Abstract
The tightly regulated expression patterns of structural cell wall proteins in several plant species indicate that they play a crucial role in determining the extracellular matrix structure for specific cell types. We demonstrate that AtPRP3, a proline-rich cell wall protein in Arabidopsis, is expressed in root-hair-bearing epidermal cells at the root/shoot junction and within the root differentiation zone of light-grown seedlings. Several lines of evidence support a direct relationship between AtPRP3 expression and root hair development. AtPRP3/β-glucuronidase (GUS) expression increased in roots of transgenic seedlings treated with either 1-aminocyclopropane-1-carboxylic acid (ACC) or α-naphthaleneacetic acid (α-NAA), compounds known to promote root hair formation. In the presence of 1-α-(2-aminoethoxyvinyl)glycine (AVG), an inhibitor of ethylene biosynthesis, AtPRP3/GUS expression was strongly reduced, but could be rescued by co-addition of ACC or α-NAA to the growth medium. In addition, AtPRP3/GUS activity was enhanced in ttg and gl2 mutant backgrounds that exhibit ectopic root hairs, but was reduced in rhd6 and 35S-R root-hair-less mutant seedlings. These results indicate that AtPRP3 is regulated by developmental pathways involved in root hair formation, and are consistent with AtPRP3's contributing to cell wall structure in Arabidopsis root hairs.
Structural cell wall proteins comprise only 10% of the dry weight of plant cell walls, yet they are thought to play an integral role in the extracellular matrix structure of many plant cells (Varner and Lin, 1989). The plant cell wall, a highly complex and dynamic structure, is crucial for proper development in that it determines the size and shape of cells and thus ultimately influences plant function. Cell walls of different cell types vary in composition and structure due to their functional specialization and are often further modified as plants adapt to environmental stresses such as wounding or pathogen infection.
The plant extracellular matrix is composed of cellulose microfibrils coated with hemicellulose molecules that interact extensively through hydrogen bonds and are embedded in a matrix of Ca2+-bridged pectin molecules (Varner and Lin, 1989; Carpita and Gibeaut, 1993). Structural cell wall proteins are thought to form an independent structure-determining network within the extracellular matrix that adds to the mechanical strength of the wall and assists in proper wall assembly. These proteins are highly repetitive in structure and are secreted into the wall as monomers, where they eventually become insolubilized in response to developmental or environmental signals (Showalter, 1993; Cassab, 1998). The nature of these crosslinks is still unknown, but there is increasing evidence for the involvement of a peroxidase-mediated reaction, possibly through the formation of intermolecular isodityrosine links (Bradley et al., 1992; Brisson et al., 1994; Schnabelrauch et al., 1996).
Pro-rich proteins (PRPs) represent one family of Pro- and Hyp-rich structural cell wall proteins that were initially identified as wound-induced gene products in carrot storage roots (Chen and Varner, 1985; Tierney et al., 1988). These proteins have subsequently been shown to be expressed in many plant species in a manner that is temporally and spatially regulated during plant development. For example, individual members of the PRP gene family are expressed during soybean leaf, stem, root, and seed coat development (Hong et al., 1989; Kleis-San Francisco and Tierney, 1990; Lindstrom and Vodkin, 1991; Wyatt et al., 1992), bean seedling growth (Sheng et al., 1991), early stages of legume root nodule formation (Scheres et al., 1990; van de Wiel et al., 1990; Wilson et al., 1994), in cell types associated with lignification in several plant species (Ye et al., 1991), in immature maize-embryos (Jose-Estanyol et al., 1992), and in young tomato fruits (Salts et al., 1991; Santino et al., 1997). The expression of PRPs is also influenced by factors associated with pathogen infection or environmental stresses such as elicitor treatment and wounding, suggesting that the synthesis of these proteins is sensitive to external stimuli (Tierney et al., 1988; Sheng et al., 1991; Creelman et al., 1992; Ebener et al., 1993; Suzuki et al., 1993).
Recently, we have characterized four genes encoding novel two-domain PRPs in Arabidopsis (AtPRPs) (Fowler et al., 1999). The expression of each of these genes was shown to be tightly regulated throughout plant development. One of these genes, AtPRP3, was detected exclusively in roots and localized to regions of the root active in forming root hairs (Fowler et al., 1999).
Root hairs are long, tubular outgrowths of root epidermal cells that are involved in the uptake of nutrients and water (Peterson and Farquhar, 1996). Each root hair is formed by a single cell and in most plant species only certain epidermal cells (trichoblasts) actually develop into root-hair-bearing cells. The regulation of root epidermal cell differentiation in Arabidopsis has been studied extensively, due in part to the simple and invariant cellular organization of its primary root. The epidermis is formed by a single layer of cells, and root hair- and non-root-hair-bearing cells are arranged in organized files along the root axis. Files of root hair cells are located over the clefts between adjacent underlying cortical cells, and are separated by one or more files of hairless cells located over single underlying cortical cells (Dolan et al., 1993, 1994).
Several pharmacological studies have implicated the hormones ethylene and auxin in the positive regulation of root hair development. Exogenous application of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) or the synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D) resulted in an increased length of root hairs (Pitts et al., 1998). In addition, ACC treatment was shown to induce the formation of ectopic root hairs (i.e. hair-bearing cells in normally hairless cell files) in a concentration-dependent manner, while inhibitors of ethylene synthesis or ethylene perception reduced root hair formation along the primary root (Masucci and Schiefelbein, 1994, 1996; Tanimoto et al., 1995). A variety of Arabidopsis mutants have also been identified that are disrupted in their normal root epidermal cell patterning (Schiefelbein et al., 1997; Schneider et al., 1997; Wada et al., 1997). For example, seedlings homozygous for recessive mutations in TTG (TRANSPARENT TESTA GLABRA) and GL2 (GLABRA) form root hairs on all epidermal cell files independent of their position relative to the underlying cortical cell walls (Galway et al., 1994; Masucci et al., 1996; Hung et al., 1998). In contrast, Arabidopsis plants overexpressing the maize R(Lc) gene show a root-hair-less phenotype (Galway et al., 1994). The latter phenotype has also been observed in seedlings carrying the root-hair-defective6 (rhd6) mutation, which affects a later stage in root epidermal development (Masucci and Schiefelbein, 1994).
In the present study we took advantage of these findings to examine the relationship between AtPRP3 expression and the process of root hair formation. We describe the cell-specific expression pattern of AtPRP3 during root development and characterize the influence of hormones and genetically altered root epidermal cell patterning on its expression. Our results indicate that AtPRP3 expression is controlled by regulatory pathways specific for root hair development, and support a role for the AtPRP3 protein contributing to cell wall structure in Arabidopsis root-hair-bearing epidermal cells.
MATERIALS AND METHODS
Genetic Stocks
In constructing AtPRP3 promoter/β-glucuronidase (GUS) lines, a 1.5-kb 5′-flanking sequence of AtPRP3 was fused to the bacterial uid gene encoding GUS and transformed into Arabidopsis ecotype Columbia, as described previously (Fowler et al., 1999). 35S-R(Lc) lines (CS 8110 and CS 8111) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). The ttg (transparent testa glabrous), gl2 (glabra2), and rhd6 (root-hair-defective6) mutants were kindly provided by J.W. Schiefelbein, University of Michigan.
Plant Growth Conditions
Arabidopsis seedlings were grown on full-strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962), pH 6.0, supplemented with 1% (w/v) Suc and 1× Gamborg's vitamins. Seeds were surface-sterilized with a 20% (v/v) bleach solution, thoroughly washed with sterile water, and placed on MS plates solidified with 0.8% (w/v) agarose. Seedlings in the light/dark experiments were grown on MS plates without Suc and solidified with 0.8% (w/v) agar. After incubating the seeds for 2 d at 4°C in the dark, seedlings were grown in a vertical orientation under continuous cool-white light at room temperature. Seedlings to be grown in the dark were exposed to cool-white light for 30 min, then wrapped in aluminum foil and incubated vertically at the same location as the light-grown seedlings.
To investigate the influence of calcium, hormones, and hormone inhibitors on AtPRP3 expression, seedlings containing the AtPRP3 promoter/GUS construct were first grown on MS medium for 3 d, and then transferred to effector-containing MS plates. As controls, some seedlings were also transferred to unsupplemented plates. After an additional growth period of 2 d, seedlings were scored for root hair formation, harvested, and assayed histochemically and quantitatively for GUS activity as described below. Agarose plates containing ACC, L-α-(2-aminoethoxyvinyl)Gly (AVG), or α-naphthaleneacetic acid (α-NAA) (all from Sigma, St. Louis) were prepared by diluting 1,000× stock solutions into the warm agarose mixture after autoclaving. In the Ca2+-depleted MS media, CaCl2 was replaced by an equal concentration of MgCl2. All experiments were performed in duplicate with a minimum of three independent AtPRP3 promoter/GUS transgenic lines, using a minimum of 60 seedlings for each treatment.
To grow plants to maturity, seedlings were started in a soil mixture (Promix:perlite:vermiculite, 3:1:1) at 20°C in an 8-h light/16-h dark photoperiod, which was changed to an 14-h light/10-h dark regime to induce flowering. For the in vitro pollen tube assay, pollen was collected from mature AtPRP3/GUS plants, and germinated as described previously (Schiefelbein et al., 1993). Pollen tubes were stained for GUS activity as described below and were analyzed on a microscope (Eclipse E-400, Nikon, Tokyo). Pollen obtained from non-transformed ecotype Columbia plants was also germinated in vitro and served as a control.
Histochemical GUS Assay
Histochemical staining of plant tissue for GUS activity was performed according to the method of Jefferson et al. (1987). Tissue samples were placed in substrate solution (50 mm sodium phosphate, pH 7.5, 15% [v/v] methanol, 2 mm 5-bromo-4-chloro-3-indolyl-glucuronide, 0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, and 0.05% [v/v] Tween 20), vacuum-infiltrated for 2 min at 85 kPa, and then incubated at room temperature for 1 to 4 h. For increased sensitivity of the enzyme assay in the pollen tube, hormone, calcium, and light/dark experiments, the cyanide salts were omitted from the substrate solution and incubation was carried out for 8 to 18 h at 37°C. Removal of pigments was achieved by several washes in 50% to 70% (v/v) ethanol. Stained tissue was analyzed with a stereomicroscope (model 2000, Zeiss, Jena, Germany) and pictures were taken on 25 or 100 ASA film (Eastman Kodak, Rochester, NY).
Quantitative Determination of GUS Activity
Quantitation of GUS activity was performed using the fluorogenic substrate 4-methylumbelliferyl-β-d-glucuronide (4-MUG). Seedlings were harvested, and after determining their fresh weight, quick-frozen in liquid nitrogen and stored at −80°C. Tissue was ground in MUG extraction buffer (0.4 mL/100 mg tissue) composed of 50 mm sodium phosphate, pH 7.0, 10 mm β-mercaptoethanol, 10 mm EDTA, pH 8.0, 0.1% (w/v) SDS, and 0.1% (v/v) Triton X-100 using micropestles (Kontes, Vineland, NJ) in Eppendorf microfuge tubes. The extract was clarified by centrifugation at room temperature for 15 min at 14,000 rpm, and the protein concentration was determined according to the method of Bradford (1976). Aliquots containing 20 μg of protein were assayed at 37°C in 50 μL of GUS reaction buffer (MUG extraction buffer with 2 mm 4-MUG) at 20-min intervals over a 60-min time course. Reactions were stopped with 950 μL of 200 mm Na2CO3 stop buffer and fluorescence was determined using a fluorometer (model 450, Turner, Krackler Scientific, Albany, NY). Prior to measuring the sample fluorescence, the fluorometer was calibrated with 50 μL of freshly prepared 1 μm 7-hydroxy-4-methylcoumarin (MU) in the carbonate stop buffer.
Analysis of AtPRP3 Expression in Arabidopsis Mutant Backgrounds
Arabidopsis root hair mutants were grown in soil as described above, and manually cross-pollinated to AtPRP3 promoter/GUS lines grown under the same conditions. Each root hair mutant line was crossed to two independent transgenic AtPRP3 promoter/GUS lines. F1 plants were checked by PCR for seedlings carrying the promoter/GUS construct, and PCR-verified lines were allowed to set seed. F2 seedlings were grown on vertically oriented MS-agarose plates containing 1% (w/v) Suc, scored for the root hair mutant phenotype, and stained for GUS activity as described above. For some of the lines, seedlings showing the appropriate phenotype were transferred into soil and allowed to set seed. Plants homozygous for both the AtPRP3/GUS construct and the root hair mutation were identified by analyzing F3 seedlings.
RESULTS
AtPRP3 Is Expressed in a Cell-Type-Specific Manner during Root Development
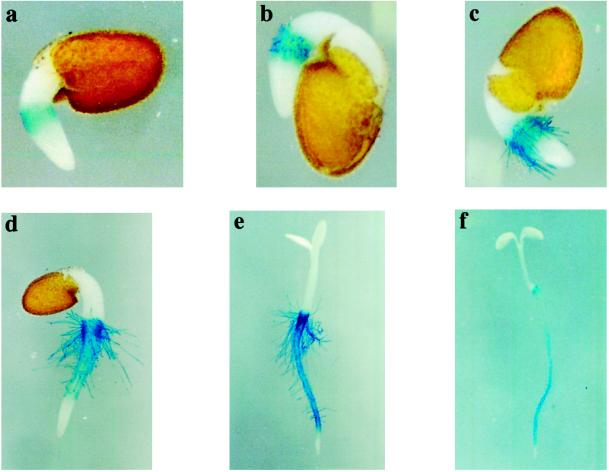
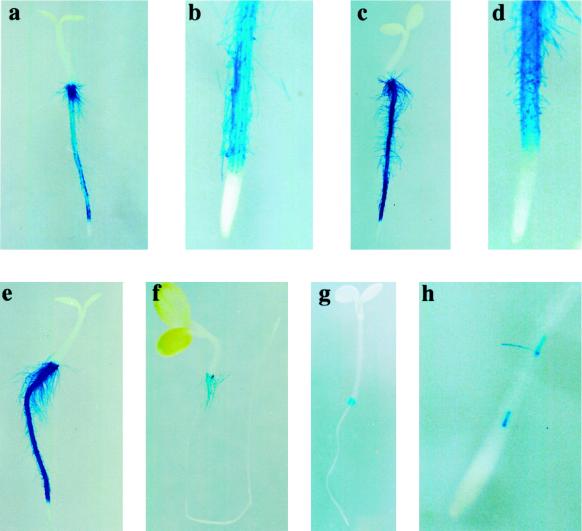
Previous studies have shown that AtPRP3 expression in Arabidopsis is localized to regions of the root that form root hairs (Fowler et al., 1999). We have further characterized the temporal and spatial pattern of AtPRP3 transcription during root growth using transgenic Arabidopsis lines expressing an AtPRP3 promoter/GUS construct (Fig. 1, a–f). Shortly after germination, AtPRP3/GUS expression was detected in the root epidermis at the hypocotyl/ primary root transition zone, where emerging root hairs were visible as small bulges of the outer root epidermal cell wall (Fig. 1a). Coinciding with the localization of the growing root hairs, AtPRP3/GUS expression was found in all epidermal cell files within this region, and was intensified with increasing elongation of the root hairs (Fig. 1, b and c). In more developed seedlings, AtPRP3/GUS staining appeared in the root-hair-bearing epidermal cell files along the primary root, which alternated with unstained, root-hair-less epidermal cell files (Fig. 1, d and e). No AtPRP3/GUS expression was detected in the root tip and, as found in the transition zone, AtPRP3 expression first appeared with the onset of root hair outgrowth. With further root growth, AtPRP3/GUS activity along the primary root was strongest in the region behind the root tip, where new root hairs developed, but decreased in the mature regions of the root (Fig. 1f). This same pattern of AtPRP3/GUS expression was observed in lateral roots (data not shown).
Figure 1.
AtPRP3 expression is linked to root hair development in Arabidopsis. Transgenic seedlings containing an AtPRP3 promoter/GUS construct were grown under continuous white light for 1 d (a–c), 2 d (d), 3 d (e), and 4 d (f), and were subsequently stained for GUS activity.
Root hair elongation occurs through a mechanism of polarized growth or tip growth (Miller et al., 1997). Another well-known example of this type of growth during plant development is found in pollen tubes (Taylor and Hepler, 1997). To determine whether AtPRP3 plays a general role in determining cell wall structure of cells elongating by tip growth, we examined pollen tubes for AtPRP3 expression. Mature pollen was germinated in an in vitro assay and stained for AtPRP3/GUS. No GUS activity was detected in growing pollen tubes under these conditions.
These results indicated that AtPRP3 may function specifically in cell walls within Arabidopsis root hairs, and led us to further investigate the relationship between AtPRP3 expression and root hair formation.
AtPRP3 Expression Is Hormonally Regulated
The plant hormones ethylene and auxin have both been implicated as positive regulators in the process of root hair formation in Arabidopsis roots (Masucci and Schiefelbein, 1994, 1996; Tanimoto et al., 1995; Pitts et al., 1998). To investigate the role of these hormones in regulating AtPRP3 expression, we characterized the effects of the ethylene precursor ACC (1-aminocyclopropane-1-carboxylic acid), the ethylene biosynthesis inhibitor l-α-(2-aminoethoxyvinyl)Gly (AVG), and the synthetic auxin α-NAA on AtPRP3/GUS expression.
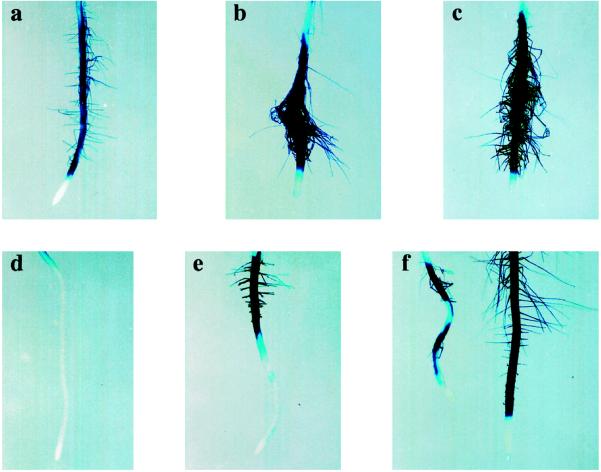
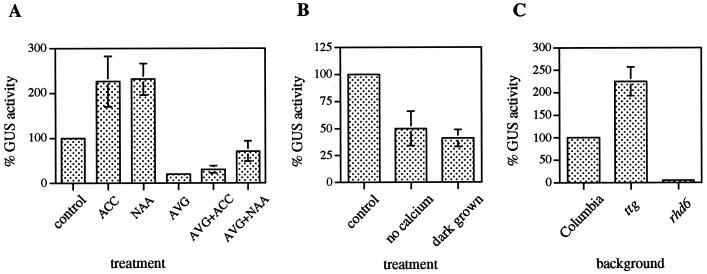
Seedlings were grown vertically on MS medium for 3 d and subsequently transferred to effector-containing MS medium for an additional 2 d of growth before being analyzed for GUS activity with both a histochemical assay (Fig. 2) and a quantitative assay (Fig. 3A). When Arabidopsis seedlings were grown in the presence of 5 μm ACC or 25 nm α-NAA, both the number and length of root hairs increased and AtPRP3/GUS activity more than doubled compared with the untreated control (Figs. 2, a–c, and 3A). In contrast, AtPRP3/GUS expression decreased to less than 20% of the control activity when seedlings were treated with 20 μm AVG (Fig. 3A), and this corresponded with a dramatic decrease in both the number and length of root hairs (Fig. 2d). The inhibition of both root hair formation and AtPRP3 expression by AVG could be partially rescued by the co-addition of either 80 μm ACC or 100 nm α-NAA to the growth medium (Fig. 2, e and f). α-NAA proved to be more effective than ACC in restoring root hair formation and AtPRP3 expression in the presence of AVG (Figs. 2 and 3A). In some of the seedlings this resulted in root hair lengths and GUS staining intensity that exceeded the untreated control (Fig. 1f). In experiments in which seedlings were germinated directly on plates containing ACC, α-NAA, or AVG, root hair formation and AtPRP3 expression in the hypocotyl/primary root transition zone was largely unaffected (data not shown). These results demonstrate that AtPRP3 expression in the differentiation zone of the primary root, but not in the hypocotyl/root transition zone, is controlled by a signal transduction pathway mediated by ethylene and auxin.
Figure 2.
Ethylene and auxin are involved in the regulation of AtPRP3 expression in the primary root. Transgenic AtPRP3/GUS seedlings were grown for 3 d and then transplanted onto medium containing no effectors (a), 5 μm ACC (b), 25 nm α-NAA (c), 20 μm AVG (d), 20 μm AVG + 80 μm ACC (e), or 20 μm AVG + 100 nm α-NAA (f). Seedlings were grown for an additional 2 d before being stained for GUS activity.
Figure 3.
The level of AtPRP3/GUS expression is modulated during Arabidopsis root development. AtPRP3/GUS seedlings were grown as indicated below, and then harvested and whole seedling extracts analyzed fluorometrically for GUS activity. A, Effect of ethylene and auxin on AtPRP3 expression. Transgenic AtPRP3/GUS seedlings were grown for 3 d and then transplanted onto medium containing no effectors (control), 5 μm ACC, 25 nm α-NAA, 20 μm AVG, 20 μm AVG + 80 μm ACC, or 20 μm AVG + 100 nm α-NAA for an additional 2 d of growth. The sd for AtPRP3/GUS expression in AVG-treated seedlings was ±6. B, Effect of Ca2+ and light on AtPRP3 expression. Transgenic AtPRP3/GUS seedlings were grown for 3 d before being transplanted onto medium with or without Ca2+ for an additional 2 d of growth. Alternatively, seedlings were grown for 3 d under continuous white light or in complete darkness. The control level of GUS activity represents that measured in light-grown seedlings in the presence of Ca2+. C, Influence of root hair mutant backgrounds on AtPRP3 expression. Transgenic lines of Columbia, ttg, and rhd6 seedlings expressing the AtPRP3/GUS construct were grown for 4 d under continuous white light. The sd for AtPRP3/GUS expression in the rhd6 mutant background was ±1.5.
Environmental Factors That Influence Root Hair Formation Modulate AtPRP3 Expression
Root hair development, especially the elongation process, has been shown to be dependent on the presence of calcium (Schiefelbein et al., 1992; Miller et al., 1997; Wymer et al., 1997). Growth of AtPRP3/GUS seedlings in Ca2+-depleted media resulted in a decrease in the number and length of root hairs compared with seedlings grown on Ca2+-containing media; AtPRP3/GUS expression was also markedly decreased in these seedlings (Figs. 4, a and b, and 3B).
Figure 4.
Environmental factors that influence root hair formation modulate AtPRP3 expression. Transgenic AtPRP3/GUS seedlings were grown for 3 d, transferred to medium with (a) or without (b) Ca2+ for an additional 2 d of growth, and stained for GUS activity. Alternatively, transgenic AtPRP3/GUS seedlings were grown horizontally on MS agar plates without Suc for 4 d under continuous white light (c) or in complete darkness (d), and then stained for GUS activity.
We also investigated the effect of light on AtPRP3 expression. Four-day-old dark-grown seedlings were shown to develop fewer root hairs and have lower levels of AtPRP3/GUS expression along their primary root than those grown under continuous white light (Figs. 4, c and d, and 3B). However, root hairs that formed in the hypocotyl/root transition zone were largely unaffected by light and stained positively for AtPRP3/GUS expression in both light- and dark-grown seedlings. The addition of Suc or Glc to the media enhanced both root hair development and AtPRP3/GUS expression in both light- and dark-grown seedlings (data not shown). These results demonstrate that AtPRP3 expression in the root differentiation zone is modulated by environmental conditions that affect root hair development, further strengthening a relationship between AtPRP3 expression and root hair formation.
AtPRP3 Expression Is Altered in Root Hair Mutant Backgrounds
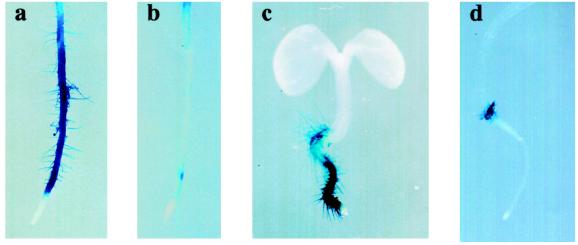
Arabidopsis seedlings with recessive mutations at the TTG or GL2 loci form root hairs in all cell files, including those that are normally hairless, suggesting that both genes are involved in the negative regulation of root hair formation in atrichoblast cells (Galway et al., 1994; Masucci et al., 1996). AtPRP3 promoter/GUS constructs were crossed into these mutant backgrounds, and F2 seedlings homozygous for the recessive root hair mutations were analyzed for GUS activity. AtPRP3/GUS expression was enhanced in both ttg and gl2 mutant seedlings, which is consistent with the observed ectopic root hair development (Figs. 3C and 5, c and e). AtPRP3/GUS expression in these seedlings was equally detected in all epidermal cell files compared with the wild-type-background seedlings, which showed alternating cell file staining (Fig. 5, b and d).
Figure 5.
AtPRP3 expression is altered in root hair mutant backgrounds. Seedlings containing an AtPRP3/GUS construct in root hair mutant backgrounds were grown for 3 d and stained for GUS activity: a and b, Columbia; c and d, ttg; e, gl2; f, rhd6; g and h, 35S-R. Cell-file-specific AtPRP3/GUS expression is shown in b, d, and h, with h representing an example of leaky AtPRP3/GUS expression corresponding to the formation of a few root hairs.
The maize R(Lc) gene encodes a myc-like transcription factor (Ludwig et al., 1989) that can complement the ttg mutation when expressed under the control of the cauliflower mosaic virus 35S promoter. In wild-type seedlings, overexpression of the maize R gene results in a hairless phenotype (Galway et al., 1994). A similar phenotype is observed in seedlings with a defect in RHD6, a gene encoding a positive regulator of root hair formation involved in root hair initiation (Masucci and Schiefelbein, 1994). AtPRP3/GUS expression along the primary root was strongly suppressed in the 35S-R and the rhd6 mutant backgrounds (Figs. 3C and 5, f and g). In both root-hair-less mutant backgrounds, occasional “leaks” occurred, with some seedlings forming a few root hairs along the root. AtPRP3 expression could be detected in these seedlings, but was restricted to the areas of root hair outgrowth (Fig. 5h). In all four root hair mutant backgrounds AtPRP3/GUS expression and root hair formation in the transition zone between the hypocotyl and root was not significantly altered compared with the wild-type background. These data provide genetic evidence that AtPRP3 expression is controlled by developmental pathways involved in root epidermal cell differentiation.
Because the ethylene/auxin-regulated pathways of root hair formation are thought to act downstream of TTG, GL2, and RHD6 (Masucci and Schiefelbein, 1994, 1996), we examined the influence of AVG, ACC, and α-NAA on AtPRP3 expression in these mutant backgrounds (Table I). In both the ttg and gl2 mutant backgrounds, the addition of 20 μm AVG to the medium resulted in a strong decrease in both root hair formation and AtPRP3/GUS expression. This phenotype could be partially reversed when either 20 μm ACC or 100 nm α-NAA was added to the medium. In the rhd6 mutant background, AtPRP3 expression was fully rescued by the addition of 5 μm ACC or 25 nm α-NAA to the medium. However, only a small increase in AtPRP3/GUS activity could be detected in the 35S-R line when exposed to ACC. In each case, the amount of AtPRP3/GUS staining correlated well with the extent of root hair formation observed.
Table I.
Comparison of AtPRP3 expression and root hair formation in root hair mutant backgrounds in response to hormone treatments
| Background | No Effectors | 5 μm ACC | 25 nm α-NAA | 20 μm AVG | 20 μm AVG + 20 μm ACC | 20 μm AVG + 100 nm α-NAA |
|---|---|---|---|---|---|---|
| Columbia | ++/++ | +++/+++ | +++/+++ | −/− | +/+ | ++/++ |
| ttg | +++/+++ | nd | nd | −/− | +/+ | +++/+++ |
| 35S-R | −/− | +/+ | −/− | nd | nd | nd |
| gl2 | +++/+++ | nd | nd | −/− | +/+ | ++/++ |
| rhd6 | −/− | +++/+++ | +++/+++ | nd | nd | nd |
Root hair mutant seedlings containing an AtPRP3/GUS construct were grown on MS media for 3 d, followed by an additional 2 d on media containing effectors as indicated. The extent of root hair formation (symbols before slash) and the level of AtPRP3 expression estimated from histochemical GUS analysis (symbols after slash) of each treatment were compared to those of an untreated Columbia wild-type background control and are expressed as −, No or very few root hairs and AtPRP3/GUS expression; +, less root hair formation and AtPRP3/GUS expression than control; ++, root hair formation and AtPRP3/GUS expression equal to control; +++, more root hair formation and AtPRP3 expression than control; nd, not determined.
DISCUSSION
Structural cell wall proteins have been implicated in modeling the extracellular matrix structure of specific cell types. We have shown that AtPRP3 expression is associated with root epidermal cell differentiation in Arabidopsis and is controlled by developmental pathways involved in root hair formation. In the Arabidopsis root, trichoblasts (cells that give rise to root hair cells) and atrichoblasts (cells that develop into root-hair-less cells) can already be distinguished within the meristematic zone of the root using characteristics such as cytoplasmic density and cell vacuolation (Galway et al., 1994). Since AtPRP3/GUS expression was never detected in the root tip but, rather, at the onset of a root hair outgrowth, AtPRP3 is likely to function primarily in determining cell wall structure during the late stages of root hair cell differentiation.
Root hairs are highly specialized structures and have been proposed to act in water and nutrient uptake as well as anchoring the plant in the soil (Peterson and Farquhar, 1996). Therefore, the root hair cell wall represents a crucial interface between the plant and its environment, and it is likely that interactions between structural cell wall proteins and other extracellular matrix components participate in determining this unique wall structure. For example, two extensin genes recently identified in tomato (Bucher et al., 1997) and cowpea (Arsenijevic-Maksimovic et al., 1997) are also uniquely expressed in root hairs. Root hair development is positively regulated by both ethylene and auxin in Arabidopsis (Masucci and Schiefelbein, 1994, 1996; Tanimoto et al., 1995; Pitts et al., 1998). We have shown that these hormones also play a positive role in the regulation of AtPRP3 expression in the root differentiation zone. Ethylene has been implicated in the proliferation of root hairs, which may aid the plant in absorption of nutrients or in stabilization of the seedling in the soil when growing under harsh environmental conditions (Ecker, 1995). Therefore, the increased expression of AtPRP3 in response to exogenous ethylene may reflect a role for AtPRP3 in strengthening the root hair cell wall under conditions of environmental stress.
Ethylene may also play a role in the stimulating effect of auxin on AtPRP3 expression. Since auxin is known to enhance ethylene biosynthesis through positively regulating individual members of the ACC synthase multigene family (Kende 1993; Abel et al., 1995; Arteca and Arteca, 1999), it may up-regulate AtPRP3 expression simply by efficiently increasing ethylene production. On the other hand, Masucci and Schiefelbein (1996) have demonstrated that in addition to an ethylene-promoted pathway, there is a separable auxin-regulated pathway involved in root epidermal cell differentiation that could contribute to the regulation of AtPRP3 expression.
In addition, ethylene may be involved in the regulation of AtPRP3 expression by certain environmental stimuli. AtPRP3 expression was shown to be down-regulated in etiolated seedlings and in seedlings grown on Ca2+-depleted media. Arabidopsis seedlings have previously been shown to form fewer root hairs on their primary root when grown in the dark, and this effect was suggested to be mediated by a decrease in ethylene sensitivity in the roots of dark- versus light-grown seedlings (Dolan, 1997). Supplementing the growth media with either Suc or Glc increased both AtPRP3 expression and the number and length of root hairs formed along the primary root in both light- and dark-grown Arabidopsis seedlings. While this effect may be solely due to the role these sugars play as major energy sources, both Glc and Suc have recently been shown to act as regulatory molecules in higher plants, and interactions between sugar-, light-, and ethylene-signaling pathways have been suggested (Koch, 1996; Mustilli and Bowler, 1997; Sokolov et al., 1998; Zhou et al., 1998). Similarly, the decrease in AtPRP3 expression observed in seedlings grown on Ca2+-depleted medium could involve an ethylene signaling pathway, since a variety of ethylene-mediated processes have been demonstrated to require Ca2+ (Raz and Fluhr, 1992).
The ethylene/auxin-regulated pathways have been shown to act at a late stage in root hair cell development (Masucci and Schiefelbein, 1996). Therefore, several Arabidopsis mutants that control root hair formation by regulating earlier stages of epidermal cell differentiation (Schiefelbein et al., 1997) were used to further investigate the regulation of AtPRP3 expression during root development. Consistent with our model for AtPRP3 functioning in determining root hair cell wall structure, AtPRP3 expression was found to be increased in the root-hair-overproducing ttg and gl2 mutant backgrounds, coinciding with the ectopic development of root hairs and the expression of AtPRP3/GUS in all cell files within the root differentiation zone. In addition, only low levels of expression were detected in the hairless seedlings overexpressing the maize R gene or carrying the rhd6 mutation. In both the ttg and gl2 mutants, AVG inhibited the expression of AtPRP3 and root hair development, indicating that AtPRP3 expression and root hair formation require an active ethylene biosynthetic pathway even in the absence of TTG and GL2 function. This was supported by the observation that ACC and auxin were able to rescue the effects of AVG on AtPRP3 expression and root hair formation in the ttg and gl2 mutant backgrounds. In contrast to the 35S-R seedling phenotype, the addition of ACC or auxin to the growth medium was also capable of reversing the hairless rhd6 phenotype (Masucci and Schiefelbein, 1994), and both of these compounds induced AtPRP3 expression in the rhd6 mutant background.
AtPRP3/GUS expression in the root hairs localized within the transition zone was not significantly affected by the hormonal and environmental factors tested or by defects in the key regulatory proteins TTG, GL2, or RHD6. This indicates that AtPRP3 expression may be controlled by two different developmental pathways. It has been shown previously that the formation of root hairs at the root/shoot junction is regulated differently from those formed along the primary root (Dolan et al., 1993). In addition, examination of Arabidopsis root hair mutants has led to the identification of genes that are involved in the formation of root hairs at either one or both of these locations, supporting the existence of two developmental pathways (Masucci and Schiefelbein, 1994; Schneider et al., 1997).
We have presented data demonstrating the close relationship between AtPRP3 expression and the developmental pathways leading to root hair formation in Arabidopsis. To our knowledge, this is the first time the expression of a root-hair-specific cell wall PRP has been described. Expression of AtPRP3 occurs during the later stages of root epidermal cell differentiation and is regulated by developmental pathways leading to root hair outgrowth. Future analysis of the biochemical properties of this protein will help us to determine the manner in which it may contribute to root hair cell wall structure.
ACKNOWLEDGMENTS
We thank the Arabidopsis Biological Resource Center for the 35S-R lines; John W. Schiefelbein for providing the rhd6, ttg, and gl2 mutants; Mary Lou Shane for excellent technical assistance; and Gary Ward, Eunice Froeliger, and Cardy Raper for helpful discussions.
Footnotes
This research was supported by the U.S. Department of Agriculture (grant no. NRICGP–95–02982). C.B. was supported by experiment station grant no. 0171655.
LITERATURE CITED
- Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana: structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- Arsenijevic-Maksimovic I, Broughton WJ, Krause A. Rhizobia modulate root-hair-specific expression of extensin genes. Mol Plant-Microbe Interact. 1997;10:95–101. doi: 10.1094/MPMI.1997.10.1.95. [DOI] [PubMed] [Google Scholar]
- Arteca JM, Arteca RN. A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (ACS6) in mature Arabidopsisleaves. Plant Mol Biol. 1999;39:209–219. doi: 10.1023/a:1006177902093. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher M, Schroeer B, Willmitzer L, Riesmeier JW. Two genes encoding extensin-like proteins are predominantly expressed in tomato root hair cells. Plant Mol Biol. 1997;35:497–508. doi: 10.1023/a:1005869717158. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Cassab GI. Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:281–309. doi: 10.1146/annurev.arplant.49.1.281. [DOI] [PubMed] [Google Scholar]
- Chen J, Varner JE. Isolation and characterization of cDNA clones for carrot extensin and proline-rich 33-kDa protein. Proc Natl Acad Sci USA. 1985;82:4399–4403. doi: 10.1073/pnas.82.13.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L. The role of ethylene in the development of plant form. J Exp Biol. 1997;48:201–210. [Google Scholar]
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Roberts K. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development. 1994;120:2465–2474. [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thalianaroot. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Ebener W, Fowler TJ, Suzuki H, Shaver J, Tierney ML. Expression of DcPRP1 is linked to carrot storage root formation and is induced by wounding and auxin treatment. Plant Physiol. 1993;101:259–265. doi: 10.1104/pp.101.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Fowler TJ, Bernhardt C, Tierney ML. Characterization and expression of four proline-rich cell wall protein genes in Arabidopsis thalianaencoding two distinct subsets of multiple domain proteins. Plant Physiol. 1999;121:1081–1091. doi: 10.1104/pp.121.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsisroot. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- Hong JC, Nagao RT, Key JL. Developmentally regulated expression of soybean proline-rich cell wall protein genes. Plant Cell. 1989;1:937–943. doi: 10.1105/tpc.1.9.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Lin Y, Zhang M, Pollock S, Marks MD, Schiefelbein J. A common position-dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis. Plant Physiol. 1998;117:73–84. doi: 10.1104/pp.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose-Estanyol M, Ruiz-Avila L, Puigdomenech P. A maize embryo-specific gene encodes a proline-rich and hydrophobic protein. Plant Cell. 1992;4:413–423. doi: 10.1105/tpc.4.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Kleis-San Francisco SM, Tierney ML. Isolation and characterization of a proline-rich cell wall protein from soybean seedlings. Plant Physiol. 1990;94:1897–1902. doi: 10.1104/pp.94.4.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Lindstrom JT, Vodkin LO. A soybean cell wall protein is affected by seed color genotype. Plant Cell. 1991;3:561–571. doi: 10.1105/tpc.3.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig SR, Habera LF, Dellaporta SL, Wessler SR. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA. 1989;86:7092–7096. doi: 10.1073/pnas.86.18.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA 2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. The rhd6 mutation of Arabidopsis thalianaalters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol. 1994;106:1335–1346. doi: 10.1104/pp.106.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsisroot. Plant Cell. 1996;8:1505–1517. doi: 10.1105/tpc.8.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DD, de Ruijter NC, Emons AM. From signal to form: aspects of the cytoskeleton-plasma membrane-cell wall continuum in root hair tips. J Exp Bot. 1997;48:1881–1896. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Mustilli AC, Bowler C. Tuning in to the signals controlling photoregulated gene expression in plants. EMBO J. 1997;16:5801–5806. doi: 10.1038/sj.emboj.7590554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev. 1996;62:1–40. [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Raz V, Fluhr R. Calcium requirement for ethylene-dependent responses. Plant Cell. 1992;4:1123–1130. doi: 10.1105/tpc.4.9.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salts Y, Wachs R, Gruissem W, Barg R. Sequence coding for a novel proline-rich protein preferentially expressed in young tomato fruit. Plant Mol Biol. 1991;17:149–150. doi: 10.1007/BF00036818. [DOI] [PubMed] [Google Scholar]
- Santino CG, Stanford GL, Conner TW. Developmental and transgenic analysis of two tomato fruit enhanced genes. Plant Mol Biol. 1997;33:405–416. doi: 10.1023/a:1005738910743. [DOI] [PubMed] [Google Scholar]
- Scheres B, Van De Wiel C, Zalensky A, Horvath B, Spaink H, Van Eck H, Zwartkruis F, Wolters AM, Gloudemans T, Van Kammen A, Bisseling T. The ENOD12 gene product is involved in the infection process during the pea-Rhizobiuminteraction. Cell. 1990;60:281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Schiefelbein J, Galway M, Masucci J, Ford S. Pollen tube and root-hair tip growth is disrupted in a mutant of Arabidopsis thaliana. Plant Physiol. 1993;103:979–985. doi: 10.1104/pp.103.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Masucci JD, Wang H. Building a root: the control of patterning and morphogenesis during root development. Plant Cell. 1997;9:1089–1098. doi: 10.1105/tpc.9.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Shipley A, Rowse P. Calcium influx at the tip of growing root-hair cells of Arabidopsis thaliana. Planta. 1992;187:455–459. doi: 10.1007/BF00199963. [DOI] [PubMed] [Google Scholar]
- Schnabelrauch LS, Kieliszewski M, Upham BL, Alizedeh H, Lamport DTA. Isolation of pI 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J. 1996;9:477–489. doi: 10.1046/j.1365-313x.1996.09040477.x. [DOI] [PubMed] [Google Scholar]
- Schneider K, Wells B, Dolan L, Roberts K. Structural and genetic analysis of epidermal cell differentiation in Arabidopsisprimary roots. Development. 1997;124:1789–1798. doi: 10.1242/dev.124.9.1789. [DOI] [PubMed] [Google Scholar]
- Sheng J, D'Ovidio R, Mehdy MC. Negative and positive regulation of a novel proline-rich protein mRNA by fungal elicitor and wounding. Plant J. 1991;1:345–354. doi: 10.1046/j.1365-313x.1991.t01-3-00999.x. [DOI] [PubMed] [Google Scholar]
- Showalter AM. Structure and function of plant cell wall protein. Plant Cell. 1993;5:9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov LN, DeJardin A, Kleczkowski A. Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana(thale cress) Biochem J. 1998;336:681–687. doi: 10.1042/bj3360681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Wagner T, Tierney ML. Differential expression of two soybean (Glycine maxL.) proline-rich protein genes after wounding. Plant Physiol. 1993;101:1283–1287. doi: 10.1104/pp.101.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L. Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J. 1995;8:943–948. doi: 10.1046/j.1365-313x.1995.8060943.x. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler P. Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Tierney ML, Wiechert J, Pluymers D. Analysis of the expression of extensin and p33-related cell wall proteins in carrot and soybean. Mol Gen Genet. 1988;211:393–399. [Google Scholar]
- van de Wiel C, Scheres B, Franssen H, van Lierop M-J, van Lammeren A, van Kammen A, Bisseling T. The early nodulin transcript ENOD2 is located in the nodule parenchyma (inner cortex) of pea and soybean root nodules. EMBO J. 1990;9:1–7. doi: 10.1002/j.1460-2075.1990.tb08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner JE, Lin LS. Plant cell wall architecture. Cell. 1989;56:231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Mybhomolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Long F, Maruoka EM, Cooper JB. A new proline-rich early nodulin from Medicago truncatulais highly expressed in nodule meristematic cells. Plant Cell. 1994;6:1265–1275. doi: 10.1105/tpc.6.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RE, Nagao RT, Key JL. Patterns of soybean proline-rich protein gene expression. Plant Cell. 1992;4:99–110. doi: 10.1105/tpc.4.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymer CL, Bibikova TN, Gilroy S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]
- Ye Z-H, Song Y-R, Marcus A, Varner JE. Comparative localization of three classes of cell wall proteins. Plant J. 1991;1:175–183. doi: 10.1111/j.1365-313x.1991.00175.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsisglucose-insensitive mutant. Proc Natl Acad Sci USA. 1998;95:1024–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]