Abstract
Sulphites are a family of additives regulated for use worldwide in food products. They must be declared on the label if they are present in concentrations greater than 10 mg kg−1, determined as sulphur dioxide (SO2). The current US regulatory method for sulphites, the optimised Monier–Williams method (OMW), produces false-positive results with vegetables from the Allium (garlic) and Brassica (cabbage) genera due to extraction conditions that are thought to cause endogenous sulphur compounds to release SO2. Recently, modifications to the OMW method (2× MW) were published that reportedly reduced this false-positive in garlic. However, no other vegetables from these genera have been investigated. In addition, an LC-MS/MS method was developed for sulphite analysis, but it has not yet been tested with these problematic matrices. Ten vegetable species were analysed using these sulphite methods (OMW titration, OMW gravimetric, 2× MW and LC-MS/MS) to determine the false-positive rate. Sulphite concentrations > 10 mg kg−1 SO2 were observed with the OMW analyses. The 2× MW method reduced the measured concentration in unsulphited samples to ≤ 10 mg kg−1 SO2 for all matrices analysed. The LC-MS/MS method showed concentrations < 10 mg kg−1 for the Brassica samples, but only displayed a slight reduction in the Allium matrices. Spiked recovery studies were conducted to determine if these methods can detect added sulphite. The 2× MW had recoveries of 17% and 42% for water and fresh garlic, respectively, and the LC-MS/MS had recoveries of 108%, 125%, 116% and 107% for water, fresh garlic, roasted garlic, and hummus, respectively. The low recoveries of the 2× MW may indicate that sulphur compounds cannot be properly quantified with this method. The ability to eliminate false-positives will enable accurate determination of added sulphite to ensure compliance with sulphite labelling requirements.
Keywords: LC-MS/MS, sulphites, food additives, false-positive
Introduction
Sulphites are a family of additives used worldwide in the food industry to reduce browning, prevent oxidation and limit microbial growth (Taylor et al. 1986). Six different forms are approved for use in food in the United States: sulphur dioxide (SO2), sodium sulphite, sodium metabisulphite, potassium metabisulphite, sodium bisulphite and potassium bisulphite (Code of Federal Regulations 2015b, 2015c, 2015d, 2015e, 2015f, 2015g). Due to their broad application, they are added to a large range of food products such as juices, dried fruit and vegetable products, baked goods, and seafood (Taylor et al. 1986). In the early 1980s, there were reports of sensitive individuals having severe allergic-type reactions following consumption of sulphite-treated foods. As a result, the USFDA mandated that sulphites be declared on the label of any product containing sulphites (determined as SO2) in excess of 10 mg kg−1 (Code of Federal Regulations 2015a). In addition, no sulphites can be added to fruits or vegetables intended to be served raw or to products considered a good source of vitamin B1, thiamine (Code of Federal Regulations 2015b, 2015c, 2015d, 2015e, 2015f, 2015g). Similar regulations are found in many other countries worldwide (Korea Food and Drug Administration 2003; Commonwealth of Australia 2011; European Commission 2011; Consolidated Regulations of Canada 2015).
The US regulation specifies that the AOAC Official Method #990.28 (AOAC International 2000), the optimised Monier–Williams (OMW) method will be used by the USFDA for all sulphite regulatory analyses. The OMW method involves heating the sample in acidified water at reflux conditions for 105 min. While refluxing, all sulphite forms are released from the food matrix, converted to SO2 gas and bubbled into a 3% hydrogen peroxide solution. The hydrogen peroxide reacts with the SO2 to form sulphuric acid, which is then titrated with sodium hydroxide to determine the concentration of sulphite in the sample. This method allows accurate determination of sulphite at the regulatory threshold for most food products with a LOD of 10 ppm SO2. However, there are some problematic matrices including the Allium (e.g., garlic, onion, leek) and Brassica (e.g., cabbage, broccoli, Brussels sprout) genera which produce false-positive results (AOAC International 2000). Both these genera are known to have high concentrations of endogenous sulphur compounds. For example, the sulphur content of garlic powder is reported as about 1% (or approximately 10,000 mg kg−1 sulphur) of its dry weight (Pentz et al. 1990). Some of these sulphurous compounds may be broken down to SO2 during the OMW analysis. SO2 produced from endogenous compounds cannot be differentiated from the SO2 produced from added sulphites.
Of all the species of the Allium and Brassica genera, garlic has been the most extensively investigated. Garlic chemistry was studied to determine the source of the SO2 production in order to evaluate method modifications that could reduce the level of false-positives (Perfetti & Diachenko 2003; Lafeuille et al. 2007). These authors determined that the production originated from three potential sources: allyl mercaptan, alliin and allicin. Alliin is the most common cysteine sulphoxide found in the cytoplasm of garlic cells. When garlic is crushed, chopped or dried, the alliinase enzyme is released from the cell’s vacuole and reacts with alliin to form sulphenic acids quickly which then produce alkyl alkanethiosulphinates (mainly allicin). This reaction can occur rapidly, especially when dried vegetables are rehydrated, even at RT. Once allicin is produced it can release varying concentrations of SO2 depending upon the pH and temperature conditions present (Lawson 1993; Perfetti & Diachenko 2003; Lafeuille et al. 2007). These compounds or variations of them exist in other Allium species, such as onion, explaining why false-positives are observed across the genus (Block 1985). Research into the Brassica genera has shown that these vegetables contain chemical compounds similar to those reported in garlic (Mazelis 1963; Hamamoto & Mazelis 1986; Marks et al. 1992; Kubec et al. 1998; Kyung & Lee 2001). Instead of alliinase, Brassica vegetables contain cysteine lyase which converts S-methyl-L-cysteine sulphoxide (SMCSO) into methyl methanethiosulphinate (MMTSO) (Mazelis 1963; Kubec et al. 1998; Kyung & Lee 2001). Due to the structural similarities between these two sets of compounds, it is thought that modifications that limit the SO2 production in one genus would also work successfully in the other genus.
The experimental conditions (pH, heating) and type of garlic (freeze dried, vacuum dried, raw) analysed can play a large role in the degree of false-positive observed with a sample (Lawson 1996). If the enzymatic activity of alliinase can be stopped, then the quantity of allicin produced will be reduced, thus limiting the extent of the false-positive. Alliinase is active over pH 4–9 and is irreversibly inactivated at pH < 3 (Lawson 1993). Alliin has also been shown to produce SO2 at pH < 2.4. Lafeuille et al. (2007) changed the OMW refluxing solvent from dilute HCl to a two-part addition of a buffered phosphoric acid/monobasic potassium phosphate solution. It was found that this kept the pH in an optimal range to prevent SO2 production from both allicin and alliin. While this reduced the false-positive, there was still a slight response when garlic samples were analysed under these conditions. Further investigation showed that another compound produced from the same pathways, allyl mercaptan, may be the cause of this residual positive response. Lafeuille et al. (2007) added a double bubbler set-up with an intermediary toluene vessel to trap any allyl mercaptan released during the distillation. This double bubbler method (2× MW) was tested with garlic, but no other problematic species were investigated.
Perfetti and Diachenko (2003) developed an LC method with post-column detection that also limited the background sulphite level in dried garlic. In this method, the samples are extracted with a 0.2 M hydrochloric acid solution which inhibits the formation of allicin. The sample is then cleaned up using a C18 SPE cartridge and all sulphites are converted to the formaldehyde adduct, hydroxymethylsulphonate (HMS). Reversed-phase ion-pairing LC is used to separate the HMS from other sample components. Once separated, HMS is reacted with a strong base and 5,5-dithio-bis-2-nitrobenzoic acid (DTNB) to produce 5-mercapto-2-nitrobenzoic acid, which can then be detected spectrophotometrically using a post-column detector. This method reduced the background levels of unsulphited dried garlic to < 20 mg kg−1 SO2. The validity of the method was not tested for other Allium species or any members of the Brassica genera.
Regulatory agencies in the United States and the European Union have been looking for an analytical method that would eliminate the false-positive produced when Allium and Brassica vegetables are analysed for sulphites. While there have been methods that attempted to distinguish between added sulphite and the decomposition of endogenous sulphur compounds, no method has been shown to be successful across multiple species. Recently, an LC-MS/MS method for the detection of sulphites was published, but it was only validated in non-problematic matrices such as apricots and shrimp. The LOQs for this method in non-problematic matrices ranged from 0.12 to 0.75 ppm SO2 depending on the matrix investigated (Robbins et al. 2015). This method has not been tested with Allium and Brassica samples.
Unspiked vegetable samples from both genera were analysed using the OMW titration and gravimetric method as well as the 2× MW and LC-MS/MS methods to determine the rate of false-positives. In addition, a range of different unsulphited garlic products were spiked with a sodium sulphite solution to determine the recovery rate of added sulphite for each of these methods. Due to the fact that dried vegetables are available on the market, as well as products containing these vegetables as ingredients, there needs to be a method that can be used for regulatory analyses that limits the false-positive response as much as necessary. To the authors’ knowledge, there are no studies in the literature that look at the false-positive response in a wide variety of vegetable types or in multiple methods. Lim et al. (2014) compared four different methods other than those compared here, but did not compare Brassica and Allium vegetables. They instead looked at false-positives observed from highly acidic products such as vinegars or pickled products. Chung et al. (2008) also investigated false-positives due to certain matrices using HPLC with fluorometric detection. They tested three different Brassica samples, but failed to investigate any Allium species which is where the largest false-positive is usually observed. Due to this lack of information in the literature, the objective of this study is to gather more information about the degree of false-positives produced by four different methods in a wide variety of Allium and Brassica vegetables.
Materials and methods
Reagents and materials
The internal standard, sodium sulphite-34S (Na234SO3, 95%), in addition to formaldehyde (37%), toluene, ammonium acetate (Sigma Ultra, minimum 98%) and sodium sulphite (Na2SO3, ≥ 98%) were acquired from Sigma-Aldrich (St. Louis, MO, USA). LC-MS-grade acetonitrile, water and methanol, in addition to glacial acetic acid, ethanol, methylene chloride, hydrogen peroxide (30%), sodium hydroxide (certified 0.1 and 0.01 N), potassium hydroxide pellets (85%), potassium dihydrogen phosphate, barium chloride, concentrated phosphoric acid and concentrated hydrochloric acid, were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA). Methyl red from Mallinckrodt Baker (Phillipsburg, NJ, USA) was used in the MW titration. Samples were diluted and extracted using 18 MΩ water obtained from an Aqua Solutions water purification system (Jasper, GA, USA). Fresh vegetables and all ingredients used for making garlic products were purchased from grocery stores located in Greenbelt, MD, USA. All food samples were stored according to the manufacturers’ suggested storage conditions.
Sample preparation
To ensure that all samples tested contained no added sulphite, fresh vegetable samples were purchased and dried in the laboratory. Ten different vegetables from the Allium and Brassica genera (broccoli, Brussels sprout, cabbage, cauliflower, chive, garlic, green onion, kale, leek, and onion) were purchased from local grocery stores. They were washed with water to remove any dirt and the edible portions were sliced and placed in a single layer on a food dehydrator (Nesco/American Harvest, Two Rivers, WI, USA). The samples were dried using the manufacturer’s recommended settings for each vegetable (Nesco American Harvest 2011). After drying, the samples were ground to a fine powder in a Waring variable speed blender (Waring Commercial, Torrington, CT, USA), placed in a capped 250 ml polypropylene centrifuge tube and stored at RT until analysis. For the spiked recovery samples, roasted garlic and garlic hummus were prepared in the laboratory to ensure no sulphites were added.
LC-MS/MS
Sulphite concentrations were determined by LC-MS/MS using the method of Robbins et al. (2015). Briefly, all free and bound sulphites were converted to a formaldehyde adduct, hydroxymethylsulphonate, by extraction of the food product with a buffered 0.2% formaldehyde solution. A stable isotope sodium sulphite internal standard solution (Na234SO3) was prepared using the formaldehyde extraction solvent and was added into the HPLC vials prior to injection.
An Acquity Ultraperformance LC System (Waters, Milford, MA, USA) equipped with a SeQuant ZIC HILIC analytical column (150 × 2.1 mm × 5 µm; The Nest Group, Inc., Southborough, MA, USA) was used for separation. The column was thermostated at 30°C and a flow rate of 0.30 ml min−1 was employed. The following 24-min gradient programme was used: 6 min hold at 90% mobile phase A (10 mM ammonium acetate in 90:10 ACN:H2O) and 10% mobile phase B (10 mM ammonium acetate in 50:50 ACN:H2O), gradient to 50% A in 4 min, 5.75 min hold at 50% A, gradient back to 90% A in 0.25 min, and equilibration at 90% A for 8 min before the next injection. All samples and standards utilised a 5 µl injection volume. An external valve (Valco Instruments Co., Houston, TX, USA) was directed to waste at 0.0, and 9.0 min and to the MS at 6.5 min. An AB Sciex 4000 QTRAP mass spectrometer equipped with an electrospray ionisation (ESI) source in negative-ion mode was used for analysis. Analyst 1.6.2 (AB Sciex, Foster City, CA, USA) controlled both systems and data analysis. The source parameters were optimised for the HMS transitions (111-81 and 111-80). The curtain gas was set to 35 arbitrary units (au); the collisionally activated dissociation (CAD) gas was run at medium; an ion spray voltage of −1200 V was used; the source temperature was 550°C; gas 1 pressure was 70 au; and gas 2 pressure was 40 au. The MS/MS data were acquired using the MRM mode (unscheduled) with unit resolution of both Q1 and Q3.
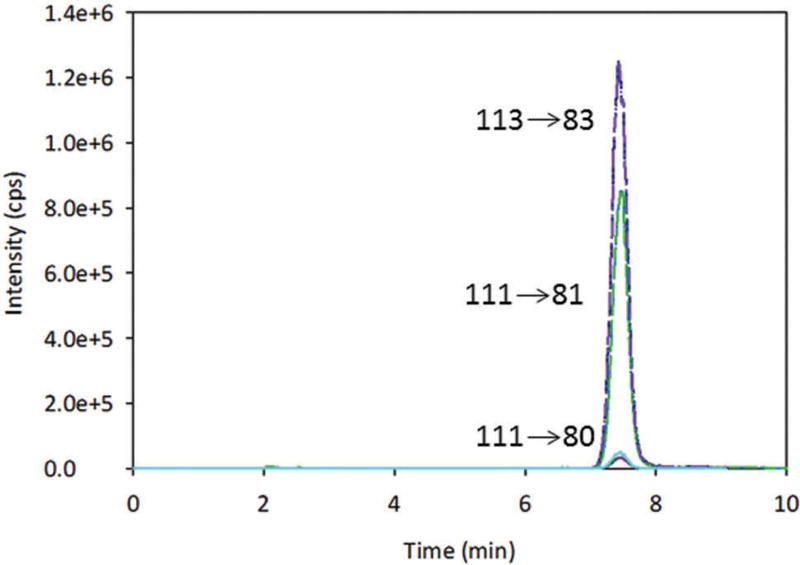
A 10-point calibration curve ranging from 0.01 to 4.5 mg kg−1 Na2SO3 in vial (0.25–114 mg kg−1 SO2 in sample) was generated for the quantitation of sulphite. A sample chromatogram is shown in Figure 1. The curve was created from the MRM ratios of the peak areas of the analyte to the internal standard (Na234SO3). A quadratic fit with 1/x2 weighting was used for the calibration curve due to the three orders of magnitude range of standard concentrations. The R2 values of all curves were greater than 0.990. Concentrations obtained using the calibration curve were adjusted by the appropriate dilution factor (250 for solid samples) and converted from Na2SO3 to SO2 using the following calculation:
where x is the concentration (ug ml−1) in the vial from the calibration curve; df is the dilution fraction for the sample; and m is the mass (g) of sample analysed. The final term of the equation is used to convert from concentration Na2SO3 to SO2. All values were reported as µg SO2 g−1 food sample.
Figure 1.
Sample LC-MS/MS chromatogram of a 0.4 ppm Na2SO3 standard with stable isotope internal standard.
Optimised Monier–Williams method (OMW)
All dried vegetable samples were analysed using the optimised MW method (AOAC method #990.28) (AOAC International 2000). Briefly, 400 ml of 18 MΩ water were added to a 1 L round-bottom flask. A 30% hydrogen peroxide stock solution was diluted 1:10 to give 30 ml of 3% hydrogen peroxide. Three drops of a methyl red indicator were added to the solution and then 0.01 N NaOH was added drop wise until a yellow end point was reached. This solution was added to a 50 ml graduated cylinder and placed under the bubbler. Nitrogen was bubbled through the entire closed apparatus for 15 min. A total of 50 g of sample (both solid and liquid) were added to the round-bottom flask and the transferring container was rinsed with 100 ml of 5% ethanol. Using the dropping funnel, 90 ml of 4 N HCl were added to the flask. The nitrogen flow was restarted and the heating mantle (Glas-col, Terre Haute, IN, USA) was turned on to a heating level that produced 80–90 drops min−1 of condensate from the condenser. The contents of the flask were boiled with these conditions for 105 min. Upon completion of the distillation, the cylinder containing the hydrogen peroxide was removed and its contents were quantitatively transferred to a 125 ml Erlenmeyer flask. The contents were titrated with either 0.1 or 0.01 N NaOH until the yellow end point was again reached. The volume of titrant needed to reach the end point was recorded. The sulphite content (as µg SO2 g−1 food sample) was determined using:
where 32.03 is the milli-equivalent weight of SO2; VB is the volume (ml) of NaOH of normality N required to reach the end point; 1000 is the factor to convert milli-equivalents to micro-equivalents; and w is the weight (g) of sample added to the round-bottom flask.
After the sulphite concentration was determined titrimetrically, a gravimetric check analysis was conducted following the AOAC method (the procedure also included in #990.28). Briefly, the contents of the titration flask were quantitatively transferred to a 250 ml beaker. Three drops of 1 N HCl were added to the beaker and the contents were swirled to ensure proper mixing. At this point, the colour of the solution should return to the pink colour observed at the end of the distillation. Approximately 1.5 ml of a 10% BaCl2 solution were added to the beaker and the contents mixed. The beaker was covered with Parafilm and allowed to sit at RT overnight. The next day the contents were passed through a pre-weighed Gooch crucible connected to a vacuum filtration flask. The beaker was rinsed several times with boiling water and ethanol. The rinses were poured through the Gooch crucible. The crucible was allowed to dry under vacuum prior to being placed in an oven at 105°C until fully dried. The crucible was then allowed to cool in a sealed desiccator. Once cool, the final mass of the crucible was recorded and the sulphite concentration was determined using the following formula:
Double bubbler Monier–Williams method (2× MW)
The concentration of SO2 present in the dried vegetable samples was determined using a modification to the OMW method published by Lafeuille et al. (2007). Briefly, OMW glassware must be modified to include a double bubbler system, as pictured in the original publication. The newly added graduated cylinder is filled with 25 ml of toluene which traps any allyl mercaptan released during the distillation. The presence of this compound in the final hydrogen peroxide vessel can cause a false-positive response. The temperature of the condenser is lowered from 5 to 0 ± 2°C. The contents of the distillation vessel were modified from the HCl solution used in the OMW method. A total of 25 g of sample are added to the distillation flask along with 120 ml of a 0.59 M phosphoric acid and 0.96 M hydrochloric acid solution. These are allowed to sit in the sealed system at RT with nitrogen bubbling through for 30 min. At this time, 480 ml of a 0.25 M potassium phosphate monobasic 0.258 M potassium hydroxide solution were added to the distillation flask. The heating mantle was turned on and the distillation was allowed to run for 105 min. As in the OMW method, the hydrogen peroxide solution is titrated with 0.01 N NaOH to equilibrium and the volume of titrant used was recorded. The concentration of SO2 present in the sample is determined using the same formula as listed above with the OMW method.
Spiked recovery study
Spiked recovery studies were conducted in water, fresh garlic cloves, roasted garlic and a garlic hummus. Each of these matrices was spiked with 10 mg ml−1 Na2SO3 solution with the volume necessary to produce a final concentration of 10 mg kg−1 SO2 in the sample. The samples were analysed by the OMW titration, LC-MS/MS and 2× MW methods. Blank samples were also analysed in triplicate and the concentrations were blank corrected prior to recovery calculations. Blank-corrected recoveries were conducted in triplicate analyses and were determined using the following formula:
Results and discussion
Method comparison
The OMW titration, OMW gravimetric, LC-MS/MS and 2× MW were used to analyse 10 different vegetables from the Allium and Brassica genera. Previous research has suggested that these species produce a false-positive when analysed using the OMW method. Further investigation was necessary to determine the extent of the false-positive observed with these four methods.
There was good agreement (no significant difference, p ≥ 0.05) observed between the OMW titration and gravimetric methods for all vegetable samples analysed. This was to be expected since the gravimetric method is often used as a check analysis for regulatory samples. RSDs ranging from 12% to 57% were observed in both genera for the OMW methods. This large deviation between replicates is common and is another indication that these methods are not the proper choices for quantifying sulphite in these matrices.
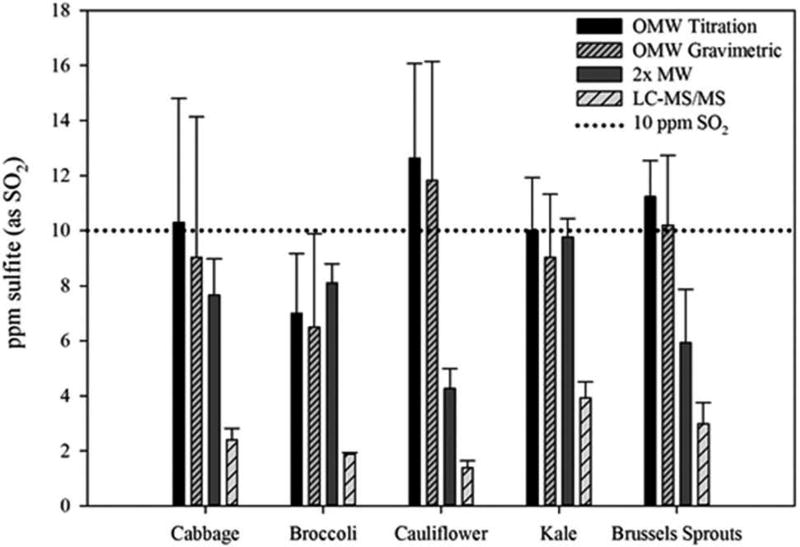
The Brassica vegetables (broccoli, Brussels sprouts, cabbage, cauliflower and kale) produced much lower SO2 concentrations than the Allium genera (Figure 2). All five samples analysed using the OMW titration method had values near the 10 mg kg−1 SO2 regulatory labelling threshold. The concentrations ranged from 7.0 ± 2.2 to 12.6 ± 3.5 mg kg−1 SO2 in broccoli and cauliflower, respectively. Similar results were seen for the OMW gravimetric with concentrations ranging from 6.5 ± 3.4 to 11.8 ± 4.3 mg kg−1 SO2 in broccoli and cauliflower, respectively. The 2× MW reduced the false-positive below the 10 mg kg−1 SO2 regulatory labelling threshold in all the Brassica vegetables, except kale. The LC-MS/MS method had the lowest SO2 detection of all four methods. The observed concentrations ranged from 1.4 ± 0.2 to 3.9 ± 0.6 mg kg−1 SO2 in cauliflower and kale, respectively.
Figure 2.
Concentration of SO2 in unsulphited Brassica vegetables detected with four different methods.
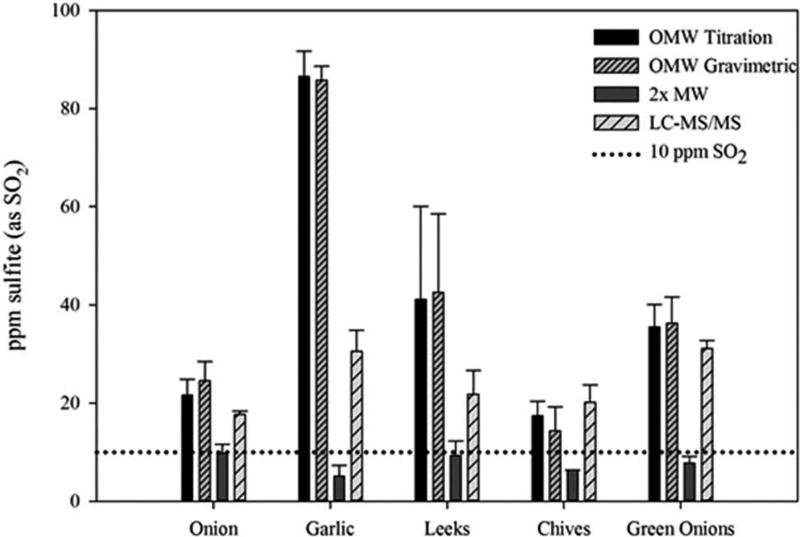
The Allium genera (chive, garlic, green onion, leek and onion) produced the greatest false-positive and poses the biggest challenge for regulatory enforcement (Figure 3). In particular, garlic had measured SO2 concentrations of approximately 90 mg kg−1 using the OMW method. This has also been seen in the literature with reported SO2 concentrations of more than 200 mg kg−1 in certain garlic samples (Perfetti & Diachenko 2003). The amount of SO2 produced through the OMW distillation can vary with soil chemistry and growing conditions of the garlic bulbs (Lawson 1996). The SO2 concentrations varied depending on the species analysed within the Allium family. This can be accounted for by the slight variation in the type and quantity of precursors and enzymes from one species to the next (Block 1985). The OMW titration results ranged from 17.4 ± 0.3 to 86.5 ± 5.2 mg kg−1 SO2 in chives and garlic, respectively. As previously mentioned, the results for the OMW gravimetric analysis followed the same pattern and concentrations as observed with the titration. Similar values to the Brassica samples were observed with the 2× bubbler method. Three of the five species were found to have SO2 concentrations significantly below the 10 mg kg−1 regulatory threshold. The two exceptions were onion and leek with concentrations of 9.8 ± 1.9 and 9.3 ± 2.9 mg kg−1 SO2, respectively.
Figure 3.
Concentration of SO2 in unsulphited Allium vegetables detected with four different methods.
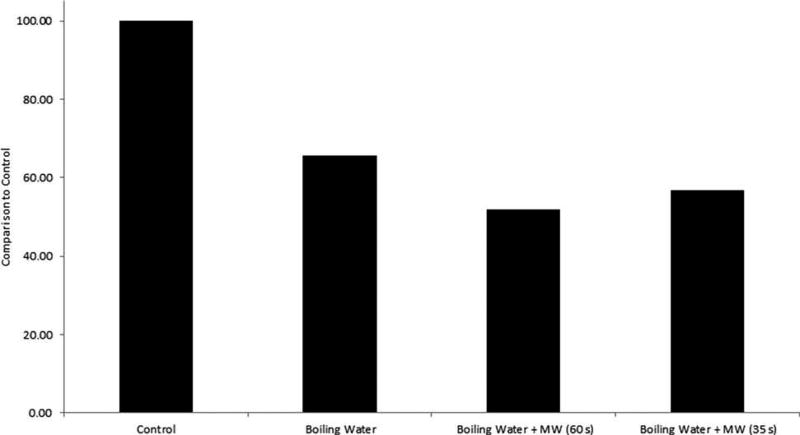
While the LC-MS/MS method showed significantly lower concentrations in garlic than were observed with the OMW titration method, these significant differences were not observed with the four other vegetable types. It should be noted, however, that garlic was the highest producer of SO2 and so there was much more reduction ability compared with the other species. Attempts at changing the extraction conditions of the LC-MS/MS to further reduce the amount of SO2 produced were unsuccessful. Previous researchers felt that the SO2 production was closely related to the pH of the assay conditions as well as to the activity of a particular enzyme (i.e., alliinase in garlic) (Perfetti & Diachenko 2003; Lafeuille et al. 2007). Manipulation of the pH between 2 and 4.5 was attempted by changing the buffering agents added to the formaldehyde extracting solution. No observable effect was observed in the SO2 concentration. In order to deactivate the enzyme, cryogenic freezing, microwaving, adding boiling water and changing the heating time and duration of the derivatisation step were all tested. Trials with the garlic powder are included in Figure 4. Although the boiling water and microwave combination proved to be the most successful at reducing the SO2 release in garlic powder, there were several drawbacks to using this approach. The microwaving had to be broken up into 10-s segments in order to keep the contents from boiling over the edge of the centrifuge tube. The method and results were difficult to duplicate and would be hard to recommend for widespread regulatory use. The boiling water method showed the best results with the dried garlic samples, but these results were not repeatable in the other vegetable powders. For this reason, the original protocol is recommended for analysis. The sample preparation steps of the original protocol may not completely reduce the formation of allicin, which is why a false-positive is still observed in these matrices.
Figure 4.
Comparison of different sample preparation methods for reducing the false-positive associated with a dried garlic powder in the LC-MS/MS method.
Spike recovery studies
False-positives associated with these vegetable families are an area of regulatory concern. However, a false-negative response when added sulphite was present would be even more of concern. The OMW titration, 2× MW and LC-MS/MS methods were investigated to determine their ability to detect added sulphites. An initial water recovery study was conducted in order to demonstrate analyst proficiency with the method. Fresh garlic, roasted garlic and garlic hummus were selected as representatives of common regulatory samples. The roasted garlic and hummus were made in the laboratory to ensure these samples did not contain added sulphite. Samples were spiked to 10 mg kg−1 with an Na2SO3 formaldehyde solution and were analysed in triplicate by each method. The blank matrix response was also determined in triplicate for each matrix and the spiked concentrations were blank corrected before a recovery value was determined.
The recoveries from water were determined to be 90% ± 3%, 108% ± 7% and 16% ± 1% from OMW, LC-MS/MS and the 2× MW methods, respectively (Table 1). The authors were not surprised that the recoveries from the OMW and LC-MS/MS methods were in an acceptable range. Both methods were included in a recent proficiency test of sulphited apricots with 144 laboratories. The OMW and LC-MS/MS methods both had z-scores less than 0.5, assuring the analysts that the methods were being performed accurately. The authors were surprised to see such a low recovery value for the 2× MW method. Lafeuille et al. (2007) did not publish any recoveries in any matrix other than garlic powder, so a comparison between laboratories was not possible. All method parameters were consistent with the published method and it seemed that the method was being performed correctly. The authors believe that there might be some interaction between the added sulphite and the components of the double bubbler where the sulphite would be bound or reacted in a way that it would not produce SO2 gas under distillation conditions.
Table 1.
Blank correcteda recovery of 10 mg kg−1 SO2 spikes from various matrices by three different analytical methods (n = 3).
| Matrix | OMWb titration | LC-MS/MS | 2× MWc |
|---|---|---|---|
| Water | 90 ± 3d | 108 ± 7 | 17 ± 1 |
| Fresh garlic | 31 ± 16 | 125 ± 1 | 42 ± 9 |
| Roasted garlic | 34 ± 8e | 116 ± 1 | Not analysedf |
| Hummus | 42 ± 4 | 107 ± 1 | Not analysed |
| White grape juiceg | 87h | 98 ± 7 | Not analysed |
| Dried apricot | 86 | 96 ± 4 | Not analysed |
Notes:
Triplicate analyses of a blank were conducted for each of the matrices. The spiked recovery value was corrected based on these values. All recoveries are expressed as per cent.
Optimised Monier–Williams.
Double bubbler Monier–Williams (Lafeuille et al. 2007).
Values are reported as mean per cent recovery ± standard deviation for triplicate measurements.
n = 4 for OMW-roasted garlic analysis.
After low recoveries were observed on the water and fresh garlic, no analysis was conducted on the remaining matrices.
The white grape juice and dried apricot recovery was previously published (Robbins et al. 2015) and is included for comparison between the problematic and the previously validated matrices.
The white grape juice and dried apricot OMW titration recovery was completed only once. The values are included only as a reference point.
The recoveries from fresh garlic were 31% ± 16%, 125% ± 1% and 44% ± 9% for the OMW, LC-MS/MS and 2× MW methods, respectively (Table 1). Both the Monier–Williams method recoveries were below 50%, showing that neither of these methods would be good for use in determining the sulphite content of Allium vegetables. These low OMW recoveries were also seen with Brassica samples analysed by Chung et al. (2008). The data collected in our study make it difficult to determine if the low false-positive rates observed with the unsulphited samples analysed by the 2× MW method were due to the inhibition of SO2 production during analysis or if the SO2 cannot be properly quantified under these analysis conditions. Lafeuille et al. (2007) used a spiking solution of sodium metabisulphite. To be certain that the formaldehyde solution was not interfering with the analysis, another set of spiked recovery experiments were conducted using an aqueous Na2SO3 solution. No significant differences were determined in either the water or the garlic samples with this change. The authors believe that the conditions for the 2× MW method may have changed the interactions between sulphite and the matrix in such a way that there is now a false-negative effect. Perfetti and Diachenko (2003) also observed a loss of sulphite under certain test conditions and believed that this may be due to allicin reacting with sulphites due to their ability to react with reducing agents. While it is thought that allicin production is limited due to the conditions of the 2× MW, this certainly could be the reasoning behind the low recoveries in the OMW method. It is possible that another component of garlic that is active under the 2× MW conditions can also react with reducing agents or bind sulphite producing these false-negative responses. Further investigation into the mechanisms behind the decreased recovery rates is necessary but beyond the scope of this work.
Due to the low recovery rates in both water and fresh garlic, only the OMW and LC-MS/MS methods were used for the roasted garlic and garlic hummus. The OMW method had recoveries of 33% ± 8% and 42% ± 4% for roasted garlic and hummus, respectively. While these recoveries are low, they are consistent with those results seen for fresh garlic. These results add to the hypothesis that there is some form of reaction or binding occurring between components of the garlic and the sulphiting compounds. The LC-MS/MS method had recoveries of 116% ± 1% and 107% ± 1% for roasted garlic and hummus, respectively. The recoveries of the LC-MS/MS samples were consistent with those observed previously in other non-problematic matrices such as apricot and white grape juice (Robbins et al. 2015). The experimental conditions and solvents of the LC-MS/MS method are quite different from those of the Monier–Williams methods. For this reason, it is not much of a surprise that the false-negative problems observed with those methods are not seen in the LC-MS/MS data.
The LC-MS/MS method reduces the false-positive observed when Allium and Brassica vegetables are analysed by the OMW titration and gravimetric method. The SO2 concentration was below the 10 mg kg−1 regulatory threshold in all Brassica species analysed and was slightly reduced in most of the Allium species investigated, though significant differences from the OMW only existed in the garlic samples. There also do not appear to be any problems recovering added sulphite from these matrices using the LC-MS/MS method, making this a viable replacement method for the tedious and time-consuming OMW methods. Further investigation into additional species, as well as additional commercially sulphited products, would be necessary before the broad adoption of this method for regulatory analyses. Once fully validated, this more rapid, specific and rugged method should help ensure more efficient and reliable compliance with worldwide sulphite regulations.
Acknowledgments
The authors thank Dr Kim Morehouse and Nicole Shyong for their assistance in purchasing and drying the vegetable samples. The authors also acknowledge Dr Christine Parker and Dr Ann Knolhoff for their helpful technical discussions. The authors also thank Dr Susan Genualdi for assistance in preparing this manuscript for publication.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Association of Official Analytical Chemists. 17. Gaithersburg (MD): AOAC International; 2000. [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–118. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- Commonwealth of Australia. Mandatory warning and advisory statements and declarations. Australia New Zealand Food Standards Code, Standard 1.2.3 2011 [Google Scholar]
- Consolidated Regulations of Canada. Food and Drug Regulations. 2015;870 Part B.01.010.2. [Google Scholar]
- Chung SWC, Chan BTP, Chan ACM. Determination of free and reversibly-bound sulphite in selected foods by high-performance liquid chromatography with fluorometric detection. J AOAC Int. 2008;91:98–102. [PubMed] [Google Scholar]
- [EC] European Commission. Commission regulation (EC) No. 1169/2011. Food Information for Consumers Regulation 2011 [Google Scholar]
- Food; exemptions from labeling. Code of Federal Regulations. 2015a Part 101.100(a)(4), Title 21. [Google Scholar]
- Hamamoto A, Mazelis M. The C-S lyases of higher plants: isolation and properties of homogeneous Cystine Lyase from Broccoli (Brassica oleracea var botrytis) buds. Plant Physiol. 1986;80:702–706. doi: 10.1104/pp.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Food & Drug Administration. General labeling standards for foods. Foods Labeling Standards. Enclosure 1 2003 [Google Scholar]
- Kubec R, Drhova V, Velisek J. Thermal degradation of S-methylcysteine and its sulphoxide - important flavor precursors of Brassica and Allium vegetables. J Agric Food Chem. 1998;46:4334–4340. [Google Scholar]
- Kyung KH, Lee YC. Antimicrobial activities of sulphur compounds derived from S-alk(en)yl-L-cysteine sulphoxides in Allium and Brassica. Food Rev Int. 2001;17:183–198. [Google Scholar]
- Lafeuille JL, Lefevre S, Achouri D. Determination of added sulphites in dried garlic with a modified version of the optimized Monier–Williamsmethod. J AOAC Int. 2007;90:1090–1097. [PubMed] [Google Scholar]
- Lawson LD. Bioactive organosulphur compounds of garlic and garlic products and their role in reducing blood lipids. ACS Symp Ser. 1993;534:306–330. [Google Scholar]
- Lawson LD. The composition and chemistry of garlic cloves and processed garlic. In: Koch HP, Lawson LD, editors. Garlic: the science and therapeutic application of Allium sativum L. related species. 3. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Lim H-S, Park S-K, Kim S-H, Song S-B, Jang S-J, Kim M. Comparison of four different methods for the determination of sulfites in foods marketed in South Korea. Food Addit Contam Part B. 2014;31:187–196. doi: 10.1080/19440049.2013.857048. [DOI] [PubMed] [Google Scholar]
- Marks HS, Hilson JA, Leichtweis HC, Stoewsand GS. S-methylcysteine sulphoxide in Brassica vegetables and formation of methyl methanethiosulphinate from Brussels sprouts. J Agric Food Chem. 1992;40:2098–2101. [Google Scholar]
- Mazelis M. Demonstration and characterization of cysteine sulphoxide lyase in the Cruciferae. Phytochemistry. 1963;2:15–22. [Google Scholar]
- Nesco American Harvest. Recipes & instructions: dehydrator & jerky maker. Two Rivers (WI): The Metal Ware Corporation; 2011. [Google Scholar]
- Pentz R, Guo Z, Kress G, Muller D, Muller B, Siegers CP. Standardisation of garlic powder preparations by the estimation of free and hydrolysable SH groups. Planta Medica. 1990;56:691. [Google Scholar]
- Perfetti GA, Diachenko GW. Determination of sulphite in dried garlic by reversed-phase ion-pairing liquid chromatography with post-column detection. J AOAC Int. 2003;86:544–550. [PubMed] [Google Scholar]
- Potassium bisulphite. Code of Federal Regulations. 2015b Part 182. 3616, Title 21. [Google Scholar]
- Potassium metabisulphite. Code of Federal Regulations. 2015c Part 182. 3657, Title 21. [Google Scholar]
- Robbins KS, Shah R, MacMahon S, de Jager LS. Development of a liquid chromatography-tandem mass spectrometry method for the determination of sulphite in food. J Agric Food Chem. 2015;63:5126–5132. doi: 10.1021/jf505525z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodium bisulphite. Code of Federal Regulations. 2015d Part 182. 3739, Title 21. [Google Scholar]
- Sodium metabisulphite. Code of Federal Regulations. 2015e Part 182. 3766, Title 21. [Google Scholar]
- Sodium sulphite. Code of Federal Regulations. 2015f Part 182. 3798, Title 21. [Google Scholar]
- Sulphur dioxide. Code of Federal Regulations. 2015g Part 182. 3862, Title 21. [Google Scholar]
- Taylor SL, Higley NA, Bush RK. Sulphites in foods: uses, analytical methods, residues, fate, exposure assessment, metabolism, toxicity, and hypersensitivity. Adv Food Res. 1986;30:1–76. doi: 10.1016/s0065-2628(08)60347-x. [DOI] [PubMed] [Google Scholar]