Abstract
Human metapneumovirus is an emerging cause of lower respiratory disease in infants, young children, and immunocompromised adults. We have previously demonstrated that human metapneumovirus infection is mediated by interaction of human metapneumovirus attachment (G) and/or fusion (F) proteins with cellular glycosaminoglycans. We report here the activity of an investigational sialidase fusion protein, DAS181, on human metapneumovirus infection of Hep-2 cells. These results suggest that human metapneumovirus infection may involve sialic acids, providing a new therapeutic strategy for human metapneumovirus for which there is currently no available treatment.
Methods
Hep-2 cells were preincubated with DAS181 or control DAS185 (a mutated sialidase) prior to inoculation with human metapneumovirus strains. Infectivity was assessed by a cell-based ELISA quantitating human metapneumovirus matrix protein. The effect of DAS181 on binding of recombinant G attachment protein was also determined.
Results
DAS181 blocked infection of human metapneumovirus strains A2, B1, and B2 at low concentrations. No effect of DAS185 was observed. Binding of MPV G protein to Hep-2 cells was also markedly inhibited by preincubation of cells with DAS181.
Conclusions
These results suggest that human metapneumovirus may utilize sialic acids as an entry cofactor. DAS181 may thus represent a new therapeutic agent useful for the treatment of human metapneumovirus.
Keywords: Human metapneumovirus, sialidase fusion protein, infectivity, sialic acids, attachment, DAS181
Background
Human metapneumovirus (hMPV) is a common cause of respiratory illness in infants and young children worldwide and is also increasingly described in adults and immunocompromised patients.1,2 We have previously demonstrated that hMPV infection is mediated by interaction of hMPV attachment (G) and/or fusion (F) proteins with cellular glycosaminoglycans (GAGS), as infection is inhibited by soluble GAGS such as heparin and chondroitin sulfates, or by enzymatic removal of cell surface GAGS with GAG lyases.3
Cell surface glycoproteins, glycolipids, and proteoglycans are utilized by a wide variety of viruses. Most carbohydrate structures used by viruses as receptors are strongly negatively charged, including sulfated GAGs or glycans containing sialic acid.4 Influenza viruses are the prototypic GAG binding viruses and utilize cell surface sialic acids as a receptor to mediate infection.5 Previous studies6 demonstrate that an investigational sialidase fusion protein, DAS181, inhibits influenza virus infection by cleaving terminal sialic acids from glycoproteins. DAS181 is comprised of a sialidase catalytic domain active on both human and avian influenza viruses and a heparin-binding domain from human amphiregulin, which localizes and retains the fusion protein to the respiratory epithelium. As a fundamentally novel host-directed approach against respiratory viruses, DAS181 has potential broad-spectrum activity, reduced risk for drug resistance, improved efficacy and safety. These characteristics combined offer benefits not currently available with other antiviral treatments.
No specific antiviral or other therapeutic agents are currently available to treat hMPV infection. In this study, we determined whether DAS181 has any effect on hMPV replication in vitro and also examined the effect of this sialidase fusion protein on binding of recombinant hMPV G attachment glycoprotein to cells.
Methods
Sialidase fusion protein
Sialidase fusion protein (DAS181) was kindly provided by Ansun BioPharma (San Diego, CA). DAS181 consists of a heparin-binding domain derived from amphiregulin (amino acid residues 125–145 in GenBank entry AAH09799) fused via its N terminus to the catalytic domain of Actinomyces viscosus sialidase (AvCD, amino acid residues 274–667 in GenBank entry X62276).6 DAS185 is a mutated sialidase expressing construct that has the identical amphiregulin tag but exhibits 400-fold reduced sialidase activity compared to DAS181. The drugs were supplied as a dry powder and solubilized in sterile PBS to a stock concentration of 50 mg/ml.
Cells and viruses
The human epithelial tumor cell line, HEp-2, and rhesus monkey kidney cells (LLC-MK2) (American Type Culture Collection, Manassas, VA) were grown in medium 199 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. Stocks of hMPV were prepared by inoculation of LLC-MK2 cells with hMPV and incubation for 14–21 days at 37℃ in 5% CO2. hMPV stocks (International Committee on Taxonomy of Viruses, ICTV, nomenclature) used for infectivity assays were as follows: V50569 and V51200 (A2 strains), V47041 and V32748 (B1 strains), and V52283 (B2 strain). All strains of hMPV were isolated from clinical samples by the diagnostic Virology Laboratory, Flinders Medical Centre, Bedford Park, South Australia. These samples were positive by RT-PCR only for hMPV and were not coinfected with influenza A; influenza B; RSV; adenovirus or parainfluenza 1, 2, or 3. All virus stocks were stored at −70℃ until use. hMPV infectivity titer was determined using an immunofluorescence assay. Briefly, cells were incubated with dilutions of the virus and counted after staining with a monoclonal Ab (MAb) to hMPV matrix protein (Chemicon, Temecula, CA) and FITC-labeled secondary antibody (Chemicon). The virus titer was calculated assuming each fluorescent focus represented one infectious unit of virus and was reported as fluorescent focus forming units (FFU) per milliliter.
hMPV infectivity ELISA
A cell-based ELISA assay was used to quantitate hMPV infection. HEp-2 cell monolayers in 96 well plates (Linbro, ICN Biomedicals, Aurora, OH) were inoculated with hMPV at a multiplicity of infection of 1 FFU per cell and incubated for 2 h at 37℃, 5% CO2. Control wells were “mock” inoculated with no virus. Cells were washed with medium 199 to remove unbound virus. Medium 199 containing 1 µg/ml trypsin was then added and cells were cultured for 48 h. Medium was removed and cells were fixed with 1% paraformaldehyde in PBS for 30 min at room temperature. Cells were washed twice with PBS, and permeabilized with 0.02% Triton X-100/PBS for 30 min at 4℃ followed by two washes with PBS. Nonspecific sites were blocked with 5% skim milk/PBS for 1 h. The wells were then incubated with hMPV matrix protein MAb diluted 1:320 (v/v) in 0.5%Tween20-PBS followed by 1:10,000 (v/v) horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (Chemicon). Each incubation was for 60 min at 37℃ and the wells were washed four times with PBS after each step. O-phenylenediamine substrate (Sigma, St Louis, MO) was added and after 30 min, 1 N H2SO4 was added and the absorbance at 490 nm was determined. Wells were inoculated in triplicate and each experiment was performed at least two times. There was a linear relationship between virus input and optical density over a greater than 100-fold range of virus inoculums.
Infectivity inhibition assays
The effect of DAS181 on hMPV infectivity was determined by a modification of the hMPV infectivity ELISA. HEp-2 cells were pretreated with various concentrations of DAS181 diluted in EBD-BSA buffer (10 mM sodium acetate pH6.0, 0.1 M NaCl, 10 mM CaCl2, 0.5 mM MgCl2, 0.5% w/v BSA) for 2 h at 37℃. After 2 h incubation cells were washed twice with 199 medium before inoculation with hMPV and assessment of infectivity as above. Results are expressed relative to virus infectivity of untreated HEp-2 cells. For titration experiments, Hep2 cells in 96 well tissue culture plates were treated with different concentration of DAS181 for 2 h at 37℃. The DAS181 containing media was removed and the plate was washed once with 199 medium, inoculated with hMPV 1.5 × 105 IFU/ml, and incubated plate in 5% CO2 incubator at 37℃ for 48 h. hMPV infection of Hep2 cells was investigated using ELISA assay.
G-protein-binding ELISA
Recombinant G protein was expressed in Pichia pastoris and purified by nickel affinity chromatography as previously described.3 The binding of G protein to cells after incubation with DAS181 was evaluated by ELISA. Briefly, triplicate HEp-2 monolayers in 96 well plates were treated with 5 or 0.5 µg/ml of DAS181 in EDB-BSA buffer for 2 h at 37℃, the solutions from each well were removed and the cells were washed twice with PBS. Both the sialidase treated and untreated cells were incubated with 100 µg/ml of biotinylated G protein at 37℃. After 1 h incubation, unbound protein was removed by being washed with 50 mM phosphate buffer, pH 7.4 (PB). Cells were then incubated with 1:1000 (v/v) HRP-conjugated streptavidin (Sigma) in 1% skim milk in PB at 37℃ for 1 h. OPD substrate was added and OD490 nm was determined. The OD of wells without G protein was subtracted as background. Results are expressed as percentage binding relative to G protein binding of untreated cells.
Results
Antiviral activity of DAS181 in vitro
The effect of DAS181 on hMPV infectivity was examined using a cell infectivity ELISA. To control for sialidase-specific activity, the mutated sialidase expressing molecule DAS185 was used at identical concentrations. HEp-2 cells were treated with 10 µg/ml of DAS181 or DAS185 for 2 h at 37℃ before inoculation with hMPV strains A2, B1, or B2. Viral infection was assessed 48 h postinoculation. As shown in Figure 1(a), for all strains of hMPV tested, infection was greatly inhibited by pretreatment with DAS181 (Figure 1(a)) whereas the sialidase defective DAS185 showed little to no activity under similar conditions. It should be noted that DAS181 had no effect on HEp-2 cells growth or viability at a concentration of 50 µg/ml, indicating that the decreased infection was not due to cell cytotoxicity (data not shown).
Figure 1.
Effect of DAS181 treatment of HEp-2 cells on hMPV (strains A2, B1, and B2) infectivity. (a) Hep-2 cells were treated with 10 µg/ml DAS181 or DAS185 for 2 h prior to inoculation with hMPV. Untreated control cells were treated with EDB–BSA buffer before inoculation with virus. Infectivity was assessed by ELISA 48 h postinoculation using MAb against hMPV matrix protein. Results shown are mean ± SD for triplicate wells of a representative experiment performed twice. OD 490 nm, optical density at 490 nm. (b) Dose-dependent inhibition of hMPV strain V32748 (B1) infectivity by DAS181. Experiments were performed as described above except Hep-2 cells were treated with varying concentrations of DAS181. Results are expressed as percent inhibition of infection relative to that of untreated cells.
We extended this finding by treating HEp-2 cells with fivefold serial dilutions of DAS181 prior to infection of cells with B1 (V32748) virus. Figure 1(b) shows that DAS181 treatment decreases hMPV infectivity in a dose-dependent manner, with concentrations as low as 0.5 ng/ml exhibiting more that 50% inhibition of infection.
Effect of DAS181 on hMPV-G protein binding to cells
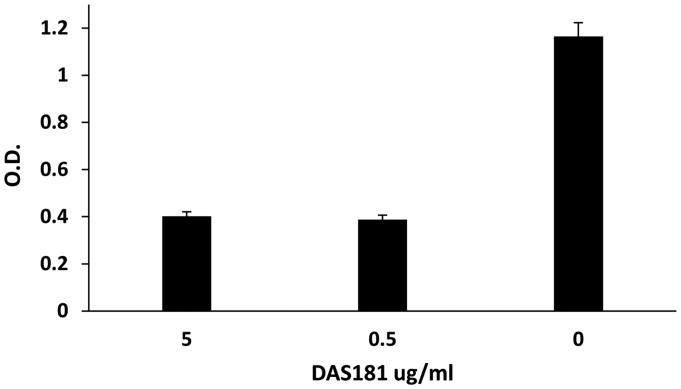
hMPV-G protein is thought to play an important role in viral attachment to host cells. We next examined if exposure to DAS181 reduced G protein binding to HEp-2 cells. HEp-2 cells were pretreated with DAS181 for 2 h prior to incubation of cells with 100 µg/ml biotinylated hMPV-G protein and binding was detected with streptavidin–HRP. Pretreatment of HEp-2 cells with DAS181 markedly inhibited viral G protein binding to cells, with concentrations of either 5 or 0.5 µg/ml inhibiting binding by approximately 75% (Figure 2).
Figure 2.
Effect of DAS181 treatment of HEp-2 cells on hMPV-G protein binding. HEp-2 cells were pretreated with DAS181 at 37℃ for 2 h. Cells were washed two times with PBS before incubation with biotinylated hMPV-G protein. After washing, bound G protein was detected by incubation with streptavidin–HRP. Results shown are mean ± SD for triplicate wells of a representative experiment performed twice. OD 490 nm, optical density at 490 nm.
Discussion
DAS181 is a recombinant fusion protein composed of a sialidase catalytic domain linked to a cationic sequence tag of 21 amino acids in length derived from the C-terminus of human amphiregulin (heparin-binding EGF). The pharmacological effect of DAS181 is the removal of sialic acid residues (SARs) from the cell surface that function as receptors for multiple respiratory viruses dependent on SAR expression. DAS181 has demonstrated inhibitory activity against over 70 influenza and 15 parainfluenza laboratory strains and clinical isolates, including influenza A (seasonal H1N1, 2009 pandemic H1N1, H2N2, H3N2, H9N2, H7N7, and H7N9 subtypes), multiple influenza B strains, multiple highly virulent influenza A H5N1 avian strains, and all subtypes of human parainfluenza (PIV-1, -2, -3, and -4).6–8 Clinical trials have also shown that DAS181 can decrease viral load in influenza-infected patients.9
hMPV along with respiratory syncytial virus (RSV) comprise the pneumovirinae subfamily of the Paramyxoviridae family. Infection is initiated by two viral surface glycoproteins, one for attachment (G) and the other for fusion (F).10 The cellular target of the G protein is glycosaminoglycans11 while the fusion protein appears to interact directly with the host cell membrane to facilitate viral entry. The results shown here support the hypothesis that DAS181 interrupts hMPV infectivity in vitro at clinically relevant concentrations. The exact nature of this effect remains to be determined. The observation that infectivity was blocked with DAS181 by not with the sialidase deficient DAS185 suggests that sialic acid-containing GAGs are required for infection. The titration of DAS181 in the infectivity assay demonstrates that extremely low concentrations are capable of inhibiting infection, with an estimated IC50 of 16 pM/ml. These results would support an enzyme-driven mechanism of action and suggest that a sialic acid-expressing molecule may be required as a coreceptor for productive hMPV infection of Hep-2 cells. In support of this, a recent report has shown that another GAG-binding virus of the Polyomavirus family does require an as yet unidentified sialylated coreceptor for productive infection.12 Subsequent work by Neu et al.13 has demonstrated the presence of a sialic acid-binding site on VP1 that is distinct from the GAG site. These authors suggest that a two-step process of attachment via GAG and entry via a sialic acid-expressing coreceptor may extend to other virus families that utilize GAG as a primary attachment site. Attachment of the related RSV to Hep-2 cells has been reported to be insensitive to the effects of tunicamycin and neuraminidase.14 However, Neu et al. have also suggested that simple treatment of neuraminidase may not sufficiently reveal this requirement for sialic acid expression, since virions may remain attached to target cells in culture until sialic expression regenerates. The results presented here open the possibility that hMPV may exploit a similar two-step strategy for viral entry.
Conclusions
In summary we have shown that the DAS181 (but not the sialidase deficient DAS185) blocks hMPV infection and binding of hMPV G protein in vitro. These findings identify a previously unknown sensitivity to sialidase treatment and suggest that DAS181 may be therapeutically active for the treatment of hMPV. As there are no current treatments for hMPV, testing in existing cotton rats or hamster models15 and subsequent human clinical evaluation of DAS181 for the treatment of hMPV should be a priority.
Acknowledgments
DAS181 and DAS185 were kindly provided by Ansun BioPharma.
Authors’ contributions
ST performed infectivity, analyzed data, and helped prepare a draft manuscript. TAS assisted with experimental design and data analysis and helped draft the manuscript. PA supervised recombinant protein expression and purification and helped draft the manuscript. DLG conceived experimental design, coordinated experimental studies, assisted with data analysis, and prepared final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by a Flinders University Faculty of Health Sciences Research Grant.
References
- 1.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J 2004; 23: S25–S32. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev 2006; 19: 546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thammawat S, Sadlon TA, Hallsworth PG, et al. Role of cellular glycosaminoglycans and charged regions of viral G protein in human metapneumovirus infection. J Virol 2008; 82: 11767–11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olofsson S, Bergström T. Glycoconjugate glycans as viral receptors. Ann Med 2005; 37: 154–172. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Y, Nagao Y, Kato H, et al. The hemagglutinins of the human influenza viruses A and B recognize different receptor microdomains. Biochim Biophys Acta 1987; 903: 417–424. [DOI] [PubMed] [Google Scholar]
- 6.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother 2006; 50: 1470–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan RW, Chan MC, Wong AC, et al. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother 2009; 53: 3935–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belser JA, Lu X, Szretter KJ, et al. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J Infect Dis 2007; 196: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 9.Moss RB, Hansen C, Sanders RL, et al. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis 2012; 206: 1844–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox RG, Williams JV. Breaking in: human metapneumovirus fusion and entry. Viruses 2013; 5: 192–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman SA, Hendry RM, Beeler JA. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol 1999; 73: 6610–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schowalter RM, Pastrana DV, Buck CB. Glycosaminoglycans and sialylated glycans sequentially facilitate Merkel cell polyomavirus infectious entry. PLoS Pathog 2011; 7e: 1002161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neu U, Hengel H, Blaum BS, et al. Structures of Merkel cell polyomavirus VP1 complexes define a sialic acid binding site required for infection. PLoS Pathog 2012; 8: e1002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez I, Melero JA. Binding of human respiratory syncytial virus to cells: implication of sulfated cell surface proteoglycans. J Gen Virol 2000; 81: 2715–2722. [DOI] [PubMed] [Google Scholar]
- 15.Wen SC, Williams JV. New approaches for immunization and therapy against human metapneumovirus. Clin Vaccine Immunol 2015; 22: 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]