Abstract
Background
Chemotherapy is an important tool for controlling enterovirus infections, but clinically effective anti-enterovirus drugs do not currently exist, mainly due to the development of drug resistance. We investigated the combination effects of enterovirus replication inhibitors in order to limit this process. In previous studies, we showed the efficacy of consecutive alternating administration of the triple combinations disoxaril/guanidine/oxoglaucine and pleconaril/guanidine/oxoglaucine against coxsackievirus B1 infection in newborn mice. Drug sensitivity tests of the viral brain isolates showed that these drug combinations prevented the development of drug resistance.
Methods
In the current study, we replaced guanidine-HCl with enteroviral RNA synthesis inhibitor MDL-860 to test the effect of a new triple combination—pleconaril/MDL-860/oxoglaucine—applied via consecutive alternating administration in newborn mice infected subcutaneously with 20 MLD50 of coxsackievirus B1.
Results
The pleconaril/MDL-860/oxoglaucine combination via consecutive alternating administration showed high activity at the 75 mg/kg MDL-860 dose: a protective effect of 50% and a pronounced suppression of brain virus titers. Moreover, along with prevention of drug resistance, a phenomenon of increased drug sensitivity was established. MDL-860 sensitivity in pleconaril/MDL-860/oxoglaucine increased 8.2 times vs. placebo (29 times vs. monotherapy) on day 7 and oxoglaucine sensitivity—4.9 times vs. placebo (by 6.8 times vs. monotherapy) on day 13. As concerns pleconaril, a demonstrable prevention of drug resistance was registered without increase of drug sensitivity. Daily, simultaneous administration of pleconaril/MDL-860/oxoglaucine showed no protective effects and led to a rapid development of drug resistance.
Conclusions
These results add new support for using consecutive alternating administration treatment courses to achieve clinically effective chemotherapy of enterovirus infections.
Keywords: Animal model, compounds, drug combination, drug resistance, picornaviridae
In recent decades, the interest on the role of enteroviruses (EVs) in human infectious pathology has increased.1–3 This is in part due to the large number of investigations carried out on a series of EV-induced infections manifested for the first time by epidemic spread in several regions of the globe, for example, the enterovirus 71 (EV 71) epidemic in Southeast Asia4,5 and EV D68 in the USA.6 Moreover, EVs are causative agents of an unusual phenomenon for the infectious pathology: one virus, in one region, during one period of time (the summer season), to cause more than 10 different clinical pictures affecting different human tissues and organs. Besides, a significant obstacle to introducing traditional epidemiologic measures was manifested—more than 80% of infected individuals were asymptomatic (lack of a clinical picture).1,7,8 This uncommon occurrence was explained by the existence of EV progeny consisting of billions of quasispecies,9,10 due to the proven mutation rate of 10−3 in EV replication,11,12 a value without analogy in the biological world.
Such quasispecies are at the basis of the rapid development of drug resistance to each established EV replication inhibitor. There is no exclusion of resistance appearance in all studies carried out with application of monotherapeutic courses.13 Despite the considerable efforts of research studies and organized clinical trials, chemotherapy for EV infections is still missing from clinical practice. But there are indisputable indications for its application: (i) the severity of a series of EV-caused illnesses, (ii) the large number of EV species and serotypes, (iii) the social importance of certain EV infections, which is connected with their widespread occurrence, and (iv) the absence of vaccinal prophylaxis, excluding the anti-poliomyelitis vaccines. Recently, progress has been made in the development of a vaccine against EV 71.14
Another special indication is the development of efficacious anti-polio drugs.15 These will be of great benefit for post-exposure prophylaxis and outbreak control.16,17
One proposed approach for preventing the development of anti-EV drug resistance is the introduction of combination chemotherapy. An investigation of multiple anti-EV inhibitory substances used in double combinations against a broad spectrum of EVs identified a considerable number of such combinations that had synergistic combined effects.13 In specific experiments, as in the synergistic combination disoxaril + enviroxime against poliovirus 1, a double drug resistance was proven.18 Herrmann and Herrmann19 postulated that the development of resistance is an obligatory indicator for considering a substance that inhibits viral replication to be a specific virus inhibitor.
In a study of newborn mice with coxsackievirus B1 (CVB1) neuroinfection treated with the VP1 ligand disoxaril (a WIN compound), drug resistance developed four to six days after virus inoculation. The disoxaril-resistant mutant was characterized by a panel of phenotypic markers (50% inhibitory concentration—IC50, plaque shape, and size, susceptibility at 50℃, and an increased pathogenicity for mice). The molecular genetic basis of the drug resistance consisted of specific replacements in amino acid consequences coded in the VP1 locus.20
Based on the results summarized so far, we decided to examine the in vivo combination effects of EV replication inhibitors with different mechanisms of action. We investigated the activity of double, triple, and quadruple combinations, either administered consecutively and alternatingly (i.e. not simultaneously) (consecutive alternating administration, CAA) or applied simultaneously and daily, in newborn mice infected with CVB1 20 MLD50. For the CAA course, we also tested the influence of the substance application order. In addition to placebo groups, monotherapeutic courses of the compounds comprising the triple combinations were used as controls. The best antiviral effect was produced by the triple combination via CAA with compounds applied in a specific sequence—an inhibitor targeting the capsid protein VP1 had to be administered first.21 Initially, the effect of the CAA course with the triple combination disoxaril + guanidine-HCl + oxoglaucine (DGO) was tested on mice inoculated with CVB1. This combination (DGO via CAA) reached a protection effect of approximately 50%. It had the same efficacy against infections with neurotropic (Nancy) and cardiotropic (Woodruff) strains of CVB3.22 We subsequently replaced the disoxaril with pleconaril (i.e. PGO), a VP1 blocker possessing its own in vivo activity, though it also has some toxicity. The PGO combination via CAA also manifested a pronounced protective effect against experimental neuroinfection with CVB1 20 MLD50.23
The in vivo antiviral effect of oxoglaucine (the third component in PGO) is distinguished by modest values (S. Spasov and A. S. Galabov, unpublished data); however, the second component—guanidine-HCl—does not generally have an individual in vivo effect.24 This fact compelled us to replace later guanidine-HCl with another inhibitor of viral RNA synthesis, the compound 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile (MDL-860).25 This Merrill-Dow Pharmaceuticals product (synthesized initially by L. Markley) is notable for its wide anti-EV scope and for its in vivo effects on cardiotropic CVB3 infection in adult mice.26
In the present work, we investigated the effect of a modified triple combination, pleconaril + MDL-860 + oxoglaucine (PMO) on experimental CVB1 neuroinfection in newborn mice.
Methods
Cells
Monolayer cultures of human epithelial type 2 (HEp-2) cells (National Bank for Industrial Microorganisms and Cell Cultures, Sofia, Bulgaria), 3.5–4.0 × 105 cells/ml, in six-well plates (CELLSTAR, Greiner Bio-One GmbH, Frickenhausen, Germany), were used for both plaque purification of viral isolates from mouse brains and determination of 50% inhibitory concentrations (IC50). Cells were grown in Dulbecco’s minimal essential medium (DMEM; Gibco BRL, Paisley, Scotland), containing 10% fetal bovine serum (FBS; Gibco BRL) and supplemented with 10 mM HEPES buffer (Merck, Germany) and antibiotics (penicillin: 100 IU/ml; streptomycin: 100 µg/ml) in a HERAcell 150 incubator (Heraeus, Germany) at 37℃, 5% CO2, and high humidity. Maintenance medium was DMEM with 0.5% heated FBS.
Virus
Coxsackievirus B1 (Connecticut-5 strain) for in vivo experiments was obtained through intracerebral passages (0.02 ml/mouse) in newborn albino mice and prepared as a 10% brain suspension in phosphate-buffered saline (PBS). The virus was passaged at least three times intracerebrally in newborn mice (without intermediary passages in cell cultures).
Mice
ICR random-bred newborn albino mice (obtained from the Experimental and Breeding Base for Laboratory Animals of the Bulgarian Academy of Sciences, Slivnitza, Bulgaria) were used. Each dam was housed in specially designed, well-ventilated acrylic cage containers, with free access to water and food, and maintained in the Animal House Facility of the Stephan Angeloff Institute of Microbiology, BAS. Animal breeding and experiments were conducted in accordance with the guidelines of Bulgaria’s Directorate of Health Prevention and Humane Behaviour toward Animals.
Compounds
Pleconaril, 3-{3,5-dimethyl-4-[3-(3-methyl-1,2-isoxazol-5-yl)propoxy]phenyl}-5-(trifluoromethyl)-1,2,4-oxadiazole (VP 63843, WIN 63843, Picovir®), was synthesized by Dr. Vadim Makarov (State Research Center for Antibiotics, Moscow, Russia). It was dissolved in polyethylene glycol 400 (PEG400).
MDL-860 was obtained from Prof. Gerhard Pürstinger (Institute of Pharmacy, University of Innsbruck, Innsbruck, Austria), and an additional amount was synthesized by Dr. Vladimir Dimitrov’s team (Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria). The compound was dissolved in PEG400.
Oxoglaucine, {1,2,9,10-tetramethoxy-7 h-dibenzo[de, g]quinolin-7-one}, an aporphinoid alkaloid from Glaucium flavum Cranz (yellow horn poppy), was obtained by Dr. Stefan Philipov from the Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences. The compound was dissolved in 1:9 v/v dimethyl sulfoxide (DMSO)/saline.
Coxsackievirus B1 infection in newborn mice: Testing the anti-enteroviral triple combination
The number of animals per experimental group was approximately 40. Prior to treatment, newborn mice received subcutaneous inoculations of CVB1 at 20 MLD50 (mouse lethal dose). CAA treatment groups received PMO compounds, administered consecutively, starting 1 h post-inoculation (day 1) and continuing through day 12. Pleconaril was administered orally, while MDL-860 and oxoglaucine were injected subcutaneously. As Table 1 shows, each compound was administered every third day, one compound per day, beginning with pleconaril, followed by MDL-860, and ending with oxoglaucine. MDL-860 was applied at different doses: 25, 50, 75, and 100 mg/kg. Each group received pleconaril at a dose of 25 mg/kg and oxoglaucine at the same dose (25 mg/kg). These doses were selected as optimal based on previous experiments in our laboratory23 [A. S. Galabov and S. Spasov, unpublished data].
Table 1.
Daily administration scheme for compounds applied in combination or alone.
| Compounds in combination or as monotherapies | Days |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compounds used in combination per day | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Consecutive combination P/M/O | Ple | Mdl | Oxo | Ple | Mdl | Oxo | Ple | Mdl | Oxo | Ple | Mdl | Oxo |
| Simultaneous triple combination | Combination of Ple, Mdl, and Oxo is simultaneously applied every day | |||||||||||
| Monotherapies of the partner substances | Each drug applied every day | |||||||||||
| Placebo | Saline solution every day | |||||||||||
Note: Compounds were applied once per day beginning 1 h post-infection (day 1).
Ple (P): pleconaril – per os; Mdl (M): MDL-860 – sc; Oxo (O): oxoglaucine – sc.
In addition to the placebo group (received 1/1 v:v PEG400/saline every day), one group received simultaneously administered PMO every day, and control groups received monotherapies of pleconaril, MDL-860, or oxoglaucine, administered daily at the doses described above. Cumulative mortality (percentage) and mean survival time (days) were recorded.
The weight (in grams) of suckling mice was also registered in dynamics as an indicator of the compounds’ toxicity in the infected body.
Preparation of virus isolates
Virus samples (three to four brain samples from each group) were collected daily beginning on day 4 and continuing through one day after treatment ended (day 13). For each group, samples were combined, and brain isolates were prepared as 10% brain suspensions in PBS.
Virus assay and plaque purification of virus isolates
Viral content of brain isolates was determined by the plaque method.27 Monolayer HEp-2 cell cultures were inoculated with 10-fold dilutions of each virus stock and left for 1 h at 37℃ for virus adsorption. The agar overlay (1.75 ml per dish) was 1% purified agar (Oxoid Ltd, Basingstoke, Haunts, UK) in DMEM, supplemented with 10% heated calf serum, 1.65 g/ml sodium bicarbonate, and antibiotics (penicillin: 100 IU/ml; streptomycin: 100 g/ml). After 48 h incubation at 37℃, a neutral red-containing agar overlay (1.5% agar with 0.02% neutral red (Fluka, Buchs, Switzerland) in physiological saline) was added. Plates were then kept at room temperature, and the number of plaque forming units (PFU) were recorded after 24 h. A single plaque from each viral stock, isolated from the appropriate dilution, was resuspended in 0.5% heated calf serum in DMEM.
Virus sensitivity to pleconaril, MDL-860, and oxoglaucine
Herrmann’s28 and Siminoff’s29 plaque-inhibition test was used on previously plaque-purified virus progenies. Monolayer HEp-2 cell cultures were inoculated with 50–60 PFU/ml of virus per well, left for 1 h at 37℃ for virus adsorption, and covered with agar overlays (described in the Virus assay and plaque purification of virus isolates section). The overlays also contained test compounds in the following concentrations: pleconaril = 0.001, 0.0032, 0.01, 0.032, 0.1, and 0.32 µM; MDL-860 and oxoglaucine = 0.1, 0.32, 1, 3.2, and 10 µM. Following incubation (48 h, 37℃), neutral red-containing agar overlays were added (described in the Virus assay and plaque purification of virus isolates section).The percentage of PFU inhibition was compared to that in the control plates (no test compound in the agar overlay). For each virus sample, the inhibitory concentration of compound required to inhibit the plaque titer by 50% (IC50) was determined. Each sensitivity test was performed three times.
Statistical analysis
Mortality was followed until day 12, and survival time was calculated as the period from day 1 (inoculation day) until one day before death. The protection index (PI) was calculated by the equation PI = ((PC − 1)/PC)× 100, where PC is the protection coefficient (% mortality in the placebo group/% mortality in the drug-treated group). Two-tailed Fisher’s exact test was used to compare survival rates between groups. One-way analysis of variance (ANOVA) with Bonferroni’s post-test was used to determine between-group differences in infectious virus titer values of the brain samples, mean survival times (MST), and IC50 values. A value of P < 0.05 was considered statistically significant for all analyses. Two-tailed unpaired t test was applied in parallel with the one-way ANOVA analysis to illustrate the susceptibility difference of isolates in pleconaril-treated groups.
Results
Effect of consecutively applied PMO combination on experimental CVB1 infection in newborn mice
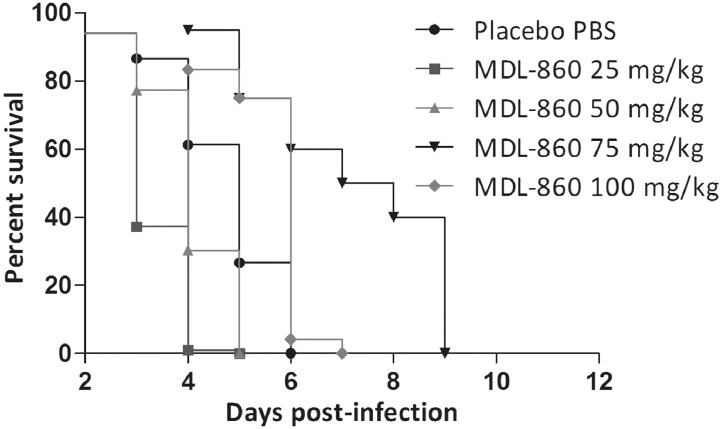
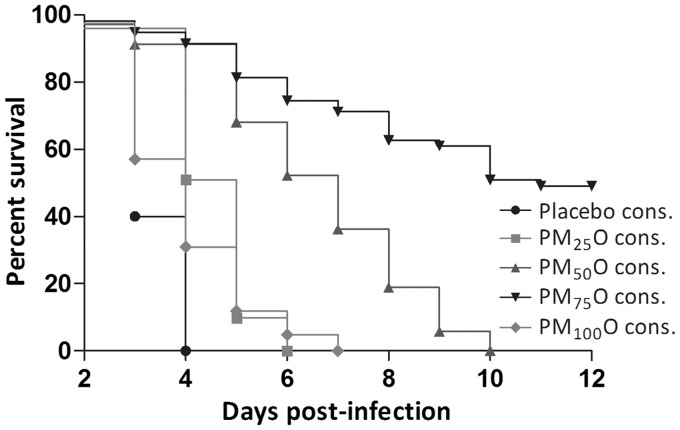
When applied as monotherapy, MDL-860 did not show a protection effect. Daily monotherapeutic MDL-860 administered in a dose–response design to newborn mice inoculated with CVB1 (20 MLD50) increased MST by 2.1 days at 75 mg/kg, and by 1.5 days at 100 mg/kg, as compared to placebo (Table 2, Figure 1). However, in the PMO via CAA scheme, the 75 mg/kg dose of MDL-860 showed high antiviral activity—a protective effect of approximately 50%, and MST increased by 6.3 days compared to placebo (Table 2, Figures 2 and 3). At 50 mg/kg MDL-860 in the triple combination, only a marked lengthening of MST by 4.6 days was found (Table 2, Figure 2).
Table 2.
Consecutive alternating administration treatment course of PMO against CVB1 neuroinfection in newborn mice.
| Experimental groups | Survivals/totala | Mortality, % | MST ± SD, daysb | PI% |
|---|---|---|---|---|
| P25M25O25 consecutively | 0/51 | 100.0 | 4.5 ± 0.8 | 0 |
| P25M50O25 consecutively | 11/67*,## | 83.6 | 8.5 ± 1.3***,### | 16.4 |
| P25M75O25 consecutively | 29/59***,^^^ | 50.8 | >10.2 ± 0.6***,^ | 49.2 |
| P25M100O25 consecutively | 0/42 | 100.0 | 4.7 ± 1.8 | 0 |
| P25M25O25 simultaneously | 0/24 | 100.0 | 3.8 ± 1.2 | 0 |
| P25M75O25 simultaneously | 0/26 | 100.0 | 4.2 ± 0.9 | 0 |
| MDL-860 25 mg/kg | 0/102 | 100.0 | 3.5 ± 0.7 | 0 |
| MDL-860 50 mg/kg | 0/66 | 100.0 | 4.1 ± 0.8 | 0 |
| MDL-860 75 mg/kg | 0/40 | 100.0 | 6.8 ± 1.3** | 0 |
| MDL-860 100 mg/kg | 0/24 | 100.0 | 6.2 ± 0.7 | 0 |
| Pleconaril 25 mg/kg | 14/45** | 68.9 | >7.9 ± 1.6*** | 31.1 |
| Pleconaril 25 mg/kg 2 days apart | 0/23 | 100.0 | 4.4 ± 0.2 | 0 |
| Oxoglaucine 25 mg/kg | 0/59 | 100.0 | 5.2 ± 0.9 | 0 |
| Placebo PBS | 0/65 | 100.0 | 4.7 ± 0.9 | 0 |
| Placebo consecutively | 0/35 | 100.0 | 3.9 ± 0.7 | 0 |
Note: Data are from at least two separate experiments. Placebo PBS refers to daily sc administration of PBS. Placebo consecutively refers to oral administration of polyethylene glycol 400 (PEG400) on day 1, followed by sc administration of PBS on days 2 and 3.
MST: mean survival time; PBS: phosphate-buffered saline; PI: protection index; PMO: pleconarilmg/kg/MDL-860 mg/kg/oxoglaucinemg/kg.
Two-tailed Fisher’s exact test.
One-way ANOVA (Bonferroni’s multiple comparison post-test).
P < 0.0001 vs. Placebo consecutively.
P < 0.001 vs. Placebo consecutively.
P< 0.05 vs. Placebo consecutively.
^^^ P < 0.0001 vs. MDL-860 75 mg/kg.
^ P < 0.01 vs. MDL-860 75 mg/kg.
P < 0.0001 vs. MDL-860 50 mg/kg.
P < 0.001 vs. MDL-860 50 mg/kg.
Figure 1.
Animal survival with daily administration of MDL-860 monotherapies at different daily doses, compared to placebo group.
Figure 2.
Animal survival with consecutive administration of combination PMO with MDL-860 at different doses, compared to placebo group.
Figure 3.
Effect of consecutive administration of PMO combination compared to simultaneous PMO administration and individual therapies of pleconaril, MDL-860, and oxoglaucine. Statistical analysis for survival rate for PMO via CAA vs. pleconaril monotherapy was performed with Log-rank (Mantel-Cox) Test (*P = 0.0111) and Gehan-Breslow-Wilcoxon Test (##P = 0.0026).
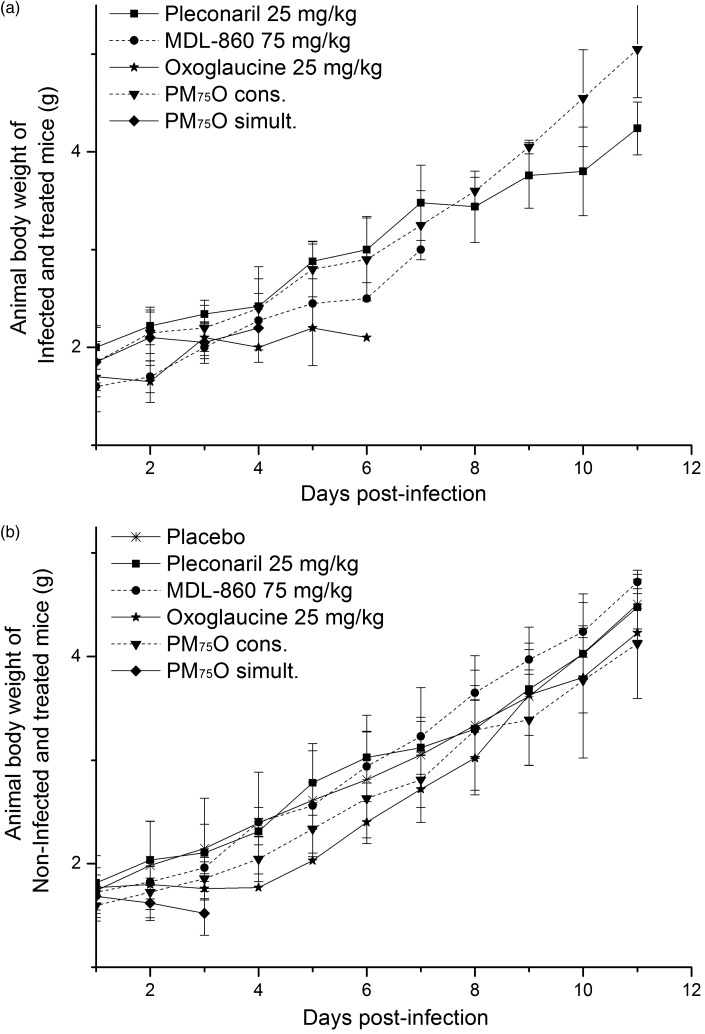
No combination toxicity was observed when the growth of body weights of the infected suckling mice treated with P25M75O25 via CAA course were traced as compared with the non-infected and treated animals (Figure 4). At the end of the observed period of time (day 11 post-infection), the mean body weight of 5.1 ± 0.4 g of infected animals treated with P25M75O25 via CAA course was registered (Figure 4(a)), while at non-infected and treated mice, it was 4.2 ± 0.5 g (Figure 4(b)). The simultaneous every day application of P25M75O25 lead to an earlier mortality in the group of non-infected animals due most probably to the combined toxicity (Figure 4(b)), while in the infected bodies, the virus infection effect is supplemented (Figure 4(a)).
Figure 4.
Individual body weight of newborn mice subjected to the treatment courses (mean values of two to three experiments): (a) infected and (b) non-infected.
The monotherapeutic course with pleconaril (25 mg/kg) manifested its23 antiviral activity (a protection effect of 31.1% and MST increased by four days), whereas oxoglaucine had no effect. The simultaneous PMO course, applied daily, was ineffective which could be related with a toxicity (Table 2, Figure 3).
Effect of PMO combination on infectious virus content in brains of treated mice
The antiviral activity of PMO via CAA against CVB1 infection was especially pronounced in comparisons of infectious virus content in the brains of the monotherapy-treated animals and the placebo group. For this analysis, the PMO with 75 mg/kg dose of MDL-860 was also the most effective combination (Figure 5(a) and (b)). After day 7 post-inoculation, the PMO via CAA group’s brain samples had a virus titer 4–5 logs lower than that of the 25 mg/kg pleconaril monotherapy group. Titers were approximately equal up through day 7.
Figure 5.
(a) Infectious virus titer of brain samples from placebo, pleconaril monotherapy (25 mg/kg), and PM75O via consecutive administration groups. Statistical analyses performed with one-way ANOVA and Bonferroni’s multiple comparison post-test ***P < 0.001. (b) Infectious virus titer of brain samples from placebo, MDL-860 monotherapy (75 mg/kg), oxoglaucine monotherapy (25 mg/kg), PM75O via consecutive administration, and PM75O via daily, simultaneous administration groups. Statistical analyses performed with one-way ANOVA and Bonferroni’s multiple comparisons post-test. **P < 0.01.
Virus brain isolates sensitivity to pleconaril following the PMO combination via CAA treatment course
Virus isolates from the brains of mice treated with the PMO combination (with 75 mg/kg MDL-860) via CAA scheme manifested a lack of drug resistance to pleconaril within days 4 to 10 followed by some increase of susceptibility to pleconaril manifested at days 11 to 13. The ratios between the IC50 values of the pleconaril group and the PMO via CAA group were as follows: day 4 = 2.9; day 5 = 2.1; day 6 = 3.8; day 7 = 6.9; day 8 = 6.4; day 9 = 6.8; day 10 = 11.1; day 11 = 16.0; day 12 = 42.0; day 13 = 49.4 (Table 3).
Table 3.
Sensitivity to pleconaril in plaque inhibition tests of virus brain isolates from newborn mice infected with CVB1.
| Group | Pleconaril IC50 values (μM) of viral brain samples | ||||
|---|---|---|---|---|---|
| Taken on day (after viral inoculation) | |||||
| 4 | 5 | 6 | 7 | 8 | |
| Placebo | 0.018 | 0.022 | a | a | a |
| Pleconaril 25 mg/kg | 0.046 | 0.037 | 0.062 | 0.164 | 0.114 |
| P25M75O25 consecutive | 0.016 ns,§ | 0.018 ns,ns | 0.016 ns,§§ | 0.024 ns,§§ | 0.018 ns,§ |
| P25M75O25 simultaneous | 0.014 | 0.042 | a | a | a |
| 9 |
10 |
11 |
12 |
13 |
|
| Placebo | a | a | a | a | a |
| Pleconaril 25 mg/kg | 0.119 | 0.135 | 0.139 | 0.387 | 0.543 |
| P25M75O25 consecutive | 0.017 ns,ns | 0.012 ns,§ | 0.009 ns,§ | 0.009###,§§§ | 0.011###,§§ |
| P25M75O25 simultaneous | a | a | a | a | a |
After day 5, no animals were alive, 100% mortality rate. Statistical analysis was performed with:
(i) one-way ANOVA (Bonferroni’s multiple comparison post-test)
P < 0.001 vs. pleconaril 25 mg/kg.
P > 0.05 vs. pleconaril 25 mg/kg.
(ii) two-tailed unpaired t test
P < 0.05 vs. pleconaril 25 mg/kg.
P < 0.01 vs. pleconaril 25 mg/kg.
P < 0.001 vs. pleconaril 25 mg/kg.
P > 0.05 vs. pleconaril 25 mg/kg.
When the pleconaril susceptibility in the brain samples from mice subjected to the monotherapeutic pleconaril course was related to the placebo group samples, a development of a pronounced resistance to pleconaril was registered since day 7 post-infection (7.4-fold increase of IC50), attaining maximum at day 13 (24.7-fold increase of IC50). The same analysis toward the triple combination via the CAA course demonstrated a lack of the pleconaril resistance till day 9 (ratio CAA to placebo around 1), and no statistically significant increase of drug sensitivity within days 11 to 13 (ratio below 0.5).
Virus brain isolates sensitivity to MDL-860 following the PMO combination via CAA treatment course
As seen in Table 4, a marked resistance to MDL-860 at a dose of 75 mg/kg was registered at monotherapeutic MDL-860 course. The influence of the PMO combination via CAA course was well demonstrable: IC50 value at MDL-860 monotherapy was 5.9 times higher on day 6 post-inoculation than that of the PMO via CAA course, and it was 28.9 times higher on day 7. The sensitivity to MDL-860 expressed as a ratio CAA to placebo was close to 1 till day 5. Then, the MDL-860 sensitivity (ratio placebo/CAA) was markedly increased: 2 times on day 6, 8.2 times on day 7, and 8.9 on day 13. The sensitivity of CVB1 isolated at day 13 during the PMO via CAA course was 6.8 times higher than the virus isolated at day 5 (P < 0.001).
Table 4.
Sensitivity to MDL-860 in plaque inhibition tests of virus brain isolates from newborn mice infected with CVB1.
| Group | MDL-860 IC50 values (μM) of viral brain samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taken on day (after viral inoculation) | ||||||||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Placebo | 3.372 | 2.843 | a | a | a | a | a | a | a | a |
| MDL-860 75 mg/kg | 2.057 | 2.582 | 8.206 | >10.0 | a | a | a | a | a | a |
| P25M75O25 consecutive | 2.165 | 2.388 | 1.418 | 0.346 | 0.319 | 0.173 | 0.153 | 0.313 | 0.316 | 0.318### |
| P25M75O25 simultaneous | 1.894 | 2.477 | a | a | a | a | a | a | a | a |
After day 5(7), no animals were alive, 100% mortality rate. Statistical analysis was performed with one-way ANOVA (Bonferroni’s multiple comparison post-test).
P < 0.001 vs. PMO consecutive day 5
Virus brain isolates sensitivity to oxoglaucine following the PMO combination via CAA treatment course
There was also increased susceptibility to the third component of the PMO combination—oxoglaucine, a substance which did not show a separate protective effect in vivo. Despite this, PMO via CAA (with 75 mg/kg MDL-860) showed a six-fold decrease in IC50 values to oxoglaucine from day 5 to day 13 (P < 0.001) (Table 5). The oxoglaucine sensitivity expressed as a ratio CAA to placebo was around 1 till day 7. Then, an increase of the oxoglaucine sensitivity (ratio CAA/placebo) was markedly increased, 4.9 times (6.8 times vs. monotherapy).
Table 5.
Sensitivity to oxoglaucine in plaque inhibition tests of virus brain isolates from newborn mice infected with CVB1.
| Group | Oxoglaucine IC50 values (μM) of viral brain samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taken on day (after viral inoculation) | ||||||||||
| 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Placebo | 0.411 | 0.743 | a | a | a | a | a | a | a | a |
| Oxoglaucine 25 mg/kg | 1.198 | 1.048 | a | a | a | a | a | a | a | a |
| P25M75O25 consecutive | 0.914 | 0.979 | 0.851 | 0.727 | 0.299 | 0.396 | 0.287 | 0.299 | 0.221 | 0.153### |
| P25M75O25 simultaneous | 1.196 | 1.817 | a | a | a | a | a | a | a | a |
After day 5, no animals were alive, 100% mortality rate. Statistical analysis was performed with one-way ANOVA (Bonferroni’s multiple comparison post-test).
P < 0.001 vs. PMO consecutive day 5.
Discussion
The replacement of guanidine-HCl with MDL-860 in the triple combination applied through the CAA treatment scheme was aimed at introducing an anti-enteroviral substance that had shown in vivo activity in a model of coxsackie A21 virus in mice, using subcutaneous application,30 and in 15 -g mice with myocarditis induced by a cardiotropic strain of coxsackievirus B3.26 An effective daily dose of 250 mg/kg was applied in both of these studies. In the present investigation of newborn mice infected with CVB1, an optimal effect, expressed by a roughly 2-day increase in MST, was attained at a daily dose of 75 mg/kg. At lower doses the compound was inactive, and at the higher dose of 100 mg/kg, its effect was diminished. The latter result could be due to toxicity, which was observed during the study on 15 g mice treated with 250 mg/kg doses of MDL-860.26
The PMO combination containing the efficacious 75 mg/kg dose of MDL-860 and applied following the CAA course demonstrated a remarkably high anti-enteroviral effect, which was expressed as a protective effect of ∼50% against massive infection (20 MLD50) with coxsackievirus B1, which provoked a lethal, fast-running encephalitis in newborn mice. Evidently, the VP1 blocker, pleconaril, played a particular role in the combination’s activity, as was established in a previous study.23 It must be stressed that the effect of P25 M75O25 in this study was higher than that of the combination P25G45O25, used against the same infection (CVB1 20 MLD50 in newborn mice) in a previous study.23 The P25 M75O25 combination also showed a markedly more pronounced suppression of virus titers in brain samples from treated mice. Besides, this effect occurred three days earlier—on day 8. Thus, the inclusion of MDL-860 appears to have improved the combination’s effect.
This study convincingly confirms another important property of consecutive alternating administration of EV replication inhibitors with different mechanisms of action: CAA not only restrains drug resistance but also increases drug sensitivity (susceptibility). Sensitivity to MDL-860 (IC50 values) increased during CAA course with PMO 8.2 times vs. placebo on day 7 and 28.9 times vs. MDL-860 monotherapy. The sensitivity to oxoglaucine at PMO CAA course was increased six times on day 13 as compared to day 4 (6.8 times vs. oxoglaucine monotherapy and 4.8 times vs. placebo). Evidently, this unusual phenomenon is a leading characteristic of the CAA treatment approach. It was observed in all studies of the in vivo anti-EV activity of triple combinations via this novel scheme of drug administration—DGO vs. CVB1,22 PGO vs. CVB1,23 and now, PMO vs. CVB1.
With regard to the pleconaril, in the present study, we found its sensitivity in the PMO combination via CAA course was completely preserved till the end of the period of observation (day 13). This is in contrast to the pronouncedly marked development of resistance during the pleconaril monotherapy course: 7 times lower IC50 values at day 7 at PMO combination via CAA course, 42 times on day 12, and by more than 49 times on day 13. Besides, no statistically significant increase of sensitivity to pleconaril was observed at days 12/13.
We consider the finding of influence on drug sensitivity as very important for promoting CAA as a treatment approach in anti-enteroviral chemotherapy.
In order to clear the mechanism of the suppression of development of drug resistance and the subsequent appearance of the drug-increased sensitivity phenomenon, we are in the course of conducting molecular genetic analyses of viral progenies within the entire period of the CAA course with PMO.
It is clear that this problem is closely related with the mode of inhibitory effect on EV replication of each substance included in the combination. It is known that the WIN compounds, disoxail/pleconaril, in the DGO and PGO combinations target the VP1 protein in the enteroviral capsid, removing the pocket factor (a lipid moiety molecule) in the VP1 hydrophobic pocket.31 The second component in the combinations DGO/PGO, guanidine-HCl, is a ligand of the 2 C protein, which suppresses daughter RNA (+) chain initiation during virus replication.32 In the present study, guanidine-HCl was replaced by MDL-860. Its mechanism of anti-EV action has not been clarified, but it is thought to interfere at an early stage, post-uncoating, in EV replication33,34; a function of the virus replicative complex, viral RNA polymerase, has been suggested.24 And finally, the third component—oxoglaucine, is an aporphinoid alkaloid isolated from the epigeous parts of the yellow horn poppy (Glaucium flavum Cranz).35,36 Oxoglaucine’s mechanism of action, established recently,37 has an enviroxime-like effect, i.e. its acts as an inhibitor of PI4KB and therefore inhibits the formation of the replication complexes of EVs.
Another open question is the interrelations between the compounds included in the triple combination via CAA treatment course. Especially interesting is the possible influence on the pharmacokinetics processes.
The double-blind placebo-controlled clinical trials of EV replication inhibitors selected through preclinical studies have so far failed. This is undoubtedly due mainly to the lack of selectivity, substantiated by well-expressed side effects in the human body. There are several examples of such trials: disoxaril (WIN51711) and WIN 54954;38 pleconaril (WIN63843)39,40; and BTA-798 (an oxime ether analogue of pirodavir).41 The results of these trials agree with the manifested lack of efficacy in in vivo models of a majority of substances that are active in vitro. More precisely, fewer than 20 of the hundreds of EV replication inhibitors have manifested some in vivo activity. These trial results show that, at present, clinically effective antivirals for treating enteroviral infections do not exist. Thus, the realization of anti-enteroviral chemotherapy is a problem for the future.
Continuing development of the treatment approach discussed here—triple anti-enteroviral combinations via consecutive alternating administration—may contribute an important perspective for establishing efficacious anti-enteroviral chemotherapy.
Acknowledgments
We thank Mme. Lucia Mukova, MS, for preparing cell cultures and Mme. Petya Stoyanova, DVM, Eleni Aksioti, and Ivanka Zahova for their technical assistance in the animal experiments.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Pallansch M, Roos R. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Knipe DM, Howley PM, Griffin DE, et al. (eds). Fields virology 2005; Vol. 1, 5th ed Philadelphia, PA: Lippincott Williams & Wilkins, pp. 839–894. [Google Scholar]
- 2.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, et al. Enterovirus surveillance – United States, 1970–2005. MMWR Surveill Summ 2006; 15: 1–20. [PubMed] [Google Scholar]
- 3.Non-Polio Enteroviruses. Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (NCIRD). Division of viral diseases, www.cdc.gov/non-polio-enterovirus/index.html (accessed 10 May 2013).
- 4.Shimizu H, Utama A, Yoshii K, et al. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1907–1998. Jpn J Infect Dis 1999; 52: 12–15. [PubMed] [Google Scholar]
- 5.Ho M. An epidemic of enterovirus 71 in Taiwan. N Engl J Med 1999; 341: 929–935. [DOI] [PubMed] [Google Scholar]
- 6.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 – Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep 2014; 63: 798–799. [PMC free article] [PubMed] [Google Scholar]
- 7.Morens DM, Pallansch MA. Epidemiology. In: Rotbart HA. (ed). Human enterovirus infections, Washington, DC: ASM Press, 1995, pp. 3–23. [Google Scholar]
- 8.Strauss J, Strauss E. Plus-Strand RNA viruses. In: ▪▪ ▪▪. (ed). Viruses and human disease, 2nd ed Burlington, MA, San Diego, CA, London, UK: Elsevier, 2008, pp. 63–136. [Google Scholar]
- 9.Duarte EA, Novella IS, Weaver SC, et al. RNA virus quasispecies: significance for viral diseases and epidemiology. Infect Agents Dis 1994; 3: 201–214. [PubMed] [Google Scholar]
- 10.Domingo E, Martin V, Perales C, et al. Coxsackie viruses and quasi species theory: evolution of enteroviruses. Curr Top Microbiol Immunol 2008; 323: 3–32. [DOI] [PubMed] [Google Scholar]
- 11.Smith DB, Inglis SC. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol 1987; 68: 2729–2740. [DOI] [PubMed] [Google Scholar]
- 12.Agol VI, Pilipenko EV, Slobodskaya OR. Modification of translation control elements as a new approach to design of attenuated picornavirus strains. J Biotechnol 1996; 44: 119–128. [DOI] [PubMed] [Google Scholar]
- 13.Galabov AS, Nikolaeva-Glomb L, Nikolova I, et al. Perspectives for effective chemotherapy of enterovirus infections. In: Najdenski H, Angelova M, Stoitsova S. (eds). New trends of microbiology (65th Anniversary of the Stephan Angeloff Institute of Microbiology), Sofia, Bulgaria: The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 2012, pp. 47–81. [Google Scholar]
- 14.Fengcai Z, Wenbo X, Jielai X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370: 818–828. [DOI] [PubMed] [Google Scholar]
- 15.Conclusions and recommendations of the Advisory Committee on Poliomyelitis Eradication, Geneva, Part II. Wkly Epidemiol Rec 2006; 81: 465–468. [PubMed]
- 16.Workshop Report. Committee on Development of a Polio Antiviral and Its Potential Role in Global Poliomyelitis Eradication, Washington DC: National Research Council of the National Academies, 2006. [Google Scholar]
- 17.Chumakov K, Ehrenfeld E, Wimmer E, et al. Vaccination against polio should not be stopped. Nat Rev Microbiol 2007; 5: 952–958. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaeva-Glomb L, Galabov AS. Synergistic drug combinations against the in vitro replication of coxsackievirus B1. Antiviral Res 2004; 62: 9–19. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann EC, Jr, Herrmann JA. A working hypothesis – virus resistance development as an indicator of specific antiviral activity. Ann NY Acad Sci 1977; 284: 632–637. [DOI] [PubMed] [Google Scholar]
- 20.Nikolova I, Petkova R, Galabov AS, et al. Disoxaril mutants of coxsackievirus B1: phenotypic characteristics and analysis of the target VP1 gene. Z Naturforschung 2011; 66c: 627–636. [DOI] [PubMed] [Google Scholar]
- 21.Vassileva-Pencheva R, Galabov AS. Avoiding drug-resistance development by novel approach of combining enteroviral substances against coxsackievirus B1 infection in mice. Antiviral Res 2010; 85: 366–372. [DOI] [PubMed] [Google Scholar]
- 22.Vassileva-Pencheva R and Galabov AS. Effectiveness of the consecutive alternative administration course of a triple antiviral combination in coxsakievirus B3 infections in mice. Drug Res 2016; 16: 1–5. [DOI] [PubMed]
- 23.Stoyanova A, Nikolova I, Galabov AS. Effect of consecutive alternating administration (CAA) of a triple anti-enteroviral combination on coxsackievirus B1 neuroinfection in mice. Antiviral Res 2015; 121: 138–144. [DOI] [PubMed] [Google Scholar]
- 24.Caliguiri LA, Tamm I. Guanidine and 2(α-hydroxibenzyl)-benzimidazole (HBB): selective inhibitors of picornavirus multiplication. In: Carter WA. (ed). Selective inhibitors of viral function, Boca Raton, FL: CRC Press, 1973, pp. 287–294. [Google Scholar]
- 25.Pürstinger G, De Palma AM, Zimmerhofer G, et al. Synthesis and anti-CVB 3 evaluation of substituted 5-nitro-2-phenoxybenzonitriles. Bioorg Med Chem Lett 2008; 18: 5123–5125. [DOI] [PubMed] [Google Scholar]
- 26.Padalko E, Verbeken E, De Clercq E, et al. Inhibition of coxsackie B3 virus induced myocarditis in mice by 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile. J Med Virol 2004; 72: 263–267. [DOI] [PubMed] [Google Scholar]
- 27.Dulbecco R. Production of plaques in monolayer tissue cultures by single particles of an animal virus. Proc Nat Acad Sci Wash 1952; 32: 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann EC. Plaque-inhibition tests for detection of specific inhibitors of DNA containing viruses. Proc Soc Exp Biol 1961; 107: 142–145. [PubMed] [Google Scholar]
- 29.Siminoff P. A plaque suppression method for the study of antiviral compounds. Appl Microbiol 1961; 9: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markley LD, Tong YC, Dulworth JK, et al. Antipicornavirus activity of substituted phenoxybenzenes and phenoxypyridines. J Med Chem 1986; 29: 427–433. [DOI] [PubMed] [Google Scholar]
- 31.McKinley MA, Pevear DC, Rossmann MG. Treatment of the picornavirus common cold by inhibitors of viral uncoating and attachment. Ann Rev Microbiol 1992; 46: 635–654. [DOI] [PubMed] [Google Scholar]
- 32.Barton DJ, Flanegan JB. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J Virol 1997; 71: 8482–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torney HL, Dulworth JK, Steward DL. Antiviral activity and mechanism of action of 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile (MDL-860). Antimicrob Agents Chemother 1982; 22: 635–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers RD, Gwaltney JM, Jr, Hayden FG. Activity of 2-(3,4-dichlorophenoxy)-5-nitrobenzonitrile (MDL-860) against picornaviruses in vitro. Antimicrob Agents Chemother 1982; 22: 639–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuzmanov BA, Philipov SA, Deligiozova-Gegova IB. Comparative photochemical and chemosystematic research of populations of Glaucinum flavum Cranz in Bulgaria. Fitologia 1998; 43: 52–57. [Google Scholar]
- 36.Nikolaeva-Glomb L, Philipov S, Galabov AS. A new highly potent antienteroviral compound. In: Georgiev VSt, Western KA, McGovan JJ. (eds). National Institute of Allergy and Infectious Diseases, NIH, Frontiers in research 2008; Vol. I, Totowa, NJ: Humana Press Inc., pp. 199–202. [Google Scholar]
- 37.Arita M, Philipov S, Galabov AS. Phosphatidylinositol 4-kinase III beta is the target of oxoglaucine and pachydipol (Ro 09-0179) for anti-picornavirus activity. Microbiol Immunol 2015; 59: 338–347. [DOI] [PubMed] [Google Scholar]
- 38.Diana GD, Rudewicz P, Pevear DC, et al. Picornavirus inhibitors: trifluromethyl substitution provides a global protective effect against hepatic metabolism. J Med Chem 1995; 38: 1355–1371. [DOI] [PubMed] [Google Scholar]
- 39.Senior K. FDA panel rejects common cold treatment. Lancet Infect Dis 2002; 2: 264. [DOI] [PubMed] [Google Scholar]
- 40.Clinical Trials.gov. U.S. National Institutes of Health. Effects of pleconaril nasal spray on common cold symptoms and asthma exacerbations following rhinovirus exposure (Study P04295), https://clinicaltrials.gov/ct/gui/show/NCT00394914 (accessed 23 June 2015).
- 41.Clinical Trials.gov. U.S. National Institutes of Health. A phase 2 multicenter, randomized, double-blind, placebo-controlled study of BTA798 in asthmatic adults with symptomatic human rhinovirus infection, https://clinicaltrials.gov/ct2/show/NCT01175226 (accessed 12 September 2013).