Abstract
Background
Current approved nucleoside therapies for genital herpes simplex virus (HSV) infections are effective but improved therapies are needed for treatment of both acute and recurrent diseases.
Methods
The effects of N-methanocarbathymidine were evaluated and compared to acyclovir using guinea pig models of acute and recurrent infection. For acute disease following intravaginal inoculation of 106 pfu HSV-2 (MS strain), animals were treated intraperitoneally beginning 24 h post-infection, and the effects on disease severity, vaginal virus replication, subsequent recurrences, and latent virus loads were evaluated. For evaluation of recurrent infection, animals were treated for 21 days beginning 14 days after infection and disease recurrence and recurrent shedding were evaluated.
Results
Treatment of the acute disease with N-methanocarbathymidine significantly reduced the severity of acute disease and decreased acute vaginal virus shedding more effectively than acyclovir. Significantly, none of the animals developed visible disease in the high-dose N-methanocarbathymidine group and this was the only group in which the number of days with recurrent virus shedding was reduced. Treatment of recurrent disease was equivalent to acyclovir when acyclovir was continuously supplied in the drinking water.
Conclusion
N-methanocarbathymidine was effective as therapy for acute and recurrent genital HSV-2 disease in the guinea pig models.
Keywords: Herpes simplex virus, genital herpes, N-methanocarbathymidine, recurrent herpes
Introduction
Genital herpes is a common sexually transmitted disease around the world.1 The synergistic relationship with human immunodeficiency virus (HIV) further enhances its impact on public health.2,3 Current approved nucleoside therapies for genital herpes reduce the severity of primary disease and virus shedding during the acute disease, but do not appear to affect subsequent recurrences.4,5 When used daily for the prevention of recurrent infections, these drugs decrease the frequency of recurrent disease and reduce but do not eliminate recurrent shedding.6–8 Similarly, they reduce, but do not prevent transmission of virus.9
N-methanocarbathymidine (N-MCT), a thymidine analog that incorporates a pseudosugar with a fixed Northern conformation, has greater antiviral activity against herpes simplex virus (HSV) type 1 and HSV-2 than acyclovir (ACV).10–12 In studies using a guinea pig model of neonatal HSV-2 infection and a lethal murine HSV-2 infection model, it was significantly more effective than ACV, especially after delayed therapy.13,14 Phosphorylation by the HSV thymidine kinase (TK)15 is similar to ACV in that the HSV TK catalyzes the conversion of both drugs to monophosphate and diphosphate, although N-MCT is metabolized to the diphosphate solely by HSV TK while ACV is not.12 We therefore evaluated the effects of N-MCT treatment on acute and recurrent HSV-2 infections using the guinea pig model of genital HSV-2.
Material and methods
Antivirals
N-MCT was kindly provided by N & N Pharmaceuticals Co. (Rockville, MD) and was dissolved in sterile saline solution for intraperitoneal (IP) administration. ACV (Sigma Co., St. Louis, MO) was obtained from the Cincinnati Children’s Medical Center pharmacy and dissolved in sterile saline solution for either daily IP or ad libitum administration (5 mg/ml) to animals in drinking water.16
Animals
Female Hartley guinea pigs (250–350 g) were obtained from Charles River Breeding Laboratories (Wilmington, MA) and housed under AAALAC-approved conditions at Cincinnati Children’s Hospital Medical Center. All procedures and protocols were approved by the Cincinnati Children’s Hospital Research Foundation Animal Care and Use Committee.
Viruses and cells
HSV-2 strain MS (ATCC-VR540) was grown in low passage primary rabbit kidney cells and titered on rabbit kidney cell monolayers as previously described.17
Intravaginal guinea pig HSV-2 infection model
The therapeutic efficacy of N-MCT against HSV-2 infection was evaluated in the guinea pig model of genital infection. Animals were inoculated intravaginally with 1 × 106 pfu of HSV-2 (MS strain) by rupturing the vaginal closure membrane with a moistened calcium alginate tipped swab (Calgiswab #3, Spectrum Labs., Los Angeles, CA) and instilling 0.1 ml of a virus suspension into the vaginal vault. Virus titers in vaginal swabs were determined on rabbit kidney cells grown in Basal Media Eagle (BME) (Gibco-Invitrogen) and 10% fetal bovine serum (FBS) (Hyclone, Thermo Fisher Scientific) as described previously.18 Primary genital skin disease was quantified using a lesion score-scale ranging from 0 representing no disease to 4 representing severe vesiculoulcerative skin disease of the perineum.18 Following recovery from primary infection, animals were examined daily from 15 to 63 days post-infection (dpi) for evidence of spontaneous recurrent herpetic lesions.18 The number of lesion days (days on which a recurrent lesion was observed on the perineum) was recorded. To evaluate recurrent virus shedding, vaginal virus swabs were collected three times weekly (Monday, Wednesday, Friday (M, W, F)), beginning at 21 dpi and continued until 63 dpi. Swabs were stored frozen (−80°C) until DNA extraction and evaluated by qPCR to measure HSV-2 DNA and to determine the frequency of viral shedding into the genital tract. At the end of the study, the guinea pigs were sacrificed, and the dorsal root ganglia (DRG) and spinal cords were harvested aseptically. The tissues were stored frozen (−80°C) until DNA was extracted for qPCR evaluation of latent virus.
N-MCT therapy of HSV-2 infected guinea pigs
N-MCT was administered either during the acute phase of infection for seven days (experiment 1) or treatment was administered starting at 15 dpi for 21 days (experiment 2).
In experiment 1, 48 female Hartley guinea pigs were divided into four groups (n = 12): group 1: vehicle control (saline), group 2: 25 mg N-MCT/kg/day, group 3: 50 mg N-MCT/kg/day, and group 4: 100 mg ACV/kg/day. The animals were treated twice daily IP for seven days starting 24 h post inoculation. After intravaginal infection, the animals were swabbed on 2, 4, 6, 8, and 10 dpi to measure acute vaginal virus titers. On 3 and 7 dpi, blood was collected by toenail bleed from six animals per group 1 h after the morning treatment for evaluation of plasma drug levels. The plasma was sent to Dr Robert Glazer for PK analysis. The animals were monitored daily for recurrent disease until 63 dpi and vaginal swabs were collected for qPCR analysis of vaginal shedding as described above. At 63 dpi, the animals were sacrificed and the DRGs and spinal cords were collected for qPCR analysis of viral DNA load.
In experiment 2, 60 Hartley guinea pigs (300–350 g) were divided into four groups (n = 15): group 1: vehicle control (saline, IP, twice daily), group 2: 50 mg N-MCT/kg/day, IP twice daily, group 3: 100 mg N-MCT/kg/day, IP once daily, and group 4: 5 mg/ml ACV, ad libitum in drinking water. The guinea pigs were inoculated intravaginally and followed during primary infection until day 14. The animals were then randomized, based on weights and primary scores, into the four treatment groups. N-MCT was prepared in saline and was administered IP. The vehicle control group received saline IP twice daily. ACV was dissolved daily (5 mg/ml) and administered ad libitum in the drinking water as this is more effective than IP inoculation.16,19 To confirm infection, a vaginal swab was collected at 2 dpi and cultured for evidence of viral replication and infection from all guinea pigs prior to randomization and treatment. Animals which were not infected were not included in the study. Treatment was initiated on day 15 and continued for 21 days until 35 dpi. The animals were examined daily for recurrent lesions and vaginal swabs were collected 3×/week (M, W, F, 15–45 dpi) and stored frozen for analysis of asymptomatic shedding by PCR. The animals were monitored until 45 dpi at which time the study was terminated. DRG and spinal cords were not collected.
Bioanalytical measurements
N-MCT levels were quantitated in guinea pig plasma samples obtained 1 h after the morning dose of N-MCT on days 3 and 7 in experiment 1 using liquid chromatography with tandem mass spectrometric detection (LC-MS/MS). Plasma was mixed with an equal volume of acetonitrile and mixed on a vortex mixer for 3 min. Samples were centrifuged at 3700 × g for 10 min and 50 µl aliquots were mixed with 500 µl water on a vortex mixer for 2 min before injection. Analyses used an Agilent 120011100 LC/MSD system using atmospheric pressure chemical ionization (APCI) with a MeOH:H2O gradient on a narrow-bore (2.1 mm lD) Zorbax C18 reverse-phase column. APCI is more sensitive and easier to interpret than electrospray ionization (ESl) for thymine-containing nucleoside analogs. Both the total-ion chromatogram and the wavelength-specific chromatogram at 273 nm indicated a single major peak that corresponded to unmetabolized N-MCT.
QPCR of HSV-2 DNA
Viral DNA levels in DRGs and spinal cords harvested at the end of each study and vaginal swab samples collected between 15 and 63 dpi were determined.20,21 Briefly, DRG and spinal cords were homogenized on ice in 500 µl of 2% FBS BME. DNA was isolated from 200 µl of the tissue homogenates and vaginal swab media using QIAamp DNA Mini Kit (Qiagen #51306) according to the manufacturer’s protocol. The samples were incubated with proteinase K for 1 h at 65°C. Viral DNA was detected using primers specific for the HSV-2 gG gene (kindly provided by Dr Debbie Long, Genocea Inc.) yielding a 71 bp DNA product. The primers were obtained from Sigma-Aldrich and the sequences were:
Forward: 5′-CGG/AGA/CAT/TCG/AGT/ACC/AGA/TC-3′; reverse: 5′-GCC/CAC/CTC/TAC/CCA/CAA/CA-3′, and probe FAM-ACC/CAC/GTG/CAG/CTC/GCC/G-tamRA.
Each PCR reaction contained 50–100 ng of sample DNA, 50 µM of each primer, and 10 µL of Taqman Gene Expression Master Mix (ABI). A Tam/Fam fluorescent dye was used and the PCR amplification was performed using a 7500 Fast Real-Time PCR system (ABI). Total volume of each sample was 20 µL. A standard curve was generated with 10-fold serial dilutions of purified HSV-2 DNA (ATCC) containing 105–100 HSV-2 copies in 50 ng of uninfected guinea pig DNA. The amplification program used included a pre-incubation step at 50°C for 2 min and at 95°C for 10 min, followed by 50 cycles consisting of a denaturation step at 95°C for 15 s, annealing at 60°C for 1 min and elongation at 72°C for 10 s. The limit of detection of the assay was between 100 and 101 copies. All samples which were negative for viral DNA were diluted 10-fold and the PCR assay was repeated.
Statistics
For comparison of means, data were analyzed by ANOVA followed by a Student’s t test comparison. The primary comparisons were the comparison of the treated groups to vehicle alone. Statistics were not adjusted for the multiple comparisons. Incidence data were compared by Fisher’s exact test. All comparisons are two-tailed. Data are presented as means and standard deviation. Pearson’s correlation coefficient was used to evaluate the correlation between latent virus loads in the DRG and recurrent lesions and recurrent virus shedding.
Results
Treatment of acute genital herpes
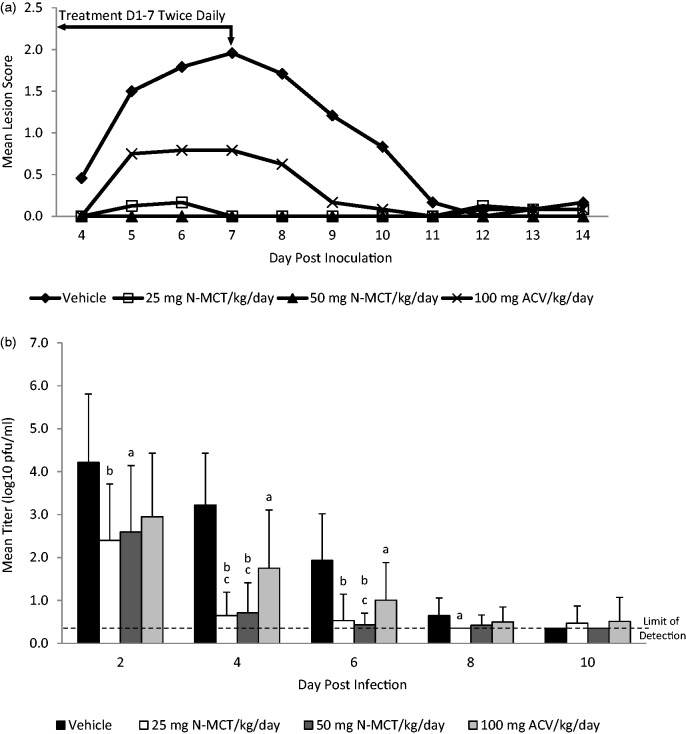
As seen in Figure 1(a), treatment with either ACV or the two N-MCT doses decreased the severity of primary disease. Treatment with ACV significantly reduced the mean lesion score from 9.9 in the vehicle group to 3.5 (p = 0.004), while treatment with 25 mg/kg N-MCT or 50 mg/kg N-MCT further reduced the score to 0.58 (p = 0.007 vs. ACV) and to 0.00 (p = 0.001 vs. ACV), respectively. Significantly, none of the animals developed visible disease in the 50 mg/kg N-MCT group compared to 8 of 12 (67%) in the ACV group (p = 0.002) and 10 of 12 (83%) in the vehicle group (p < 0.001). Similarly, while reductions in virus replication were seen for each treatment, the reduction with N-MCT was greater than ACV and virus levels were reduced more rapidly by N-MCT compared to ACV (Figure 1(b)). For example, at 4 dpi, virus titers were reduced from 3.3 log10 pfu/ml in the vehicle group to 1.75 log10 pfu/ml by ACV, but further reduced by another log to 0.71 log10 pfu/ml (p < 0.05 vs. ACV) and 0.65 log10 pfu/ml (p < 0.05 vs. ACV) by 25 mg/kg and 50 mg/kg of N-MCT, respectively.
Figure 1.
Effect of treatment on acute infection (days 0–14). Following intravaginal inoculation of 1 × 106 pfu of HSV-2 (MS strain) animals were treated with either saline, 25 mg N-MCT/kg/day, or 50 mg N-MCT/kg/day, or 100 mg ACV/kg/day. The animals were treated twice daily IP for seven days starting 24 h post inoculation. Primary genital skin disease was quantified using a lesion score-scale ranging from 0 representing no disease to 4 representing severe vesiculoulcerative skin disease of the perineum. Vaginal swabs were obtained on the days indicated. (a) Severity of acute disease. (b) Titer of virus detected in vaginal swabs.
ap vs. vehicle < 0.05, bp vs. vehicle < 0.001, cp vs. 100 mg ACV/kg/day < 0.05.
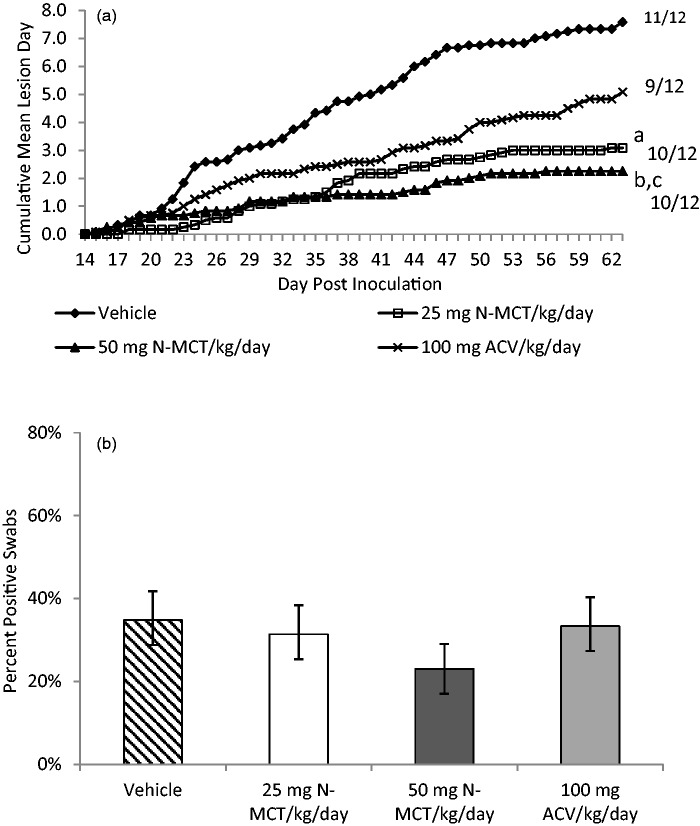
Treatment of the acute disease did not reduce the number of animals that developed subsequent recurrences. However, as shown in Figure 2(a), treatment with both 25 and 50 mg/kg/day of N-MCT significantly reduced the number of days with recurrent lesions from 7.6 ± 3.8 days in the vehicle group to 3.1 ± 3.0 days (p = 0.004) and 2.3 ± 1.9 days (p < 0.001) for the 25 mg/kg and 50 mg/kg N-MCT groups, respectively (Figure 2(a)). The difference between the ACV group (5.1 ± 3.8 recurrent lesion days) and the 50 mg/kg dose was also significant (p = 0.03). Of importance, only treatment with 50 mg/kg/day N-MCT decreased the number of days that recurrent virus shedding was detected from 34.8% in the vehicle group to 23.0% (p = 0.01) (Figure 2(b)). Animals in the 50 mg/kg/day N-MCT group were also the only group to shed less virus on the days that virus was detected by PCR (1.27 ± 0.70 log10 genomic copies/µg DNA for the 50 mg/kg/day N-MCT group compared to 2.17 ± 0.42 log10 genomic copies/µg DNA for the vehicle group, p < 0.001) (data not shown).
Figure 2.
Effect of treatment of acute disease on subsequent recurrent infection (days 15–62). Following intravaginal inoculation of 1 × 106 pfu of HSV-2 (MS strain) animals were treated with either saline, 25 mg N-MCT/kg/day, or 50 mg N-MCT/kg/day, or 100 mg ACV/kg/day. The animals were treated twice daily IP for seven days starting 24 h post inoculation. We then assessed animals daily for the presence of recurrent lesions. (a) Mean number of days with recurrent lesions. Number to the right is the number of animals with any recurrent lesion/total number of animals in each group. The p values refer to the comparison of the mean number of recurrences per animal. (b) Recurrent virus shedding (percent of days that virus was detected in vaginal swabs). The p values compare the percent of positive swabs in the groups.
ap vs. vehicle = 0.004, bp vs. vehicle < 0.001, cp vs. 100 mg ACV/kg/day < 0.05, dp vs. vehicle < 0.01.
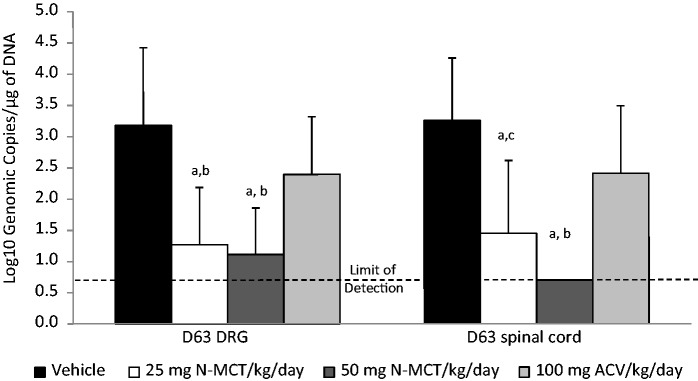
Similar to the data for recurrent lesions and recurrent shedding, N-MCT treatment during the acute disease leads to a significant decrease in the latent viral load of the DRG and the spinal cord (Figure 3). Viral genome was detected in 10 of 12 animals in the vehicle group, with a mean viral load of 3.18 ± 1.25 mean log10 genomic copies/µg DNA, whereas latent viral DNA was detected in 4/12 of the 25 mg/kg/day N-MCT group and only 2 of 12 of the 50 mg/kg/day N-MCT group (1.15 ± 0.69 mean log10 genomic copies/µg DNA (p < 0.001) and 0.98 ± 0.66 mean log10 genomic copies/µg DNA (p < 0.001, respectively). ACV treatment did not significantly reduce the viral load in the DRG (2.28 ± 1.05 mean log10 genomic copies/µg DNA, p = 0.068). Similar findings were seen for the spinal cord (Figure 3). Significantly, treatment with 50 mg/kg/day N-MCT completely prevented latent infection of the spinal cord.
Figure 3.
Effect of treatment of acute disease on the quantity of latent virus detected in the dorsal root ganglia (DRG) and spinal cord. Following the observation of animals for recurrent disease animals were sacrificed and the quantity of virus evaluated by qPCR. ap vs. vehicle < 0.001, bp vs. 100 mg ACV/kg/day < 0.005, cp vs. 100 mg ACV/kg/day < 0.05.
N-MCT pharmacokinetics
APCI is more sensitive and easier to interpret than ESl for thymine-containing nucleoside analogs. Both the total-ion chromatogram and the wavelength-specific chromatogram at 273 nm indicated a single major peak that corresponded to unmetabolized N-MCT. Table 1 shows the concentrations that were obtained at doses of 25 mg/kg and 50 mg/kg N-MCT. Plasma concentrations of N-MCT were proportional to dose, and equivalent concentrations obtained at 3 and 7 days of treatment. Plasma levels of N-MCT were 45- to 85-fold higher than its IC50 of 0.1 µg/ml against HSV-2.22
Table 1.
Concentrations of N-MCT (ng/ml) in guinea pig plasma.
| Sample No. | Dose (mg/kg) | Day 3 | 7 |
|---|---|---|---|
| 1 | 25 | 3800 | 4000 |
| 2 | 25 | 6240 | 5200 |
| 3 | 25 | 3060 | 4540 |
| 4 | 25 | 3750 | 4500 |
| 5 | 25 | 4850 | 3510 |
| 6 | 25 | 5580 | 5910 |
| Mean | 4550 | 4610 | |
| SE | 500 | 350 | |
| 7 | 50 | 7550 | 7610 |
| 8 | 50 | 8680 | 7320 |
| 9 | 50 | 6590 | 8770 |
| 10 | 50 | 10,600 | 6450 |
| 11 | 50 | 7450 | 8430 |
| 12 | 50 | 10,100 | 10,100 |
| Mean | 8500 | 8110 | |
| SE | 650 | 520 |
Correlation of latency to recurrent disease and recurrent virus shedding
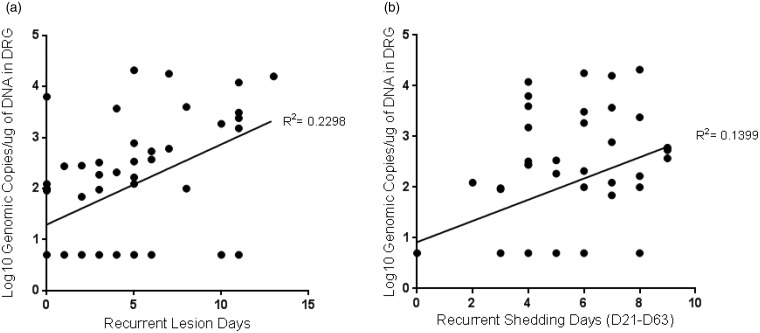
Data evaluating the relationship between the quantity of latent virus (the pool of virus available to reactivate) and the frequency of recurrent disease or recurrent virus shedding are limited. We therefore used the data from the above experiment to address the hypothesis that there would be a positive correlation between these data. We found that the number of recurrent lesion days (p < 0.001) and recurrent shedding days (p = 0.009) were correlated to the amount of latent virus (Figure 4).
Figure 4.
Correlation of recurrent illness to the quantity of latent virus detected in the DRG. (a) Correlation with recurrent lesion days. (b) Correlation with recurrent virus shedding (the number of days virus was detected in vaginal swabs collected during the recurrent disease observation period).
Treatment of recurrent genital herpes
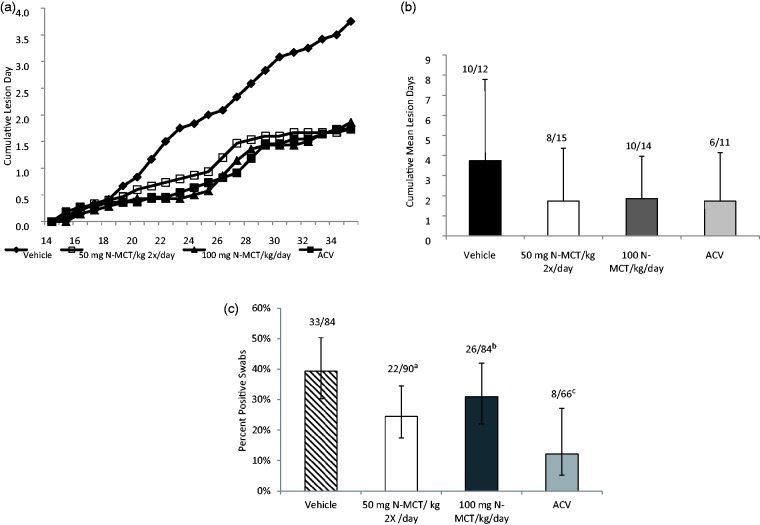
In the next experiment, we examined whether treatment of latently infected guinea pigs with N-MCT would reduce the number of recurrent lesion days or recurrent shedding days (Figure 5a, b). In this study, animals were administered 100 mg N-MCT daily, either twice daily (50 mg/kg/dose) or once a day (100 mg/kg/dose) starting at 15 dpi and continued through 35 dpi. During this treatment period, both N-MCT treatments reduced the recurrent lesion days by about 50% from 3.8 ± 4.1 in the vehicle to 1.7 ± 2.6 and 1.9 ± 2.1, respectively, for the twice a day and once a day N-MCT groups. The reduction by continuous oral administration of ACV in the drinking water was similar (1.7 ± 2.4). These reductions were not significantly different compared to the vehicle group (p = 0.13–0.17). Twice daily N-MCT did significantly reduce the number of days of recurrent virus shedding from 39.3% in the vehicle group to 24.4% (p < 0.05) (Figure 5(c)). Continuous ACV (12.1%) was, however, the most effective therapy, although it was administered on a continuous basis equivalent to approximately 450–900 mg/kg/day.
Figure 5.
Effect of treatment of recurrent infection. Animals were infected and treatment begun on day 15 for 21 days. Animals received either saline, IP, twice daily, 50 mg N-MCT/kg/day, IP twice daily, 100 mg N-MCT/kg/day, IP once daily, or 5 mg/ml ACV, ad libitum in drinking water. Animals were observed daily for evidence of recurrent lesions. (a) Cumulative recurrent lesion days. (b) Mean number of days with recurrent lesions. Lines above the columns are the number of animals with recurrent shedding/total animals in each group. (c) Recurrent virus shedding (percent of days that virus was detected in vaginal swabs). Number above columns is the number of positive swabs/the total number of swabs obtained. Error bars are 95% confidence intervals. The p values compare the percent of positive swabs in the groups.
ap vs. vehicle < 0.05, bp vs. ACV < 0.007, cp vs. vehicle < 0.003.
Discussion
Treatment of primary genital herpes should reduce the severity of lesions and, if provided early enough in the disease process, lower the amount of virus that becomes latent. Presumably lowering the burden of latent virus would lead to reduction in virus that can reactivate and cause recurrent disease and recurrent virus shedding. In the studies presented here, IP administration of N-MCT was significantly more effective than high dose ACV in reducing the severity of primary genital herpes disease and in preventing the development of primary disease. Further, N-MCT more effectively reduced local replication in the genital tract and the amount of latent virus in the DRG, the site of latency, compared to ACV. Reductions in recurrent disease and recurrent virus shedding were also greater with N-MCT. These effects were undoubtedly related to the high plasma levels of N-MCT achieved after IP administration, which far exceeded its IC50 against HSV-2.22 Thus, there were clear advantages to N-MCT compared to ACV when used early for the treatment of primary genital herpes. This is consistent with the improved outcomes seen in neonatal HSV disease in guinea pigs when N-MCT was compared to ACV.14 IP administration was chosen to mimic IV administration and provide the greatest opportunity for success. Previous studies have shown that oral administration is also effective against HSV infections.13 To date there have not been any reports of toxicity in the preclinical evaluations.
There is also a need to improve the effectiveness of antiviral treatment when used for recurrent HSV disease. Present day use of nucleoside analogs reduces recurrent disease and decreases, but does not prevent recurrent virus shedding.6–8 In the studies presented here, N-MCT reduced the number of recurrent lesion days by a similar amount compared to ACV, when ACV was continually supplied in the drinking water. N-MCT also reduced the number of days with recurrent virus shedding but was not as effective as ACV. However, the continual administration of ACV in the drinking water provided an advantage to this drug compared to N-MCT, as most recurrent HSV disease is due to frequent short duration events.23
The use of two antivirals that decreased latent virus load when used to treat primary genital herpes in guinea pigs provided an opportunity to correlate latent virus load and recurrent disease and shedding. Previous experiments using ACV had shown a high correlation of latent virus load and in vivo reactivation of latent HSV-1 in mice following heat shock.24 To our knowledge, this is the first comparison of recurrent virus shedding and latent virus load in the guinea pig genital model. The correlation between detection of reactivated HSV-1 in the trigeminal ganglia and the burden of latent virus reported previously24 was considerably higher than the correlation of latent HSV-2 and spontaneous reactivation reported here. This is most likely due to the need for events following reactivation that are required to produce the end points measured here, lesions and genital tract shedding compared to the previous report detecting reactivated virus in the ganglia. Thus, in our model of spontaneous recurrences, after reactivation the virus must exit the neurons and replicate in the genital tract to produce lesions or enough virus to detect in vaginal swabs. These additional steps could provide variability that leads to the reduced correlation that we report here. It is also possible that HSV-2 or genital HSV infections have sites of persistent virus other than the DRG.
In summary, N-MCT had advantages compared to ACV for treating primary genital HSV-2 infections and was as effective as continuous treatment with ACV. N-MCT should be further evaluated in clinical trials for the treatment of genital herpes infections.
Acknowledgments
We would like to thank Julie Earwood and Jennifer Clark for their technical assistance.
Funding
This work was supported by the National Institute of Health Contract: Mouse and Guinea Pig Models for Herpesviruses: National Institutes of Health (NIH/NIAID) antiviral testing program (National Institute of Health Contract No.: HHSN272201000008I) to Cincinnati Children’s Hospital Medical Center.
Conflict of interest
Aquilur Rahman is employed by N & N Pharmaceuticals Inc. RIG and DIB are consultants for N&N Pharmaceuticals Inc. All others have no conflict of interest.
References
- 1.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 2008; 86: 805–812, A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis 2008; 8: 490–497. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20: 73–83. [DOI] [PubMed] [Google Scholar]
- 4.Bryson YJ, Dillon M, Lovett M, et al. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med 1983; 308: 916–921. [DOI] [PubMed] [Google Scholar]
- 5.Wald A, Benedetti J, Davis G, et al. A randomized, double-blind, comparative trial comparing high- and standard-dose oral acyclovir for first-episode genital herpes infections. Antimicrob Agents Chemother 1994; 38: 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas JM, Critchlow C, Benedetti J, et al. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med 1984; 310: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 7.Wald A, Zeh J, Barnum G, et al. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996; 124: 8–15. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Selke S, Warren T, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis 2006; 33: 529–533. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350: 11–20. [DOI] [PubMed] [Google Scholar]
- 10.Marquez VE, Hughes SH, Sei S, et al. The history of N-methanocarbathymidine: the investigation of a conformational concept leads to the discovery of a potent and selective nucleoside antiviral agent. Antiviral Res 2006; 71: 268–275. [DOI] [PubMed] [Google Scholar]
- 11.Prichard MN, Keith KA, Quenelle DC, et al. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob Agents Chemother 2006; 50: 1336–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalah L, Huleihel M, Manor E, et al. Metabolic pathways of N-methanocarbathymidine, a novel antiviral agent, in native and herpes simplex virus type 1 infected Vero cells. Antiviral Res 2002; 55: 63–75. [DOI] [PubMed] [Google Scholar]
- 13.Quenelle DC, Collins DJ, Rice TL, et al. Efficacy of orally administered low dose N-methanocarbathymidine against lethal herpes simplex virus type-2 infections of mice. Antivir Chem Chemother 2011; 22: 131–137. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein DI, Bravo FJ, Clark JR, et al. N-Methanocarbathymidine is more effective than acyclovir for treating neonatal herpes simplex virus infection in guinea pigs. Antiviral Res 2011; 92: 386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schelling P, Claus MT, Johner R, et al. Biochemical and structural characterization of (South)-methanocarbathymidine that specifically inhibits growth of herpes simplex virus type 1 thymidine kinase-transduced osteosarcoma cells. J Biol Chem 2004; 279: 32832–32838. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein DI, Stanberry LR, Harrison CJ, et al. Antibody response, recurrence patterns and subsequent herpes simplex virus type 2 (HSV-2) re-infection following initial HSV-2 infection of guinea-pigs: effects of acyclovir. J Gen Virol 1986; 67 (Pt 8): 1601–1612. [DOI] [PubMed] [Google Scholar]
- 17.Bourne N, Stanberry LR, Kern ER, et al. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob Agents Chemother 2000; 44: 2471–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanberry LR, Bernstein DI, Burke RL, et al. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis 1987; 155: 914–920. [DOI] [PubMed] [Google Scholar]
- 19.Ellis MN, Barry DW. Oral acyclovir therapy of genital herpes simplex virus type 2 infections in guinea pigs. Antimicrob Agents Chemother 1985; 27: 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein DI, Cardin RD, Bravo FJ, et al. Topical SMIP-7.7, a toll-like receptor 7 agonist, protects against genital herpes simplex virus type-2 disease in the guinea pig model of genital herpes. Antivir Chem Chemother 2014; 23: 189–196. [DOI] [PubMed] [Google Scholar]
- 21.Skoberne M, Cardin R, Lee A, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol 2013; 87: 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquez VE, Siddiqui MA, Ezzitouni A, et al. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J Med Chem 1996; 39: 3739–3747. [DOI] [PubMed] [Google Scholar]
- 23.Schiffer JT, Swan DA, Corey L, et al. Rapid viral expansion and short drug half-life explain the incomplete effectiveness of current herpes simplex virus 2-directed antiviral agents. Antimicrob Agents Chemother 2013; 57: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawtell NM, Thompson RL, Stanberry LR, et al. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis 2001; 184: 964–971. [DOI] [PubMed] [Google Scholar]