Abstract
Introduction: Despite extensive testing, the efficacy of low-frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) of temporo-parietal targets for the treatment of auditory verbal hallucinations (AVH) in patients with schizophrenia is still controversial, but promising results have been reported with both high-frequency and neuronavigated rTMS. Here, we report a double-blind sham-controlled study to assess the efficacy of high-frequency (20 Hz) rTMS applied over a precise anatomical site in the left temporal region using neuronavigation. Methods: Fifty-nine of 74 randomized patients with schizophrenia or schizoaffective disorders (DSM-IV R) were treated with rTMS or sham treatment and fully evaluated over 4 weeks. The rTMS target was determined by morphological MRI at the crossing between the projection of the ascending branch of the left lateral sulcus and the superior temporal sulcus (STS). Results: The primary outcome was response to treatment, defined as a 30% decrease of the Auditory Hallucinations Rating Scale (AHRS) frequency item, observed at 2 successive evaluations. While there was no difference in primary outcome between the treatment groups, the percentages of patients showing a decrease of more than 30% of AHRS score (secondary outcome) did differ between the active (34.6%) and sham groups (9.1%) (P = .016) at day 14. Discussion: This controlled study reports negative results on the primary outcome but demonstrates a transient effect of 20 Hz rTMS guided by neuronavigation and targeted on an accurate anatomical site for the treatment of AVHs in schizophrenia patients.
Keywords: Transcranial magnetic stimulation, neuronavigation, magnetic resonance imaging, Auditory Hallucinations Rating Scale, placebo, trial, 20 Hz
Introduction
When schizophrenia patients fail to optimal antipsychotic therapy, repetitive transcranial magnetic stimulation (rTMS) may be an adjuvant therapy for the treatment of auditory verbal hallucinations (AVHs).1 RTMS is primarily delivered at a low frequency (1 Hz) based on the assumptions of 2 hypotheses. First, that low- and high-frequency stimulation in the temporal cortex may induce inhibitory and excitatory effects respectively, as has been observed in the primary motor cortex.2 Second, neuroimaging and neurophysiological studies have indicated that AVHs are correlated with cerebral hyperactivity in the left temporal cortex, suggesting that inhibitory effects may be required to alleviate AVHs.3–5 However, the efficacy of 1 Hz rTMS remains controversial,6–11 and there may be insufficient evidence for 1 Hz rTMS as an adjunctive therapy to antipsychotic medication.12 Only 5 of 21 controlled studies aiming to demonstrate 1 Hz rTMS efficacy13 reported positive results,14–18 and recent results achieved with high frequency rTMS could call the use of a low frequency stimulation into question and revive interest in rTMS for the treatment of AVH. The first open study using a high-frequency (20 Hz) demonstrated a drastic reduction in AVH19 that was maintained for 6 months in one patient.20 Recent observations also suggest that high-frequency or thêta-burst rTMS can produce inhibitory effects in healthy subjects21 for the treatment of both AVH22,23 and tinnitus.24 Additionally, the treatment can be intensified by delivering high frequency stimulation over a 2-day period rather than over 2 weeks, to rapidly affect AVHs. This delivers a number of stimuli roughly similar to that routinely given at low frequency (1 Hz) over approximately 2 weeks in just 2 days. A major advantage of a paradigm consisting of a series of stimulations delivered in a few days is the reduction of administration duration, which improves both the adherence to the treatment and its availability. These “high dose” or “accelerated” protocols have been well tolerated and depressive patients show a rapid decline of symptoms.25–28 However, these protocols have not yet been tested for the treatment of AVHs in schizophrenia patients.
In addition to changes in frequency, rTMS efficacy could also be increased by an accurate target that takes between-subject anatomical variability into account. The left temporo-parietal region is the proposed target in most studies, and it is usually located using the traditional T3P3 site according to the International 10–20 system of EEG electrode positioning, which is known to be a rough estimation.29 To address the wide anatomical variability between subjects, some authors have proposed using neuronavigation integrating morphological or functional imaging data to locate the stimulation target.18,30–33 These studies indicate the pitfalls of the T3P3 method and the use of functional cerebral imaging to guide localization and these results clearly point to the need for better and more accessible localization techniques for rTMS optimization. Therefore, we aim to determine an accurate anatomical site in the left temporo-parietal region identified as the best stimulation target33 that can be easily and reproducibly targeted in clinical practice.
Finally, while promising results have been separately reported with high-frequency22,23 or neuronavigated rTMS,18 there are no reports comparing the efficacy of high-frequency neuronavigated rTMS with sham stimulation in a double-blind placebo-controlled study. We suggest that the combination of both high frequency (20 Hz) stimulation and neuronavigation may be an optimal strategy for reducing AVHs in patients with schizophrenia.
Methods
Subjects
Seventy-four patients with schizophrenia or schizoaffective disorders were included in 7 University centers across France (Caen, Creteil, Dijon, Paris, Poitiers, Rennes, and Rouen).
The patients included in this randomized placebo-controlled double-blind trial had a diagnosis of schizophrenia or schizoaffective disorders (DSM-IV R) assessed with the MINI, were aged from 16 to 65 years old, had a severity score of hallucinations on the Auditory Hallucination Rating Scale (AHRS)15 >10, and had clinically stabilized disease defined by the absence of antipsychotic treatment modifications within the last 2 months.
Exclusion criteria were pregnancy or active breastfeeding, brain tumor, history of epilepsy, previous rTMS treatment, and metal objects in the body.
This study was approved by the human ethics committee (CPP, Nord Ouest), by the French Agency for Health (ANSM), and was registered on www.clinicaltrials.gov under number NCT01022489. All patients provided their written informed consent to participate.
Procedures
Patients were randomized to receive either active 20 Hz rTMS or sham treatment after providing written informed consent, and participants and clinical staff were blind to the treatment allocation. The randomization list consisted of a sequence of blocks containing the 2 trial arms (active and sham treatment) randomly assigned, with permuted blocks of size 4. Each hospital has its own randomization list. The rTMS administrator assigned each participant to the active or sham arms, and was blind to the treatment along with the investigator/assessor. The patient was the only person with access to the randomization list and had no contacts or role in assessing AVHs. The blindness procedure was maintained throughout the follow-up period.
Patients were evaluated at 6 visits over 4 weeks to evaluate treatment efficacy. Assessment was performed using the AHRS14 immediately preceding the first treatment session on day 1 (D1), after the last session (D2), and at D7, D14, D21, and D30. Additionally, the Positive and Negative Syndrome Scale (PANSS),34 the Scale to assess Unawareness of Mental Disorder (SUMD),35 the self-evaluation of insight (IS),36 and the Clinical Global Impression (CGI) were also applied at baseline (D0), D14, and D30. Handedness was assessed with the Oldfield scale,37 and subjects were categorized into right-handed (score ≥ 50) and nonright-handed (score < 50). Side effects were recorded during each session and independently at days 2 and 7.
rTMS
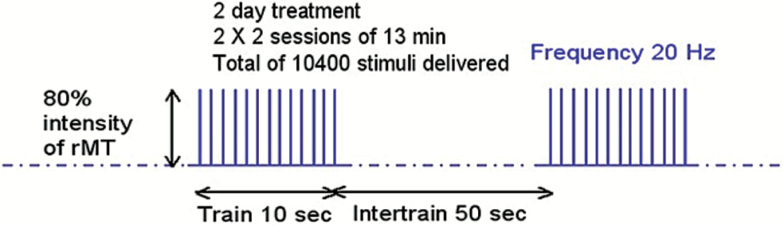
Two types of devices were used in this multicenter trial, the Magstim Rapid2 (The Magstim Company Limited, Carmarthenshire, UK) and the MagPro X-100 (MagVenture, Farum, Denmark) with a figure-8 coil. The high-frequency protocol (20 Hz) consisted of 13 trains with a duration of 10 s and 200 pulses in each train (figure 1). The intertrain interval was 50 s, resulting in 2600 total pulses and a total duration of 13 min. Four 13-min rTMS sessions were performed, with 2 sessions a day. We individually measured the resting motor threshold (rMT) before each session using electromyography recordings on the first dorsal interosseus muscle, and set the stimulation intensity at 80% of the rMT.
Fig. 1.
20 Hz rTMS paradigm. rMT, resting motor threshold; sec, seconds; min, minutes; Hz, Hertz.
The same procedure was performed in the sham group including the rMT assessment and the neuronavigation system for target localization, and sham coils were used for the 2 stimulators. Both sham coils deliver a very slight magnetic field, have the same appearance and sound, and provide the same tactile sensations as the active coils.
rTMS Site Location
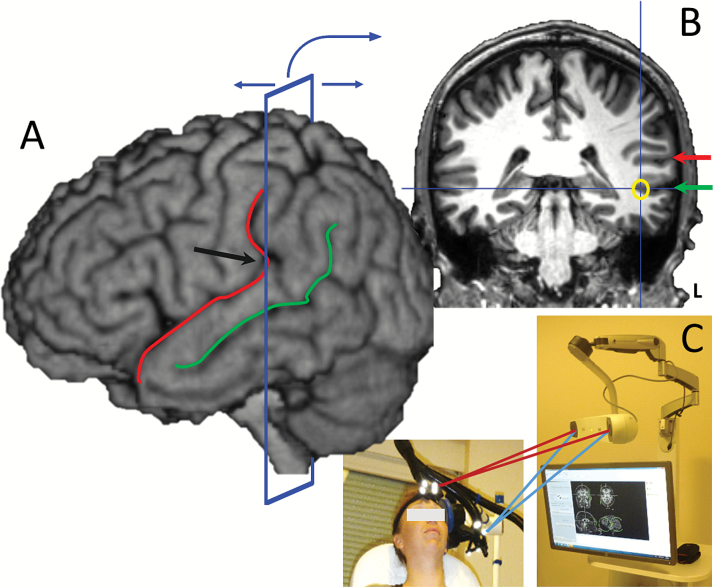
The anatomical target was determined in accordance with a previous study using a language task during functional magnetic resonance imaging (fMRI).19 In this study, a significant AVH reduction was obtained by stimulating over the area of maximal task-evoked fMRI activation, which was located along the left superior temporal sulcus (STS). We also observed that this functional cluster generally corresponded to the same anatomical location across patients, while the classical location of T3P3 based on the 10–20 EEG system showed wide anatomical variability between subjects. The cortical target indicated by fMRI was always located at the crossing between the projection of the ascending branch of the left lateral sulcus (LS) and the left STS and it could be easily and quickly identified (in under 2 min) according to the individual MRI and using a frameless stereotaxic device, even by a nonexpert in neuroimaging. Therefore, we used this targeting procedure in the present study (figure 2; supplementary data video 1).
Fig. 2.
Location of the rTMS stimulation target by structural MRI and neuronavigation (A) Before rTMS treatment, the lateral sulcus (red line) and the superior temporal sulcus (green line) are located on the 3D MRI view using neuronavigation software. Then the coronal plane is moved in the posterior direction until it crosses the point where the lateral sulcus becomes vertical (black arrow). (B) The lateral sulcus (red arrow) and the superior temporal sulcus (green arrow) are located based on the 3D view on the coronal plane. Finally, the rTMS target is defined at the bottom of the superior temporal sulcus (yellow circle). (C) The neuronavigation tool is used during treatment to precisely locate the coil over the target.
Outcome Measurements
The primary outcome was defined as the percentage of patients who presented a decrease of more than 30% in the AHRS frequency item at 2 successive evaluations, spaced 1 week apart. The secondary outcomes were the percentage of patients who presented a decrease of more than 30% in the total AHRS score after 2 weeks (D14), the variation in total AHRS scores between D1 and D2, D7, D14, D21, and D30, the variation in CGI, SUMD, and positive, negative, general, and total PANSS scores between D0, D14, and D30, and the recording of adverse effects during the rTMS sessions, after the last session, and at D7.
The rTMS administrator used open-ended questions and a visual analog scale (VAS) to assess adverse effects and the overall painfulness of the procedure after each treatment session. The blind investigator used the UKU rating scale38 (after the last rTMS session at D2) and at D7 to assess potential delayed side effects.
Statistical Analyses
Based on preliminary results obtained from an open study,20 we determined that 72 patients were needed in the present study for a power at 90% and an alpha risk at 0.05. We used the Student’s t-test to evaluate differences between groups, with Levene’s test to check heteroscedasticity. We applied Pearson’s chi-square test or Fisher’s exact test to compare qualitative or categorical variables. Mixed-effects models with repeated measures (MMRM) were then used with the “group” and “day” factors to make pairwise comparisons between the 2 groups and different measurement times. The odds ratio (OR) reflects the odds of a successful outcome in the active treatment group relative to the odds of a similar outcome in the sham group, and was used to measure effect size.39 OR was adjusted for the neuro-navigation system, stimulator type, and center variables.
All tests were 2-tailed and the probability significance threshold was set at P < .05. IBM—All statistical analyses were performed using SPSS 22.0 for Windows. All protocol and data are available for evaluation at the Caen CHU (Centre de Recherche Clinique, CRC).
Results
Dropout
Fifteen of the 74 patients (20%) included and randomized dropped out, 6 from the sham group and 9 from the active group. Fourteen did not receive all 4 sessions of rTMS due to cerebral anatomical abnormalities (n = 4), technical issues with the rTMS (n = 4), withdrawal of consent (n = 3), and improvement of AHRS (n = 1). There was one protocol violation by a single patient related to a violation of inclusion criteria, who was therefore excluded from the analyses and 2 patients were lost before the beginning of rTMS treatment. The remaining 59 patients were treated and fully evaluated in either the active treatment group (n = 26) or in the sham group (n = 33) and were included in the analyses (see supplementary data flow diagram).
Baseline Comparison
Patient demographics are summarized in table 1. There was no difference between groups in age, gender, age of onset, duration of illness, marital status, employment status, severity of hallucinations as assessed with the AHRS scores, severity of illness assessed as with the PANSS total score, antipsychotic doses in chlorpromazine-equivalents, or diagnosis. However, the sham group had been slightly but not significantly hospitalized more often, had a longer duration of illness, and had fewer years of education than the active group.
Table 1.
Population Characteristics
| Variables | Active Group | Sham Group | P |
|---|---|---|---|
| (n = 26) | (n = 33) | ||
| M ± DS | M ± DS | ||
| Age (years) | 35.3 ± 8.3 | 39.6 ± 11.4 | .113 |
| Years of school education | 12.5 ± 3.1 | 11.0 ± 3.0 | .058 |
| Number of psychiatric hospitalizations | 4.6 ± 3.9 | 7.6 ± 7.1 | .059 |
| Age of onset (years) | 23.0 ± 6.3 | 23.0 ± 7.4 | .983 |
| Disease duration (years) | 11.0 ± 7.7 | 15.2 ± 9.6 | .079 |
| Chlorpromazine equivalent (mg) | 630 ± 411 | 771 ± 557 | .292 |
| AHRS D0 | 28.6 ± 6.0 | 27.8 ± 4.5 | .529 |
| PANSS total score D0 | 85.19 ± 32.15 | 91.58 ± 30.69 | .441 |
| % | % | ||
| Gender (male) | 65.4 | 45.4 | .127 |
| Employment status (unemployed) | 76.9 | 84.8 | .307 |
| Marital status (single) | 80.8 | 67.6 | .421 |
| Diagnosis | |||
| Schizophrenia (DSM-IV R) | 76.9 | 75.8 | .917 |
| Schizoaffective disorders (DSM-IV R) | 23.1 | 24.2 | |
Primary Outcome
The percentage of patients showing a decrease of more than 30% in the AHRS frequency item at 2 successive ratings was not significantly different between the active (50%) and the sham groups (48.5%).
Secondary Outcome
The percentage of patients showing a decrease of more than 30% in the total AHRS score is shown in table 2. The difference in the percentage of these responders between the active group (34.6%) and the sham group at D14 (9.1%) (P = .016) was significant, and this difference remained significant after adjustment for neuronavigation system, type of stimulator, and center, with an adjusted OR at 5.60 (95% CI: 1.28–0.022, table 3). The percentage of responders was not significantly different between the 2 groups at D2, D7, D21, or D30.
Table 2.
Percentage of Respondersa at Day 2, 7, 14, 21 and 30
| Active Group, n = 26 | Sham Group, n = 33 | P | |
|---|---|---|---|
| Day 2 | 19.2 | 21.2 | .851 |
| Day 7 | 30.8 | 21.2 | .403 |
| Day 14 | 34.6 | 9.1 | .016 |
| Day 21 | 26.9 | 18.2 | .421 |
| Day 30 | 26.9 | 18.2 | .421 |
aResponders: variation in AHRS scores ≤−30% (relative variation of the score between days 2, 7, 14, 21, 30 and day 1).
Table 3.
Percentage of Responders With a Decrease of AHRS Scores Greater Than 30% (Relative Variation) Relative to Active and Sham Groups, Adjusted for the Other Variables by Logistic Regression
| OR | 95% CI | P | |
|---|---|---|---|
| Groups | |||
| Active vs sham | 5.60 | 1.28–24.54 | .022 |
| Neuronavigation system | .657 | ||
| NexStim vs ANT neuro | 2.08 | 0.30–14.47 | .460 |
| Syneika vs ANT neuro | 3.47 | 0.23–53.03 | .371 |
| Type of transcranial magnetic stimulator | |||
| MagPro vs MagStim | 1.14 | 0.12–10.40 | .910 |
| University hospital centers | |||
| Ranked from low to high success rates | 1.11 | 0.68–1.83 | .673 |
Note: Hosmer–Lemeshow test: P = .804.
Variation in AHRS total score (table 4) was significant (P < .001), but not different between the active and sham groups (P = .978), and time × group interaction was significant (P < .001).
Table 4.
AHRS Score Variation in the Sham (n = 33) and the Active Groups (n = 26)
| Groups | Mean | SD | |
|---|---|---|---|
| Day 1(1) | Sham | 26.92 | 6.054 |
| Active | 27.96 | 5.01 | |
| Day 2(2) | Sham | 22.91 | 7.53 |
| Active | 24.27 | 6.10 | |
| Day 7(3) | Sham | 22.30 | 7.65 |
| Active | 21.73 | 8.77 | |
| Day 14(4) | Sham | 23.36 | 6.44 |
| Active | 21.67 | 8.76 | |
| Day 21(5) | Sham | 23.42 | 6.57 |
| Active | 22.98 | 7.91 | |
| Day 30(6) | Sham | 22.57 | 6.64 |
| Active | 22.61 | 6.46 |
Note: Course of the scores: P < .001. P < .001: (1) vs (2), (1) vs (3), (1) vs (4), (1) vs (5), (1) vs (6). For all other comparisons, P was not statistically significant. Score between active and sham group: P = .978. Interaction time/groups: P < .001.
The variation of the scores for the other scales were broadly similar to that seen for total AHRS scores, except for the SUMD and Birchwood scale which showed no significant difference in the variations of the total scores (supplementary data table 1). No time × group interactions were observed for any scale.
Procedure Painfulness and Adverse Events
Adversed effects are summarized in the Table 5. The active treatment was perceived as slightly but significantly more painful than the sham treatment (2.8 ± 1.6 vs 1.4 ± 1.7), and adverse events directly caused by stimulation (squeezing, local pain, clenched jaw, or blepharospasm) were reported more often during active treatment. Other adverse events (eg, body position discomfort) were reported more often during the sham treatment. Although there were more adverse events observed during the active than the sham procedure, there were no major side effects reported in any groups.
Table 5.
Frequency of Adverse Effects and Painfulness of the Treatment
| Active Group (n = 104) | Sham Group (n = 132) | ||
|---|---|---|---|
| Local signs | |||
| Tingling | 9.6% | 3.8% | |
| Squeezing | 11.5% | 2.3% | P < .01 |
| Pain | 10.6% | 3.8% | P < .05 |
| Regional signs | |||
| Hemi-facial pain and squeezing | 11.5% | 8.3% | |
| Clenched jaw | 28.8% | 1.5% | P < .0001 |
| Blepharospasm | 14.4% | 0.8% | P < .001 |
| General signs | |||
| Headache | 13.5% | 6.8% | |
| Asthenia | 1.0% | 1.5% | |
| Other complaints | |||
| Noise | 6.7% | 12.9% | |
| Uncomfortable body position | 0.0% | 5.3% | P < .05 |
| Motor threshold | 1.9% | 2.3% | |
| Other | 4.8% | 6.8% | |
| No complaints | 32.7% | 56.1% | P < .0001 |
| Treatment painfulness | |||
| VAS (mean of 4 sessions) | 2.8 ± 1.6 (n = 21) | 1.4 ± 1.7 (n = 28) | P < .05 |
There were also no differences in side effects as assessed with the UKU at D2, but depression and sleepiness/sedation were reported significantly more often in the sham group at D7 (16% vs 0%, P = .04 and 19% vs 0%, P = .02).
Discussion
This is the first controlled study testing the efficacy of 20 Hz rTMS guided by neuronavigation and targeted to a specific anatomical site for the treatment of AVHs in patients with schizophrenia. The primary outcome was defined as percentage of patients showing a 30% decrease of the AHRS frequency item at 2 successive evaluations and did not show a significant difference between active and sham treatment groups. However, we did observe a transient efficacy at day 14, with 34.6% and 9.1% of patients in the active and sham groups showing a decrease of more than 30% of AHRS scores, respectively (P = .016).
High Frequency
These results confirm a previous uncontrolled observation19 and indicate that high-frequency rTMS over a specific site on the left STS can lead to a significant improvement relative to placebo in AVHs. The results suggest that rTMS acts selectively on AVHs, since no changes were observed in insight level or negative, positive, or general psychopathological symptoms of schizophrenia. This result supports the hypothesis that high-frequency rTMS applied to the left temporal cortex can modulate cortical activity in the same direction as low-frequency rTMS in this clinical condition defined here as patients with schizophrenia and AVHs who are stimulated at this precise left temporal site.21 As the efficacy of low frequency rTMS for the treatment of AVH is controversial and has led to a low-level recommendation in Europe (Level C), the use of high frequency stimulation could revive an interest in rTMS for the treatment of AVHs in schizophrenia patients.
Two previous studies comparing high frequency or cTBS with low frequency stimulation reported similar and significant decreases in AVHs.22,23 However, these studies were not double-blind or sham-controlled, and the placebo effect is known to be high in this context.13
There are some limitations to the results presented here, specifically that the observed efficacy was transient at day 14 and was not observed on AVH frequency, in contrast to our expectations.
Our primary endpoint was defined as improvement in the frequency of AVHs at 2 successive evaluations, and was informed by a study by Hoffman et al40 that reported significant improvements with 1 Hz rTMS compared to sham stimulation in hallucination frequency but not in hallucination change score. Two additional studies have also failed to demonstrate any efficacy of 1 Hz rTMS on AVH frequency.41,42 Therefore, evaluating rTMS on the hallucination frequency only might be too limiting, given that the AHRS frequency item does not take hallucinations that occur on a less than daily basis into account.
The percentage of responders based on total AHRS score did show a significant difference between the active and the sham groups at D14 following rTMS procedure, but this difference did not persist over time. Since this result was only observed at D14, some comments on the delay and the transient nature of the between-group difference in responder rate are warranted. First, the significant difference in responders might be due to the combination of a delayed effect of the active treatment along with a simultaneous decrease of placebo responders in the sham group at D14. This delayed effect was also observed in the treatment of AVHs after the first week,43 at 2-week follow-up,18 and even at 3 weeks following active cTBS,44 and a similar temporal pattern has been observed in depressive patients.28 Placebo responders could also vary over time, and analysis of the placebo response for each subject allows us to investigate the observed dip in placebo responses seen at day 14 (see supplementary data figure 1). In this study, 13 patients in the sham group were observed to be placebo responders in least at 1 out of the 5 evaluations, and 3 types of responders were identified. Three patients from the sham group were placebo responders at all evaluations except one (either at the first or the last evaluation), could be defined as maintained placebo responders, and were observed at D14 in particular (type 1). Five patients were placebo responders at 1, or more often at 2 evaluations but not at D14, and presented AVHs at D14 and at least 2 other evaluations (type 2). These patients may reflect the natural variations of AVH scores over time, rather than a real placebo effect. Five patients were early placebo responders at only D2 and/or D7, and not at later evaluations (type 3). This early and brief response over 1 week following sham rTMS has been reported previously.43 These type 3 placebo responders could account for the “dip” in overall placebo responders observed at D14 in the sham group. We note that the difference in responders between the active and sham groups could not be explained by differences in demographic characteristics. In fact, given the observation of a greater placebo response with higher baseline clinical severity scores,45 we expected a higher level of placebo responders in the sham group, since those patients presented with slightly higher doses of antipsychotics, PANSS scores, and more prior hospitalizations. Finally, rTMS effect sustainability has not been evaluated under double-blind conditions in previous studies. In fact, reporting an efficacy of active treatment over sham conditions evaluated the efficacy of rTMS at one endpoint only, either at the end of the treatment procedure14–17,40 or at 1 and 2 weeks after the end of treatment.18 Notably, a transient effect was reported with active cTBS showing an effect after 3 weeks but not after 6 weeks after treatment.44 This delayed and transient effect account for the negative results reported in studies based only on early or late assessments after treatment.46–49 However, the transient effect seen in the present study could also be due to an insufficient number of rTMS sessions. As described, rTMS exposure in this study was limited to 4 sessions, while “high dose” or “accelerated” protocols typically suggest more sessions to achieve maximum efficacy.26 The view toward maximizing the number of rTMS sessions was also suggested by data in an unmasked extension study for the treatment of AVHs.40
The fact that efficacy was not maintained over time could support the application of maintenance rTMS sessions every 2 or 3 weeks. The delayed clinical response lasting beyond the time of stimulation could be explained by an effect of 20 Hz rTMS on brain plasticity.50 This hypothesis is corroborated by the long-lasting effects observed in various markers of neuroplasticity in animals following high-frequency cortical stimulation, including brain-derived neurotrophic factor and glutamate receptors.51
Site of Stimulation
The second issue addressed in the current study is the method of rTMS targeting to a precise anatomical site. The target was defined as the site for which stimulation was found to produce the most significant effects on AVHs, and was located in the left temporo-parietal cortex,33 an area that could correspond to a hub region for both language processing and AVHs. Unfortunately, no AVHs were registered during the scans, so we were unable to investigate overlap between this anatomical location and functional clusters induced by AVHs. However, in this study this region was rigorously identified on each patient’s morphological magnetic resonance imaging (MRI) using a simple procedure (see supplementary data video 1). This method is certainly more accurate on an anatomical basis than using the EEG 10–20 system, and has the additional advantage of ease relative to performing fMRI in clinical practice. Previous studies have shown the value of neuronavigation based on individual morphological or fMRI for improving rTMS outcomes in healthy subjects52 as well as in patients with depression53,54 or schizophrenia.55 Previous rTMS studies have shown conventional 10–20 EEG-based targeting requires significantly more patients to obtain significant results, relative to studies using neuronavigation.56 This also helps to explain the negative results reported in previous studies with small sample sizes using procedures other than neuronavigation,46 even though high-frequency (20 Hz) stimulation was applied.57
Tolerance
Although 20 Hz was preformed, the treatment presented here was tolerated quite well. While the presence of side effects as assessed with VAS was significantly higher in the active group relative to the sham group, they were all minor. No major adverse events were observed, there were no seizures, and the treatment was not discontinued in any patient due to side effects. Although no seizures following rTMS applied to the temporal areas have been reported, we designed the rTMS parameters for the present study according to the international safety guidelines for rTMS,58 paying particular attention to the duration of intertrain intervals (50 s) with respect to the stimulation intensity (80% of rMT) to avoid the risk of seizure.58,59 We also did not find any cognitive side effects, in agreement with the literature60 which includes rTMS studies performed in patients with AVHs.15,61,62 Unexpectedly, more depression and sleepiness/sedation were reported with the UKU rating scale in the sham group than in the active group at D7. No other differences between groups were observed. The side effects reported with VAS were counter-balanced with the nocebo effects assessed with the UKU scale, and suggest that the blindness procedure was effective. This data further support the safety profile of high-frequency rTMS application for other clinical indications, such as neuropathic pain, tinnitus, and stroke.63
Limits of the Study
This study has some limitations. A high number of patients that dropped out prior to starting the rTMS procedure (20%), although there were no dropouts during the follow-up period. The fact that randomization was not done just before the beginning of the treatment could explain the high dropout level and the slight (but not significant) difference between groups in past hospitalizations, illness duration, and years of education. This reduced the power of the study from 90% to 77% and could explain the lack of a significant result for the primary outcome. However, for the secondary outcome the P-value was equal to .016, the OR was significant, and the power was close to 80% indicating relevant differences between groups. The absence of a group treated with low-frequency (1 Hz) rTMS prevents us from drawing conclusions regarding the relative merits of high-frequency over low-frequency rTMS applied over the cortical target site used in this study. The fact that patients were not asked about which group they were a part of at the end of the treatment could be considered a weakness of the blindness procedure. Finally, the number of stimulation sessions could be insufficient for a long-lasting effect, and which might explain the transient nature of the effect we observed.
In conclusion, these results confirm the value of high-frequency rTMS (20 Hz) applied to the left temporal cortex for the treatment of AVHs. However, this efficacy is transient, and additional clinical trials are necessary to further support this innovative therapy and to improve its efficacy, potentially by long-term management based on a more prolonged procedure.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The financial grant was supported by the French Health Ministry in a National “Programme Hospitalier de Recherche Clinique” (PHRC, No. 06-03) and the Regional Council of Basse-Normandie.
Conflicts of interest
Sonia Dollfus is an expert and consultant or participated in educational conferences for the following industrial laboratories: Gedeon Richter, Roche, Takeda, Fabre, Janssen, and ONO Pharma. Olivier Guillin is expert or consultant for Takeda, JNJ, Servier, Lundbeck, Fabre, Otsuka. Benoit Trojak received honoraria for participation in clinical trials and oral presentations from DA pharma, Ethypharm, Lundbeck, Janssen, and Lilly. Marion Plaze participated in educational conferences or has received travel grant from Lilly, Otsuka, Lundbeck, Janssen, and Astra-Zeneca. Cécilia Nauczyciel received honoraria for participation in oral intervention from Astra Zeneca. Vincent Meille received honoraria for participation in clinical trials and oral presentations from DA pharma, Ethypharm, Lundbeck, Janssen, and Lilly. Marie Odile Krebs participated in advisory boards or educational conferences and has received travel grant or compensation from F. Hoffmann–La Roche, Ltd., Otsuka, Lundbeck, Janssen, and Astra-Zeneca. Jean-Marie Batail has relationships (travel/accommodations expenses covered/reimbursed/educational conferences) with Otsuka, Lundbeck, Janssen, and Lilly who might have an interest in the work submitted in the previous 3 years. Raphaël Gaillard has served as consultant, member of the scientific advisory board and/or speaker for Astra Zeneca, Pierre Fabre, Janssen, Lilly, Otsuka, Roche, SANOFI, Servier and he has received research support from Servier and Sisley. Samar S. Ayache, Perrine Brazo, Nathalie Chastan, Benoit Crepon, Christophe Delmas, Olivier Etard, Gael Fouldrin, Emmanuel Gerardin, Laurent Guillaume, Rémy Guillevin, Carole Guillevin, Ghina Harika-Germaneau, Nemat Jaafari, Jean-Pascal Lefaucheur, Nicolas Lafay, Elise Leroux, Aurélie Montagne Larmurier, Rémy Morello, Clément Nathou, Annick Razafimandimby, Maud Rotharmel, Ghassen Saba, and Issa Wassouf have no conflicts of interest to disclose.
Supplementary Material
Acknowledgments
We thank Nicolas Delcroix and Antoine Nourry from UMS 3408 (GIP Cyceron) for their assistance in brain imaging and Yannick Chene for his administrative help. We also thank the staff of the Clinical Research Center (CRC) and the DRCI form Caen CHU for their help in all the centers.
References
- 1. Miyamoto S, Lieberman JA, Fleischhacker WW, Aoba A, Marder SR. Antipsychotic drugs. In: Tasman A, Kay J, Lieberman JA, eds. Psychiatry. Chichester, UK: John Wiley & sons; 2003:1928–1964. [Google Scholar]
- 2. Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. [DOI] [PubMed] [Google Scholar]
- 3. Dierks T, Linden DE, Jandl M et al. . Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. [DOI] [PubMed] [Google Scholar]
- 4. Reulbach U, Bleich S, Maihofner C, Kornhuber J, Sperling W. Specific and unspecific auditory hallucinations in patients with schizophrenia: a magnetoencephalographic study. Neuropsychobiology. 2007;55:89–95. [DOI] [PubMed] [Google Scholar]
- 5. Silbersweig DA, Stern E, Frith C et al. . A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. [DOI] [PubMed] [Google Scholar]
- 6. Aleman A, Sommer IE, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68:416–421. [DOI] [PubMed] [Google Scholar]
- 7. Tranulis C, Sepehry AA, Galinowski A, Stip E. Should we treat auditory hallucinations with repetitive transcranial magnetic stimulation? A meta-analysis. Can J Psychiatry. 2008;53:577–586. [DOI] [PubMed] [Google Scholar]
- 8. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slotema CW, Aleman A, Daskalakis ZJ, Sommer IE. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: update and effects after one month. Schizophr Res. 2012;142:40–45. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Liang W, Yang S, Dai P, Shen L, Wang C. Repetitive transcranial magnetic stimulation for hallucination in schizophrenia spectrum disorders: a meta-analysis. Neural Regen Res. 2013;8:2666–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demeulemeester M, Amad A, Bubrovszky M, Pins D, Thomas P, Jardri R. What is the real effect of 1-Hz repetitive transcranial magnetic stimulation on hallucinations? Controlling for publication bias in neuromodulation trials. Biol Psychiatry. 2012;71:e15–e16. [DOI] [PubMed] [Google Scholar]
- 12. Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database Syst Rev. 2015;8:CD006081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dollfus S, Lecardeur L, Morello R, Etard O. Placebo response in repetitive transcranial magnetic stimulation trials of treatment of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Bull. 2016;42(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355:1073–1075. [DOI] [PubMed] [Google Scholar]
- 15. Hoffman RE, Gueorguieva R, Hawkins KA et al. . Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005;58:97–104. [DOI] [PubMed] [Google Scholar]
- 16. Poulet E, Brunelin J, Bediou B et al. . Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry. 2005;57:188–191. [DOI] [PubMed] [Google Scholar]
- 17. Brunelin J, Poulet E, Bediou B et al. . Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res. 2006;81:41–45. [DOI] [PubMed] [Google Scholar]
- 18. Klirova M, Horacek J, Novak T et al. . Individualized rTMS neuronavigated according to regional brain metabolism ((18)FGD PET) has better treatment effects on auditory hallucinations than standard positioning of rTMS: a double-blind, sham-controlled study. Eur Arch Psychiatry Clin Neurosci. 2013;263:475–484. [DOI] [PubMed] [Google Scholar]
- 19. Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S. Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res. 2009;113:77–83. [DOI] [PubMed] [Google Scholar]
- 20. Dollfus S, Larmurier-Montagne A, Razafimandimby A et al. . Treatment of auditory hallucinations by combining high-frequency repetitive transcranial magnetic stimulation and functional magnetic resonance imaging. Schizophr Res. 2008;102:348–351. [DOI] [PubMed] [Google Scholar]
- 21. Nathou C, Etard O, Simon G, Dollfus S. How do high- and low-frequency repetitive transcranial magnetic stimulations modulate the temporal cortex. Psychophysiology. 2015;52:192–198. [DOI] [PubMed] [Google Scholar]
- 22. de Weijer AD, Sommer IE, Lotte MA et al. . High frequency rTMS; a more effective treatment for auditory verbal hallucinations?Psychiatry Res. 2014;224:204–210. [DOI] [PubMed] [Google Scholar]
- 23. Kindler J, Homan P, Flury R, Strik W, Dierks T, Hubl D. Theta burst transcranial magnetic stimulation for the treatment of auditory verbal hallucinations: results of a randomized controlled study. Psychiatry Res. 2013;209:114–117. [DOI] [PubMed] [Google Scholar]
- 24. Khedr EM, Rothwell JC, Ahmed MA, El-Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatry. 2008;79:212–215. [DOI] [PubMed] [Google Scholar]
- 25. Holtzheimer PE III, McDonald WM, Mufti M et al. . Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety. 2010;27:960–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baeken C, Marinazzo D, Wu GR et al. . Accelerated HF-rTMS in treatment-resistant unipolar depression: insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15:286–297. [DOI] [PubMed] [Google Scholar]
- 27. George MS, Raman R, Benedek DM et al. . A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul. 2014;7:421–431. [DOI] [PubMed] [Google Scholar]
- 28. Duprat R, Desmyter S, Rudi de R et al. . Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission?J Affect Disord. 2016;200:6–14. [DOI] [PubMed] [Google Scholar]
- 29. Herwig U, Satrapi P, Schönfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. [DOI] [PubMed] [Google Scholar]
- 30. Schönfeldt-Lecuona C, Grön G, Walter H et al. . Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15:1669–1673. [DOI] [PubMed] [Google Scholar]
- 31. Jardri R, Lucas B, Delevoye-Turrell Y et al. . An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry. 2007;12:320. [DOI] [PubMed] [Google Scholar]
- 32. Sommer IE, de Weijer AD, Daalman K et al. . Can fMRI-guidance improve the efficacy of rTMS treatment for auditory verbal hallucinations?Schizophr Res. 2007;93:406–408. [DOI] [PubMed] [Google Scholar]
- 33. Hoffman RE, Hampson M, Wu K et al. . Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17:2733–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 35. Paillot C, Ingrand P, Millet B et al. . French translation and validation of the Scale to assess Unawareness of Mental Disorder (SUMD) in patients with schizophrenics. Encephale. 2010;36:472–477. [DOI] [PubMed] [Google Scholar]
- 36. Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62–67. [DOI] [PubMed] [Google Scholar]
- 37. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 38. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. [DOI] [PubMed] [Google Scholar]
- 39. Fleiss JL. Measures of effect size for categorical data. In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. New York: Russel Sage; 1994:245–260. [Google Scholar]
- 40. Hoffman RE, Wu K, Pittman B et al. . Transcranial magnetic stimulation of Wernicke’s and Right homologous sites to curtail “voices”: a randomized trial. Biol Psychiatry. 2013;73:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SH, Won L, Young-cho C et al. . A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett. 2005;376:177–181. [DOI] [PubMed] [Google Scholar]
- 42. Rosa MO, Gattaz WF, Rosa MA et al. . Effects of repetitive transcranial magnetic stimulation on auditory hallucinations refratory to clozapine. J Clin Psychiatry. 2007;68:1528–152. [DOI] [PubMed] [Google Scholar]
- 43. Chibbaro G, Daniele M, Alagona G et al. . Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett. 2005;383:54–57. [DOI] [PubMed] [Google Scholar]
- 44. Plewnia C, Zwissler B, Wasserka B, Fallgatter AJ, Klingberg S. Treatment of auditory hallucinations with bilateral theta burst stimulation: a randomized controlled pilot trial. Brain Stimul. 2014;7:340–341. [DOI] [PubMed] [Google Scholar]
- 45. Agid O, Siu CO, Potkin SG et al. . Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry. 2013;170:1335–1344. [DOI] [PubMed] [Google Scholar]
- 46. Koops S, van Dellen E, Schutte MJ, Nieuwdorp W, Neggers SF, Sommer IE. Theta burst transcranial magnetic stimulation for auditory verbal hallucinations: negative findings from a double-blind-randomized trial. Schizophr Bull. 2016;42:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slotema CW, Blom JD, de Weijer AD et al. . Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry. 2011;69:450–456. [DOI] [PubMed] [Google Scholar]
- 48. de Jesus DR, Gil A, Barbosa L et al. . A pilot double-blind sham-controlled trial of repetitive transcranial magnetic stimulation for patients with refractory schizophrenia treated with clozapine. Psychiatry Res. 2011;188:203–207. [DOI] [PubMed] [Google Scholar]
- 49. Blumberger DM, Christensen BK, Zipursky RB et al. . MRI-targeted repetitive transcranial magnetic stimulation of Heschl’s gyrus for refractory auditory hallucinations. Brain Stimul. 2012;5:577–585. [DOI] [PubMed] [Google Scholar]
- 50. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression?Depress Anxiety. 2015;32:182–192. [DOI] [PubMed] [Google Scholar]
- 51. Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Neggers SF, Langerak TR, Schutter DJ et al. . A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage. 2004;21:1805–1817. [DOI] [PubMed] [Google Scholar]
- 53. Fitzgerald PB, Hoy K, McQueen S et al. . A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology. 2009;34:1255–1262. [DOI] [PubMed] [Google Scholar]
- 54. Rusjan PM, Barr MS, Farzan F et al. . Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kindler J, Homan P, Jann K et al. . Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73:518–524. [DOI] [PubMed] [Google Scholar]
- 56. Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Gaebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21:207–221. [DOI] [PubMed] [Google Scholar]
- 57. Kimura H, Kanahara N, Takase M, Yoshida T, Watanabe H, Iyo M. A randomized, sham-controlled study of high frequency rTMS for auditory hallucination in schizophrenia. Psychiatry Res. 2016;241:190–194. [DOI] [PubMed] [Google Scholar]
- 58. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. [DOI] [PubMed] [Google Scholar]
- 59. Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–471. [DOI] [PubMed] [Google Scholar]
- 60. Martis B, Alam D, Dowd SM et al. . Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125–1132. [DOI] [PubMed] [Google Scholar]
- 61. d’Alfonso AA, Aleman A, Kessels RP et al. . Transcranial magnetic stimulation of left auditory cortex in patients with schizophrenia: effects on hallucinations and neurocognition. J Neuropsychiatry Clin Neurosci. 2002;14:77–79. [DOI] [PubMed] [Google Scholar]
- 62. Fitzgerald PB, Benitez J, Daskalakis JZ et al. . A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol. 2005;25:358–362. [DOI] [PubMed] [Google Scholar]
- 63. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation?Nat Rev Neurosci. 2007;1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.