Abstract
Background
Virus replication strongly depends on host metabolic machinery and essential cellular factors, in particular, on amino acid profiles. Amino acids play an important role in the pathogenesis of all virus-related infections both as basic substrates for protein synthesis and as regulators in many metabolic pathways, including gene expression. The inhibitory effects of deficiency or excess of these essential elements on virus replication are widely appreciated. Although the same interrelationship between host cellular factors and HIV have been recognized for a long time, the effects of amino acids on HIV-1 RNA replication dynamic is not yet well documented. Our aim was to determine in this pilot study the direct effect of L-lysine amino acid on HIV-1 RNA replication in vitro in HIV-infected patients.
Methods
A total of 100 HIV-1-infected males without highly active antiretroviral therapy (HAART) were monitored in our center. The patients were in stage A of the disease according to the 1993 Centers for Disease Control (CDC) classification system for HIV-infection. Patients with HIV were enrolled in one stage (A) of the disease with the average amount CD4 lymphocytes in the range of 200–300 cells/µL at the time of sample acquisition. For evaluation of the effects of essential L-lysine amino acid on HIV-1 RNA replication level, we used a model of amino acid-excess system in vitro following incubation of plasma samples for 24 h at 25°C. Quantitative HIV-1 RNA assay was performed using (RT-PCR) reverse-transcriptase polymerase chain reaction (Rotor-Gene Q, QIAGEN, Germany).
Results
The mean HIV-1 RNA levels were significantly higher in the enriched peripheral blood mononuclear cells plasma samples HIV-infected subjects after 24 h incubation at 25°C temperature than in the plasma samples the same patients studied on the date of blood tests (p < 0.0001). The number of HIV-1 RNA copies increased in 1.5 times. We observed that in plasma of the same HIV-infected patients after adding L-lysine and following incubation in vitro, viral load increased significantly in comparison with standard samples (p < 0.0001). The increased viral load was found in 100/92 (92%) of HIV-infected subjects. The average number of HIV-1 RNA copies in samples had increased by 4.0 times. However, we found no difference in HIV-1 RNA levels after replacement of L-lysine for L-arginine in comparison samples in the same HIV-infected patients. It is obvious that the addition of L-arginine does not increase viral replication in vitro as L-lysine amino acid supplement does. Additionally, no increase in viral load was determined after adding L-lysine and non toxic doses of its inhibitor (L-lysine alpha-oxidase) in plasma samples.
Conclusions
The results show that L-lysine amino acid excess is characterized by significant increased of HIV-1 RNA copies in enriched peripheral blood mononuclear cells plasma samples of HIV-infected patients. There was evidence for an association between L-lysine supplementation and HIV-1 RNA replication and the level changes of this host essential nutritional element play a key role in the synthesis of the virus proteins and in transcription initiation of the retrovirus life cycle. High intake of L-lysine amino acid may increase the risk of high viral load, subsequent acceleration of immunosuppression and HIV progression. Overall results demonstrate that the simple L-lysine-related model in vitro can be widely used for practical purposes to evaluate HIV-1 RNA replication dynamic, disease prognosis and new approaches in treatment of the patients with human immunodeficiency virus. Although the impact mechanism of L-lysine amino acid on the viral load in the pathogenesis of HIV-infection is at present conjectural and requires further development, the results highlight an interesting target in antiviral therapy, and this statement remains to be proved in further research and clinical trials.
Keywords: HIV, plasma L-lysine, viral load
Introduction
Despite the considerable efforts of the world community and the optimistic expectations of achieving the main goals in the response to new cases of HIV-infection (such as universal access to prevention, treatment, care, and support) the HIV/AIDS pandemic remains a major health problem. UNAIDS estimates that about 35.0 million people are living with HIV, with 2.1 million new infections in 2013 and an estimated 1.5 million deaths from HIV-related illnesses in the same year.1,2
The development of new methods and approaches in the treatment of HIV-infection in the era of highly active antiretroviral therapy remains an urgent and pressing need. Understanding the pathogenesis of HIV infection and finding points of vulnerability in the relationships between the infecting organism and the virus is a key to achieving full control of HIV infection.
Viral infections usually cause the host significant metabolic disturbances associated with direct toxic and cytopathic effects like intracellular parasites using the host cell resources for their energy, construction, and information needs.3–20 Monitoring of these metabolic changes is important for understanding the disease pathogenesis and getting a new approach for the possible potential effect on the infectious agent.
Significant changes in the host protein and lipid metabolism, catabolic effects and the negative nitrogen equilibrium lead to alterations in amino-acid profiles due to viral infection.21–23 At the same time, observational studies indicate that amino acid imbalances in the extracellular medium were found to be critical in the life cycle of some viruses and are related to increased risk of diseases rapidly progressing.24–30 However, there are few data in the literature about infection-related amino acid imbalance in HIV-infected patients.31–35 In virus infection, this basic element is not only useful for cellular and virus protein synthesis36–38 but also act as regulators of gene expression via the mechanism of amino-acid-regulated transcription factors.39–41
The presence or absence of amino acids in the extracellular medium and its concentrations in the cell modulate specific transcription factors to promote or repress the synthesis of multiple genes.42–44 The same mechanism is well known in HIV pathogenesis. HIV requires a cellular tRNALys as a primer for initiation of reverse transcription and HIV is not infectious unless the cellular tRNALys interacts directly on the specific region of the viral RNA genome.45–49 At the same time, only the presence of a sufficient concentration of the covalent amino acid in the cytoplasm activates the cellular tRNA.50–57 We assume that it is a universal rule that also applies to the HIV infection pathogenesis.
For today, the data on the relationship between the plasma amino acid profile and viral load in HIV-infected patients requires further confirmation and the purpose of the present study was to detect a possible direct effect of the L-lysine amino acid on the HIV-1 RNA replication in vitro.
Material and methods
Patients
We performed a case-control study of 100 HIV-1-infected males (age range, 23–38 years; mean ± s.d.; 32 ± 4.3 years), who were periodically monitored in the Municipal Center of HIV/AIDS prophylaxis, Surgut, Russian Federation. The patients were in stage A of the disease according to the 1993 Centers for Disease Control (CDC) classification system for HIV-infection. We ascertained patients’ general attributes (age, sex), medical history, co-infections and the simultaneous survey period. From the entire cohort, all patients did not receive highly active antiretroviral therapy (HAART).
Blood samples for this study were collected during routine clinical control and analytical monitoring, which included immunological and virological evaluation. The plasma samples of 100 HIV-1-infected males were separated into next groups as follows.
Three test samples from each of 100 HIV-1-infected patients (group 1):
plasma samples 1 after 24 h incubation at 25°C.
plasma samples 2 after adding 10.0 mcg L-lysine and following incubation for 24 h at 25°C.
standard plasma samples studied on the date of blood tests.
Three test samples from each of 15 the same HIV-1-infected patient (group 2 or controls 1):
plasma samples 1 after 24 h incubation at 25°C.
plasma samples 2 after adding 10.0 mcg L-arginine instead of L-lysine and following incubation for 24 h at 25°C.
standard plasma samples studied on the date of blood tests.
Three test samples from each of 15 the same HIV-1-infected patient (group 3 or controls 2):
plasma samples 1 after 24 h incubation at 25°C.
plasma samples 2 after adding 10.0 mcg L-lysine and L-lysine alpha-oxidase (0.3 mcg/mL) with following incubation for 24 h at 25°C.
standard plasma samples studied on the date of blood tests.
Author warrants that the Municipal Center of HIV/AIDS prophylaxis has approved the protocol for any investigation involving humans and that all experimentation was conducted in conformity with ethical and humane principles of research. Blood samples were obtained after informed consent from the patients.
Laboratory studies
Fasting venous blood samples for measurement of HIV-1 RNA levels were collected in 5.0 mL tubes containing 1.6 mg/mL K2 EDTA (BD Vacutainer®, USA) and were centrifuged (3500 r/min; 10 min). Aliquots, the plasma of 1000 µL, were pipetted into Eppendorf tubes and were enriched peripheral blood mononuclear cells (PBMC) the same patient, and immediately analyzed on the date of blood tests in the same laboratory (control samples). The plasma samples 1 were analyzed after 24 h incubation at 25°C. The plasma samples 2 were enriched peripheral blood mononuclear cells (PBMC) the same patient and were analyzed after adding L-lysine in quantity 10.0 mcg and following incubation for 24 h at 25°C.
Quantification HIV-1 RNA assay was performed by quantitative competitive (RT-PCR) reverse-transcriptase polymerase chain reaction using the commercially available Amplisens® HIV-monitor-FRT kit (Amplisens®, Russian Federation) and real-time PCR cycler (Rotor-Gene Q, QIAGEN, Germany). No significant differences in plasma HIV-1 RNA levels were observed in repeat samples obtained from any given patient. The overall reproducibility of results was consistent to within ±7%.
Statistical analysis
Plasma HIV-1 RNA copies/mL values follow a Gaussian distribution (Kolmogorov-Smirnov) in total cohort, so the Student’s t-test was applied to compare viral load in comparison groups, with a 95% confidence interval. Data are reported as mean ± s.d. (standard deviation), and p-values below 0.05 were considered to indicate statistical significance. Statistical analyses were performed using the statistical software package Biostat®.
Results
HIV-1 RNA replication in plasma samples of HIV-infected patients in vitro (groups 1, 2, 3)
We ascertained patients’ general attributes (age, sex), medical history, and stage of HIV infection, co-infections and the simultaneous survey period, without previous antiretroviral therapy.Clinical data, stages and immunological status of HIV-infected subjects are summarized in Table 1.
Table 1.
Clinical data, hematological, and immunological characteristics of HIV-infected patients.
| Characteristic (mean ± s.d.) | HIV-infection patients in stage A (CDC, 1993) |
||
|---|---|---|---|
| Group 1 (n = 100) | Group 2 (n = 15) | Group 3 (n = 15) | |
| Age (years) | 32 ± 4.3 | 31 ± 5.8 | 31 ± 5.8 |
| Sex (F/M) | 0/100 | 0/15 | 0/15 |
| Antiviral treatment | 0 | 0 | 0 |
| Duration of HIV (months) | 31 ± 12.3 | 28 ± 10.1 | 28 ± 10.1 |
| HIV-1 risk factor (IDU) | 100 | 15 | 15 |
| CD4 (cells/µL) | 238.4 ± 58.9 | 241.1 ± 52.3 | 241.1 ± 52.3 |
| CD8 (cells/µL) | 997.0 ± 128.3 | 934.2 ± 157.6 | 934.2 ± 157.6 |
The amount of HIV-1 RNA copies were evaluated in three plasma samples from each of 100 HIV-infected individuals divided into three groups:
plasma samples 1 after 24 h incubation at 25°C;
plasma samples 2 after adding L-lysine or L-arginine (or L-lysine alpha-oxidase) and following incubation for 24 h at 25°C;
standard plasma samples studied on the date of blood tests.
We observed that the mean HIV-1 RNA levels were significantly higher in the plasma samples after 24 h incubation at 25°C temperature than in the plasma samples the same patients studied on the date of blood tests (p < 0.0001). The increase viral load was determined in 100/98 (98%) of HIV-infected subjects. The number of HIV-1 RNA copies increased in 1.5 times. Viral load was not increased after replacement of L-lysine for L-arginine or adding its inhibitor (L-lysine alpha-oxidase) in plasma samples of HIV-infected subjects. No previous studies are available for comparison.
The data obtained showed that viral replication in patients with HIV in one stage A has significant differences. The results of this study allow us to quantify and evaluate the dynamic of viral replication using a comparison of plasma samples after 24 h incubation at 25°C and standard sample in the same patient.
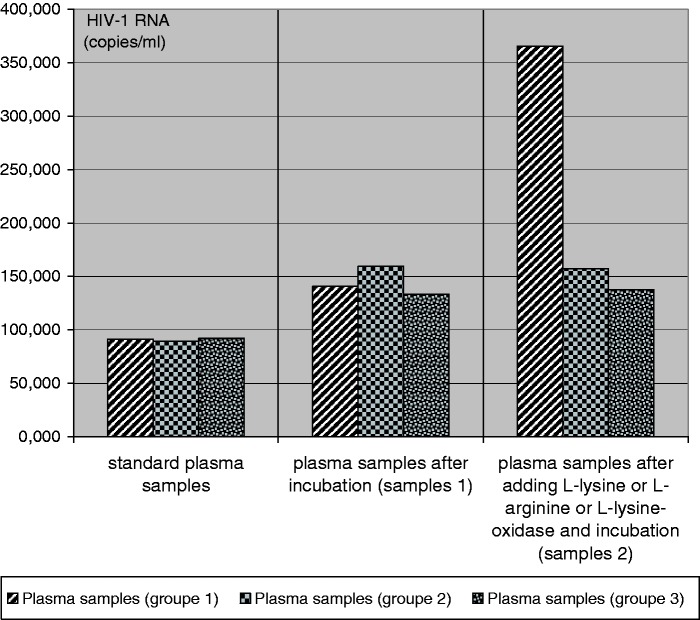
Table 2 and Figure 1 summarize the results on virus replication in samples 1, 2 of HIV-infected patients (groups 1, 2, 3).
Table 2.
Compared HIV-1 RNA levels in plasma samples 1, 2 of HIV-infected patients from groups 1, 2, 3.
| Standard plasma samples | Samples 1 (plasma samples after incubation) | Samples 2 (plasma samples after adding L-lysine or L-arginine or L-lysine alpha-oxidase and incubation) | |
|---|---|---|---|
| Group 1 | n = 100 | n = 100 | n = 100 |
| HIV-1 RNA (copies/mL) | 91,300 ± 12,100a,b | 140,500 ± 16,400a,c | 365,500 ± 42,400b,c |
| L-lysine supplement (mcg/mL) | – | – | 10.0 |
| Incubation of samples | – | 24 h at 25°C | 24 h at 25°C |
| Group 2 (controls 1) | n = 15 | n = 15 | n = 15 |
| HIV-1 RNA (copies/mL) | 89,150 ± 9900d,e | 159,300 ± 14,120d,f | 157,400 ± 12,950e,f |
| L-arginine supplement (mcg/mL) | – | – | 10.0 |
| Incubation of samples | – | 24 h at 25°C | 24 h at 25°C |
| Group 3 (controls 2) | n = 15 | n = 15 | n = 15 |
| HIV-1 RNA (copies/mL) | 92,000 ± 10,300e | 133,300 ± 12,050e | 137,400 ± 11,350 |
| L-lysine supplement (mcg/mL) and L-lysine alpha-oxidase (mcg/mL) | – | – | 10.0 + 0.3 |
| Incubation of samples | – | 24 h at 25°C | 24 h at 25°C |
Controls versus samples 1, p < 0.0001.
Controls versus samples 2, p < 0.0001.
Samples 2 versus samples 1, p < 0.0001.
Controls versus samples 1, p < 0.01.
Controls versus samples 2, p < 0.001.
Samples 2 versus samples 1, p = 0.8.
Values are given as mean ± s.e. (range).
Figure 1.
Compared HIV-1 RNA levels in plasma samples 1, 2 of HIV-infected patients (groups 1, 2, 3).
The figure shows that L-lysine amino acid excess is characterized by significant increased of viral load in plasma samples of HIV-infected patients before and after antiretroviral therapy. The mean HIV-1 RNA levels were significantly higher in the samples 1 after 24 h incubation at 25°C than in the standard plasma samples (controls) of the same patients. The number of HIV-1 RNA copies increased in 1.5 times after 24 h of incubation. Viral load increased significantly in samples (2) of the same HIV-infected patients after adding L-lysine amino acid in comparison with the samples (1 and controls). The average number of HIV-1 RNA copies in samples 2 had increased by 4.0 times. We found no difference in viral load after replacement of L-lysine for L-arginine between samples 1 and 2. It is obvious that the addition of L-arginine does not increase viral replication in vitro as L-lysine amino acid supplement does. We found no significant increase in viral load after adding L-lysine (10.0 mcg/mL) and nontoxic doses of its inhibitor (L-lysine alpha-oxidase, 0.3 mcg/mL) in plasma samples HIV-infected patients (controls 2).
HIV-1-infected patient (group 1, n = 100)
The mean HIV-1 RNA copies/mL were significantly higher in the plasma samples 1 after 24 h incubation at 25°C than in the standard plasma samples of the same patients studied on the date of blood tests (140,500 ± 16,400 and 91,300 ± 12,100, p < 0.0001, Student’s t-test). The number of HIV-1 RNA copies increased in 1.5 times after 24 h of incubation.
We found that in samples 2 (365,500 ± 42,400) after adding L-lysine amino acid and following incubation, viral load was higher in comparison with the samples 1 and standard samples (140,500 ± 16,400 and 91,300 ± 12,100, p < 0.0001, and p < 0.0001 respectively, Student’s t-test). The average number of HIV-1 RNA copies in samples 2 has increased in 4.0 times (p < 0.0001, Student’s t-test) in the total cohort.
L-lysine amino acid was added in an amount of 10.0 mcg in each plasma sample. Amino acid of 10.0 mcg was optimal for the virus replication process in this experiment. Additional quantities of L-lysine did not cause a further increase viral replication.
HIV-1 RNA replication in plasma samples with replacement of additional L-lysine to L-arginine (group 2, n = 15)
The survey shows a significant increase of HIV-1 RNA copies/mL in plasma samples 1 and 2 the same HIV-infected patients (159,300 ± 14,120 and 183,400 ± 203,300) compared to controls (89,150 ± 9900, p < 0.01 and p < 0.001, Student’s t-test). However, we found no difference in viral load after replacement of L-lysine for L-arginine between samples 1 and 2 (195,900 ± 216,300 and 157,400 ± 12,950, P = 0.8, Student’s t-test).
It is obvious that the addition of L-arginine does not promote viral replication in vitro as L-lysine amino acid supplement does.
HIV-1 RNA replication in plasma samples with adding of L-lysine and its inhibitor (group 3, n = 15)
Additionally, we found no significant increase in viral load after adding L-lysine amino acid (10.0 mcg/mL) and nontoxic doses of its inhibitor (L-lysine alpha-oxidase, 0.3 mcg/mL) in plasma samples the same HIV-infected patients. It has been shown that the presence of L-lysine alpha-oxidase catalyzes the cleavage of L-lysine in the samples of plasma and inhibits HIV replication in vitro. The average number of HIV-1 RNA copies has increased by 1.3 times or from 92,000 to 137,400 copies/mL.
Discussion
Virus replication strongly depends on host metabolic machinery and essential cellular factors, in particular, on amino acid profiles. Amino acids play an important role both as basic substrates for protein synthesis and as regulators in many metabolic pathways, including gene expression in virus diseases.18–23 Specific abnormalities or deficiencies of these essential elements, associated with virus-related conditions are widely appreciated.15–17,19–31 The available data clearly showed the inhibition of the multiplication and abortive replication cycle of some viruses by low levels of amino acids in extracellular medium.24–30 The production of significant amounts of empty virus particles, free of viral nucleic acids has been reported for several virus systems in the absence of essential amino acids in cell cultures.21–26
Not many data exist on the amino acids imbalance in HIV infection32–38 but numerous studies have thoroughly examined the key role of tRNALys as a primer activation of reverse transcriptase in the HIV pathogenesis.45–48 There is strong evidence of the presence, in each viral particle, a number of host tRNALys able to make a rapid and guaranteed next replication initiation. HIV-1 virions appear to contain approximately 8 to 25 tRNALys molecules per two copies of viral genome.49 Meanwhile, any amino acid tRNA activity depends on the concentration of homonymous covalent substrate in the host cytoplasm.57 This is one of the vulnerable and weak points in the life cycle of HIV and in the absence or insufficient quantity of this essential intracellular element, an “effective” replication of the virus will be under threat.
We hypothesized previously that L-lysine presence in extra and related intracellular media can be particularly significant for pathogenesis human immunodeficiency virus and directly related to HIV viral load. The previous findings support this hypothesis and confirm the postulate that the plasma L-lysine concentration affects the activity and dynamic of HIV-1 replication. Our results show that advanced stages of HIV disease are characterized by significant changes of plasma L-lysine concentrations with the disease stages of HIV infection and levels of this basic amino acid negatively correlating with viral load.58
This study has shown that HIV-1 RNA levels were significantly higher in the plasma samples after incubation. The number of HIV-1 RNA copies increased an average 1.5 times.
Viral load is constantly changing during HIV infection and is a widely recognized marker in the monitoring of disease progression and treatment efficacy. Now the HIV-1 RNA level is determined as the standard static parameter, which does not reflect the real dynamic of the virus replication process. Using the simple technique will let us to assess the dynamic of HIV replication for better monitoring of the disease in the context of clinical trials.
The next stage of the experiment allowed us to determine the reproduction rates by the addition of L-lysine amino acid in vitro. We observed that after adding L-lysine and following incubation, viral load was significantly higher in comparison with the standard samples in the same HIV-infected patients. At this stage of the study, the average number of HIV-1 RNA copies has increased by 4.0 times.
Additionally, we found no difference in viral load after replacement of L-lysine for L-arginine. It is obvious that the addition of L-arginine does not promote viral replication as L-lysine supplement does and these findings confirm the exceptional role of L-lysine amino acid in the virus reproduction. This assertion also is supported by the following experiment: no increase in viral load was determined after adding L-lysine and non toxic doses of its inhibitor in plasma samples. The available data also confirm that the presence of L-lysine alpha-oxidase catalyzes the cleavage of amino acid and to inhibit HIV replication.59
Overall study results show that L-lysine excess in vitro is characterized by a significant increase of HIV-1 RNA copies in plasma samples of HIV-infected patients. There was evidence for an association between L-lysine supplementation and HIV-1 RNA reproduction and level changes of this host essential nutritional element play a key role in the retrovirus life cycle. Based on the obtained data, we can confidently assert that the intake limitation or deficiency of L-lysine can reduce HIV replication level in vitro and in vivo.
Although the impact mechanism of L-lysine amino acid on the viral load in the pathogenesis of HIV-infection is at present conjectural and requires further development, the results highlight an interesting target in antiviral therapy, and this statement remains to be proved in further research and clinical trials.
Acknowledgments
The author thanks the laboratory staff of the Municipal Center of HIV/AIDS prophylaxis (Surgut, Russian Federation) for their technical support and for their performance of routine hematological analyses.
Conflict of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Report on the global AIDS epidemic, 2013.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS). World AIDS Day Report – Results, 2013.
- 3.Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev 1996; 17(5): 518–532. [DOI] [PubMed] [Google Scholar]
- 4.Nunez M. Hepatitis C virus and metabolic disturbances. IAPAC 2005; 11(7): 216–217. [PubMed] [Google Scholar]
- 5.Macallan DC. Metabolic syndromes in human immunodeficiency virus infection. Horm Res 2001; 55(Suppl 1): 36–41. [DOI] [PubMed] [Google Scholar]
- 6.Maloberti A. Metabolic syndrome in human immunodeficiency virus-positive subjects, prevalence, phenotype, and related alterations in arterial structure and function. Metab Syndr Relat Disord 2013; 11: 403–411. [DOI] [PubMed] [Google Scholar]
- 7.Delgado T, Sanchez EL, Camarda R, et al. Global metabolic profiling of infection by an oncogenic virus, KSHV induces and requires lipogenesis for survival of latent infection. LoS Pathog 2012; 8: e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotler DP. Human immunodeficiency virus-related wasting, malabsorption syndromes. Semin Oncol 1998; 25: 70–75. [PubMed] [Google Scholar]
- 9.Hsu CS. Impact of hepatitis B virus infection on metabolic profiles and modifying factors. J Viral Hepat 2012; 19: e48–57. [DOI] [PubMed] [Google Scholar]
- 10.Ritter JB, Wahl AS, Freund S, et al. Metabolic effects of influenza virus infection in cultured animal cells: Intra- and extracellular metabolite profiling. BMC Syst Biol 2010; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhvi R, Markusen JF, Ky B, et al. Assessment of virus infection in cultured cells using metabolic monitoring. Cytotechnology 1996; 22(1–3): 79–85. [DOI] [PubMed] [Google Scholar]
- 12.Peeples M, Levine S. Metabolic requirements for the maturation of respiratory syncytial virus. J Gen Virol 1980; 50: 81–88. [DOI] [PubMed] [Google Scholar]
- 13.Becker Y, Asher Y. In vitro synthesis of DNA in nuclei isolated from herpes simplex virus-infected cells, untreated and treated with metabolic inhibitors. Virology 1975; 63: 209–220. [DOI] [PubMed] [Google Scholar]
- 14.Malinowski FC. The significance of metabolic competitors in the treatment of virus diseases. Can J Med Technol 1962; 24: 147–152. [PubMed] [Google Scholar]
- 15.Tamm I. Metabolic antagonists and selective virus inhibition. Clin Pharmacol Ther 1960; 1: 777–796. [PubMed] [Google Scholar]
- 16.Grunfeld C, Feingold K. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. Engl J Med 1992; 327: 329–337. [DOI] [PubMed] [Google Scholar]
- 17.Kotler DP. Preservation of short-term energy balance in clinically stable patients with AIDS. J. Clin Nutr 1990; 51: 7–13. [DOI] [PubMed] [Google Scholar]
- 18.Macallan DC. Wasting in HIV infection and AIDS. J Nutr 1999; 129(1S Suppl): 238–242. [DOI] [PubMed] [Google Scholar]
- 19.Beisel WR, Sawyer WD, Ryll ED, et al. Metabolic effects of intracellular infections in man. Ann Internal Med 1967; 67: 744–779. [DOI] [PubMed] [Google Scholar]
- 20.Wannemacher RW, Pekarek JR, Bartelloni PJ, et al. Changes in individual plasma amino acids following experimentally induced sand fly fever virus infection. Metabolism 1972; 21: 67. [DOI] [PubMed] [Google Scholar]
- 21.Wigand R, Kümel G. Amino acid requirement of adenovirus multiplication. J Gen Virol 1978; 39: 281–292. [DOI] [PubMed] [Google Scholar]
- 22.Tankersley RW. Amino acid requirements of herpes simplex virus in human cells. J Bacteriology 1964; 87: 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldblum N, Ravid Z, Becker Y. Effect of withdrawal of arginine and other amino acids on the synthesis of tumor and viral antigens of SV4o virus. J Gen Virol 1968; 3: 143–146. [DOI] [PubMed] [Google Scholar]
- 24.Everitt E, Sundquist B, Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural protein. J Virol 1971; 8: 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inglis VBM. Requirement of arginine for the replication of herpes virus. J Gen Virol 1968; 3: 9–17. [DOI] [PubMed] [Google Scholar]
- 26.Loh PC, Oie HK. Role of lysine in the replication of reovirus, I. Synthesis of complete and empty virions. J Virol 1969; 4: 890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito T. Antiviral effect of arginine against herpes simplex virus type 1. Int J Mol Med 2009; 23(4): 495–499. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki H, Tsujimoto K, Koyama AH, et al. Arginine facilitates inactivation of enveloped viruses. J Pharm Sci 2008; 97: 3067–3073. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda K, Yamasaki H, Suzuki Y, et al. Novel strategy with acidic arginine solution for the treatment of influenza a virus infection. Exp Ther Med 2010; 1: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feigin RD, Dangerfield HG. Whole-blood amino acid changes respiratory-acquired Pasteurella tularensis infection in man. J Infect Dis 1967; 117: 346. [DOI] [PubMed] [Google Scholar]
- 31.Griffith RS, DeLong DC, Nelson JD. Relation of arginine-lysine antagonism to Herpes simplex growth in tissue culture. Chemotherapy 1981; 27: 209–213. [DOI] [PubMed] [Google Scholar]
- 32.Miller CS, Foulke CN. Use of lysine in treating recurrent oral Herpes simplex infections. Gen Dent 1984; 32: 490–493. [PubMed] [Google Scholar]
- 33.Wannemacher RW., Jr Key role of various individual amino acids in host response to infection. Am J Clin Nutr 1977; 30: 1269–1280. [DOI] [PubMed] [Google Scholar]
- 34.Laurichesse H. Threonine and methionine are limiting amino acids for protein synthesis in patients with AIDS. J Nutr 1998; 128: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 35.Hortin GL, Landt M, Powderly WG. Changes in plasma amino acid concentrations in response to HIV-1 infection. Clin Chem 1994; 40: 785–789. [PubMed] [Google Scholar]
- 36.Schneider RJ, Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem 1987; 56: 317–332. [DOI] [PubMed] [Google Scholar]
- 37.Willems M, Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology 1966; 30: 355–367. [DOI] [PubMed] [Google Scholar]
- 38.Kääriäinen L, Ranki M. Inhibition of cell functions by RNA-virus infections. Annu Rev Microbiol 1984; 38: 91–109. [DOI] [PubMed] [Google Scholar]
- 39.Avin-Wittenberg T, Galili G. Metabolism: Amino acid regulatory wisdom. Nat Chem Biol 2012; 8: 23–24. [DOI] [PubMed] [Google Scholar]
- 40.Fafournoux P, Bruhat A, Jousse C. Amino acid regulation of gene expression. Biochem J 2000; 351: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilberg MS, Hutson RG, Laine RO. Amino acid-regulated gene expression in eukaryotic cells. FASEB J 1994; 8: 13–19. [DOI] [PubMed] [Google Scholar]
- 42.Caldara M, Minh PN, Bostoen S, et al. ArgR-dependent repression of arginine and histidine transport genes in Escherichia coli K-12. J Mol Biol 2007; 373: 251–267. [DOI] [PubMed] [Google Scholar]
- 43.Calvo JM, Matthews RG. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev 1994; 58: 466–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho BK, Federowicz S, Park YS, et al. Deciphering the transcriptional regulatory logic of amino acid metabolism. Nat Chem Biol 2011; 8: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tisné C, Roques BP, Dardel F. The annealing mechanism of HIV-1 reverse transcription primer onto the viral genome. J Biol Chem 2004; 279: 3588–3595. [DOI] [PubMed] [Google Scholar]
- 46.Qin Y, Morrowa CD. Essential regions of the tRNA primer required for HIV-1 infectivity. Nucleic Acids Res 2000; 28: 4783–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo F, Cen S, Niu M, et al. Specific inhibition of the synthesis of human Lysyl-tRNA synthetase results in decreases in tRNALys incorporation, tRNALys annealing to viral RNA, and viral infectivity in human immunodeficiency virus type 1. J Virol 2003; 77: 9817–9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marquet R, Isel C, Ehresmann C, et al. tRNAs as primer of reverse transcriptases. Biochimie 1995; 77: 113–124. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y. Incorporation of excess wild-type and mutant tRNA(3Lys) into human immunodeficiency virus type 1. J Virol 1994; 68: 7676–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scornik OA. Faster protein degradation in response to decreased steady-state levels of aminoacylation of tRNA is in Chinese hamster ovary cells. J Biol Chem 1983; 258: 882–886. [PubMed] [Google Scholar]
- 51.Allen RA, Raines PL, Regen DM. Regulatory significance of transfer RNA charging levels. Ibid 1969; 190: 323–336. [DOI] [PubMed] [Google Scholar]
- 52.Arnstein HRV, Barwick CW, Lange JD, et al. Control of protein synthesis by amino acid supply. FEBS Lett 1986; 194: 146–150. [DOI] [PubMed] [Google Scholar]
- 53.Vaughan MH, Hansen BS. Control of initiation of protein synthesis. Ibid 1973; 248: 7078–7096. [PubMed] [Google Scholar]
- 54.Flaim KE. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effect of amino acid deficiency on peptide chain initiation, polysomal aggregation and distribution of albumin mRNA. J Biol Chem 1982; 257: 2939–2946. [PubMed] [Google Scholar]
- 55.Ogilvie A, Huschka U, Kersten W. Control of protein synthesis in mammalian cells by aminoacylation of tRNA. Biochim Biophys Acta 1979; 565: 293–304. [DOI] [PubMed] [Google Scholar]
- 56.Arfin SM. A role for asparagyl tRNA in the regulation of asparagine synthetase in a mammalian cell line. Proc Natl Acad Sci USA 1977; 74: 2367–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hershko AA, Ciechatiover A. Mechanisms of intracellular protein breakdown. Ann Rev Biochem 1982; 51: 335–364. [DOI] [PubMed] [Google Scholar]
- 58.Butorov EV. Relationship between plasma l-lysine concentrations and levels of HIV-1 RNA. Virulence 2013; 4(7): 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lukasheva EV, Berezov TT. L-Lysine alpha-oxidase: physicochemical and biological properties. Biochemistry (Mosc) 2002; 67(10): 1152–1158. [DOI] [PubMed] [Google Scholar]