Recognizing - the judgment of previous occurrence. Psychol Rev 1980;87:252–271. [Google Scholar]
- 3. Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 4. Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev Jan. 2007;114:152–176. [DOI] [PubMed] [Google Scholar]
- 5. Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merkow MB, Burke JF, Kahana MJ. The human hippocampus contributes to both the recollection and familiarity components of recognition memory. Proc Natl Acad Sci U S A. 2015;112:14378–14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dede AJ, Wixted JT, Hopkins RO, Squire LR. Hippocampal damage impairs recognition memory broadly, affecting both parameters in two prominent models of memory. Proc Natl Acad Sci U S A. 2013;110:6577–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. J Neurosci. 2011;31:15693–15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby LA, Yonelinas AP, Ranganath C, Ragland JD. Recollection and familiarity in schizophrenia: a quantitative review. Biol Psychiatry. 2013;73:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee J, Green MF. Social preference and glutamatergic dysfunction: underappreciated prerequisites for social dysfunction in schizophrenia. Trends Neurosci Sep. 2016;39:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harvey PO, Lepage M. Neural correlates of recognition memory of social information in people with schizophrenia. J Psychiatry Neurosci. 2013;38:130007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertrand MC, Sutton H, Achim AM, Malla AK, Lepage M. Social cognitive impairments in first episode psychosis. Schizophr Res Sep. 2007;95:124–133. [DOI] [PubMed] [Google Scholar]
- 13. Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 2013;170:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guillaume F, Guillem F, Tiberghien G, et al. Use of the process dissociation procedure to study the contextual effects on face recognition in schizophrenia: familiarity, associative recollection and discriminative recollection. Psychiatry Res. 2007;149:105–119. [DOI] [PubMed] [Google Scholar]
- 15. Martin CD, Baudouin JY, Franck N, Guillaume F, Guillem F, Tiberghien G, Huron C. Impairment not only in remembering but also in knowing previously seen faces and words in schizophrenia. Psychiatry Res. 2011;188:18–23. [DOI] [PubMed] [Google Scholar]
- 16. Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. [DOI] [PubMed] [Google Scholar]
- 17. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 18. Haut KM, van Erp TG, Knowlton B, et al. Contributions of feature binding during encoding and functional connectivity of the medial temporal lobe structures to episodic memory deficits across the prodromal and first-episode phases of Schizophrenia. Clin Psychol Sci. 2015;3:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 20. First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 21. McGlashan TH.Structured Interview for Prodromal Syndromes (SIPS). New Haven, CT: Yale University; 2001. [Google Scholar]
- 22. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. [DOI] [PubMed] [Google Scholar]
- 23. Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int J Methods Psychiatr Res. 1993;3:227–243. [Google Scholar]
- 24. Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knowlton BJ, Squire LR. Remembering and knowing: two different expressions of declarative memory. J Exp Psychol Learn Mem Cogn. 1995;21:699–710. [DOI] [PubMed] [Google Scholar]
- 26. Iacoboni M, Lieberman MD, Knowlton BJ, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 2004;21:1167–1173. [DOI] [PubMed] [Google Scholar]
- 27. Eldridge LL, Sarfatti S, Knowlton BJ. The effect of testing procedure on remember-know judgments. Psychonomic Bull Rev. 2002;9:139–145. [DOI] [PubMed] [Google Scholar]
- 28. Mayer JD, Salovey P, Caruso DR.Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) User’s Manual. Toronto, Ontario, Canada: MHS Publishers; 2002. [Google Scholar]
- 29. Sergi MJ, Fiske AP, Horan WP, et al. Development of a measure of relationship perception in schizophrenia. Psychiatry Res. 2009;166:54–62. [DOI] [PubMed] [Google Scholar]
- 30. Nuechterlein KH, Green MF.MATRICS Consensus Cognitive Battery. Los Angeles, CA: MATRICS Assessment, Inc.; 2006. [Google Scholar]
- 31. Bell M, Tsang HW, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophr Bull. 2009;35:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allen DN, Strauss GP, Donohue B, van Kammen DP. Factor analytic support for social cognition as a separable cognitive domain in schizophrenia. Schizophr Res. 2007;93:325–333. [DOI] [PubMed] [Google Scholar]
- 33. van Erp TG, Lesh TA, Knowlton BJ, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. [DOI] [PubMed] [Google Scholar]
- 37. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.