Short abstract
In this review, our recent advances in the development of nucleoside di- and nucleoside triphosphate prodrugs is summarized. Previously, we had developed a successful membrane-permeable pronucleotide system for the intracellular delivery of nucleoside monophosphates as well, the so-called cycloSal-approach. In contrast to that work in which the delivery is initiated by a chemically driven hydrolysis reaction, for the di- and triphosphate delivery, an enzymatic trigger mechanism involving (carboxy)esterases had to be used. The other features of the new pronucleotide approaches are: (i) lipophilic modification was restricted to the terminal phosphate group leaving charges at the internal phosphate moieties and (ii) appropriate lipophilicity is introduced by long aliphatic residues within the bipartite prodrug moiety. The conceptional design of the di- and triphosphate prodrug systems will be described and the chemical synthesis, the hydrolysis properties, a structure–activity relationship and antiviral activity data will be discussed as well. The advantage of these new approaches is that all phosphorylation steps from the nucleoside analogue into the bioactive nucleoside triphosphate form can be bypassed in the case of the triphosphate prodrugs. Moreover, enzymatic processes like the deamination of nucleosides or nucleoside monophosphates which lead to catabolic clearance of the potential antivirally active compound can be avoided by the delivery of the higher phosphorylated nucleotides.
Keywords: Biological activity, cell uptake, nucleoside analogues, prodrugs, triphosphate
Introduction
Since many decades, nucleoside analogues are used in anticancer and antiviral chemotherapy, and they represent the backbone to combat several viral infections nowadays.1–4 However, nucleoside analogue drugs have to be anabolized in cells by virus-encoded or, in most cases, by host cell kinases to undergo stepwise addition of phosphate groups to yield the active nucleoside triphosphate analogue.5,6 Owing to the substrate specificity of the kinases, the activation of nucleoside analogues often proceeds insufficiently. Furthermore, the clinical efficacy is hampered by limitations such as low biological half-lives due to catabolic transformation, variable bioavailability after oral administration, or the development of resistant virus strains.7,8 Within the stepwise kinase-catalyzed phosphorylation process, often the first phosphorylation step catalyzed by salvage pathway enzyme thymidine kinase (TK) has been identified as the limiting step, e.g. for the anti-HIV drug 3′-deoxy-2′,3′-didehydrothymidine (d4T, 1), the formation of its monophosphate metabolite 3.9 However, in the case of 3′-azido-3′-deoxythymidine (AZT, 2), not the formation of the monophosphate derivative 4 is critical but the formation of the AZT-diphosphate metabolite (AZTDP, 6) by host cell kinase thymidylate kinase (TMPK) is the bottleneck (Scheme 1);10,11 recently, we showed that d4U- or ddU-diphosphate were very poor substrates for cellular nucleoside diphosphate kinase (NDP-K), which proved that even the conversion of nucleoside analogue diphosphates as 5 or 6 to nucleoside analogue triphosphates such as 7 or 8 can be rate limiting as well.12 Moreover, for many of the synthesized nucleoside analogues reported in the literature, the detailed metabolism to yield the triphosphate has not been studied and is therefore unknown. Many of these compounds are often tested as the nucleoside analogue in in vitro bioactivity assays, and if they proved to be not active, they are discarded. The main reason for the lack of activity may be associated with the lack of efficient phosphorylation. To overcome the phosphorylation bottleneck, the design of prodrugs of the phosphorylated metabolites might be an option to restore antiviral or antitumor activity. This concept has been successfully introduced for monophosphorylated nucleoside analogue metabolites (Scheme 1).13–21 Two examples of such nucleoside monophosphate prodrug systems are the phosphoramidate strategy developed by Cahard et al.,16 which is nowadays also used successfully for the chemotherapy of HCV in the extraordinary drug sofosbuvir and the cycloSal-technology 9 introduced by us.19 The cleavage mechanism of the cycloSal-compounds was based on chemically induced steps (Scheme 2), while the phosphoramidates are cleaved by a complex series of enzymatic and chemical steps. Interestingly, before our own work, lipophilic delivery forms for the downstream phosphorylation metabolites following the same principle were reported in a few reports only.22,23
Scheme 1.
Metabolism of thymine-bearing pyrimidine nucleosides and the different pronucleotide approaches for the three phosphorylated metabolites of nucleoside analogues.
Figure 2.
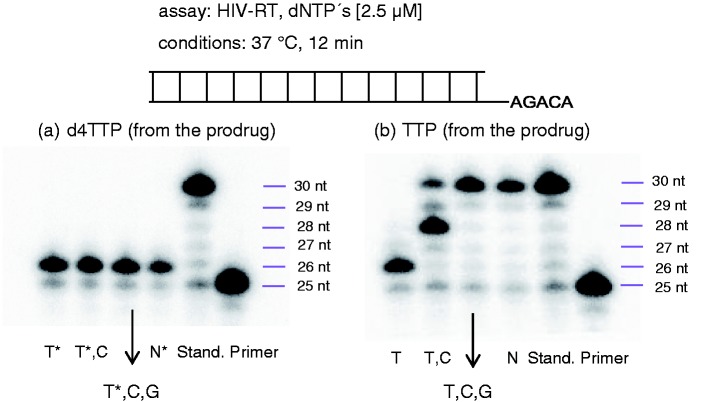
RT primer extension assay using the HIV polymerase RT: (a) Study with the hydrolysis product of TriPPPro-d4TTP; (b) study with the hydrolysis product of TriPPPro-TTP; lanes (1) only the hydrolysis product, (2) hydrolysis product + dCTP, (3) hydrolysis product + dCTP/dGTP, (4) hydrolysis product + dCTP/dGTP/dATP, (5) all four commercial dNTPs, and (6) primer.
To bypass the second phosphorylation step, we developed symmetric and non-symmetric nucleoside diphosphate prodrugs, the so-called DiPPro-approach 10 (Scheme 1).12,24–27 These prodrugs showed not only very good antiviral activity in HIV-infected TK-deficient human T-lymphocyte CEM/TK− cell cultures but also formed the nucleoside diphosphate in, e.g. cell extracts in high amounts. In order to improve the selectivity of these diphosphate delivery systems, we reported on non-symmetric DiPPro-compounds. Using these derivatives, a highly selective delivery was achieved.26 Recently, we disclosed the first delivery of nucleoside triphosphates through a prodrug technology (TriPPPro-approach 11; Scheme 1).28,29
It was demonstrated with d4T 1 as a model nucleoside analogue that the corresponding TriPPPro-compounds even retained pronounced anti-HIV activity in CEM/TK− cell cultures, whereas the parent 1 was virtually inactive in these cells due to the inherent lack of cytosolic TK. In both cases of these pronucleotides, the membrane permeability was achieved by the following principle: at the terminal phosphate group of the diphosphate (β-phosphate) or the triphosphate (γ-phosphate), two acceptor-substituted benzyl esters were covalently linked. The one or two other phosphate groups in the nucleoside di- or triphosphates were not modified with a lipophilic mask and thus were still charged. The intracellular enzymatically driven cleavage of the two masks by a first cleavage of the phenolic acyl ester and a subsequent spontaneous cleavage of the remaining part of the mask led to the selective release of d4TDP 5 or d4TTP 7 from DiPPro- 10 or TriPPPro-compounds 11. This delivery mechanism is summarized in Scheme 2.
Scheme 2.
General structures and designed delivery mechanisms of cycloSal- 9, DiPPro- 10, and TriPPPro-compounds 11.
Thereby, we discovered a unique way to deliver the nucleoside di- or the finally bioactive triphosphate of nucleosides into cells. In contrast to the common applications of nucleoside analogues today, with the approach to directly deliver the nucleoside triphosphate, we are entirely independent of the intracellular phosphorylation process.
As we are involved since many years in the development of nucleotide prodrugs (e.g. the cycloSal-nucleoside monophosphate prodrug technology), many of our previous results were very helpful when we started working on the di- or triphosphate prodrug idea.
The major problem in the development of nucleoside analogue di- and triphosphates in contrast to the monophosphate prodrug compounds is the presence of the phosphate anhydride bonds in the di- or triphosphate moieties. In order to neutralize the charges present in these phosphorylated compounds to get a lipophilic derivative, a phosphate triester derivative has to be prepared in the case of the monophosphate prodrugs. This was achieved in the phosphoramidate or in the cycloSal-approaches. Starting from these compounds, the delivery mechanism involves enzymatic or chemical steps which finally led to phosphate ester bond cleavages. However, in order to prepare a “neutralized” nucleoside di- or triphosphate derivative, not only the phosphate ester bonds are present but also the phosphate anhydride bonds linking the two or three phosphate moieties, respectively. Chemically such anhydride bonds are quite unstable which makes a selective cleavage of the phosphate ester bonds almost impossible. Indeed, we tried to prepare fully masked nucleoside diphosphates first but rapidly encountered the problem of quick hydrolysis of the anhydride moieties. Therefore, we decided to abandon the concept to use fully neutralized derivatives but to use partially charged compounds instead. The advantage for that was that the charges stabilize the anhydride bonds by two effects: (a) the negative charge prevents a nucleophilic reaction at the phosphate groups due to electrostatic repulsion and (b) the negative charges convert the phosphate moieties into bad leaving groups. Both effects led to a significant stabilisation of the di- or triphosphate moieties. Actually, nature “uses” the same principle in the nucleoside triphosphates such as ATP; in their charged form, these compounds are only kinetically stable but as soon as they are complexed by cations like magnesium (Mg2+), the charges are neutralized and the reactivity of these compounds increases drastically.
Nucleoside diphosphate prodrugs based on the cycloSal-concept
Initially, we tried to transfer our cycloSal-technology (compound 12, Scheme 3) to the design of potential nucleoside diphosphate prodrugs. However, although the application of the cycloSal-approach finally failed, the results obtained formed the basis for the development of the further concept that led to the development of the DiPPro-prodrugs.
Scheme 3.
Hydrolysis pathways of cycloSal-NDP-prodrugs 12.
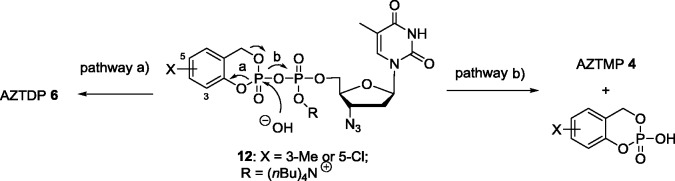
For the synthesis of those compounds, we formed the pyrophosphate moiety starting from a corresponding nucleoside monophosphate synthesized by the Sowa and Ouchi method,30 and these were then coupled with a P(V)-reagent (phosphorochloridate chemistry) bearing the cycloSal-moiety. It was important that the nucleoside monophosphate has to be in the corresponding di(nBu4N)+-salt form – although these are very hygroscopic – in order to achieve an efficient coupling reaction with the P(V)-reagent. Because 3-methyl-cycloSal-nucleotides had proven to be the best suitable monophosphate pronucleotides in our previous work, we used this substitution pattern in the cycloSal-moiety here as well. For the synthesis, the nucleoside monophosphate salt was dissolved in DMF/pyridine and cycloSal-phosphorochloridate was added at −60 °C. After the starting nucleotide was completely consumed, the reaction products were purified on RP-18 columns eluting with gradients of water/methanol. Due to the extensive purification required, the yields of compounds 12 were poor (∼25%).
In order to test the formation of the diphosphate, hydrolysis studies were performed in aqueous buffer solution (25 mM phosphate buffer (PBS) at pH 7.3) followed either by HPLC analysis or by 31P-NMR spectroscopy. To our surprise, starting from the 3-methyl-substituted compound 12, 99% of AZTMP was formed via pathway b, and additionally, only 1% of AZTDP via pathway a (Scheme 3) was detected. Obviously, the approach of the incoming nucleophile to the β-phosphate atom led still to a strong preference for the phosphate displacement instead of the release of the phenolic group. This led to the wrong product, and all attempts to overcome this problem proved unsuccessful. Even when we switched the substituent to a 5-chloro atom (electron-withdrawing group), the product ratio was still in favor to the formation of the monophosphate (75% AZTMP vs. 25% AZTDP). Moreover, the improved AZTDP formation was also accompanied with a significant reduction in chemical stability. Consequently, we concluded that it did not seem feasible to overcome the intrinsic chemical instability of the phosphate anhydride bond in order to generate a selective delivery pathway induced by an initial nucleophilic reaction at the β-phosphate group even if a negative charge is present at the α-phosphate group.
Bis(acyloxybenzyl)-nucleoside diphosphates 10: The symmetric DiPPro-compounds
Taking the results summarized above into account, we next developed a lipophilic masking strategy for nucleoside diphosphates as shown in compound 10 (Scheme 4).
Scheme 4.
General structure and designed hydrolysis pathway of DiPPro-compounds 10.
The main difference is that the delivery mechanism is not chemically driven but is now initiated by an enzymatic reaction within the masking group so that the phosphate anhydride group is not “touched” in the initial triggering cleavage reaction. We concentrated our work on using esterases/lipases for the trigger due to the high intracellular concentration of these enzymes. The approach was developed by first introducing two identical acceptor-substituted benzyl esters to the β-phosphate of the nucleoside diphosphate (Scheme 4) and we kept the initial idea of using the remaining charge at the α-phosphate moiety to markedly stabilize the pyrophosphate group. Upon enzymatic hydrolysis of the phenolic acyl-ester in the benzyl residue, an “Umpolung” reaction occurred, which converted the acyl ester into a strong hydroxyl donor-substituent in the aromatic ring of the benzyl masking group. Subsequently, a spontaneous cleavage of the benzyl-C-O-bond to phosphate group occurred leading to the formation of the mono-masked acyloxybenzyl-NDP intermediate 13. Repetition of this process formed the NDPs without involving the pyrophosphate moiety in the process. By that, a good chemical stability resulted, but on the other hand, an efficient formation of the NDPs also in CEM cell extracts was obtained.24,27,31
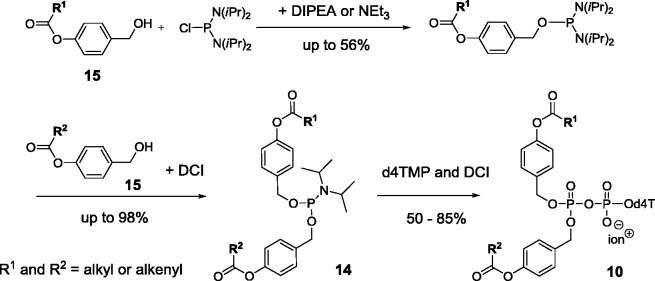
For the synthesis, again the pyrophosphate moiety was formed starting from nucleoside monophosphates, which in contrast to the synthesis of cycloSal-nucleoside diphosphates were coupled with a reactive P(III)-reagent that already comprised the bis(acyloxybenzyl) groups. In this reaction, first a P(III)–P(V) intermediate was formed which is then oxidized to yield the pyrophosphate.24,25,27 As above, for the preparation of DiPPro-compounds 10, (nBu)4N+-salts of nucleoside monophosphates were reacted with bis(4-acyloxylbenzyl)phosphoramidites 14 in the presence of dicyanoimidazole (DCI) as a weak acid catalyst.27,32 The synthesis of compounds 14 was achieved by reacting two equivalents of the benzyl alcohols 15 with diisopropylaminodichlorophosphite (not shown in Scheme 5). Optionally, two different acyloxybenzyl alcohols were reacted with bis(diisopropylamino)chlorophosphane in a two-step procedure. The latter route yielded the non-symmetrically masked prodrugs.26 After completion of the reaction, tBuOOH was added for the oxidation (Scheme 5). DiPPro-NDP prodrugs 10 bearing different nucleoside analogues were isolated in chemical yields up to 72%.
Scheme 5.
Synthesis of the DiPPro-nucleoside diphosphates 10 (shown for d4T as an example).
Next, a series of hydrolysis studies were performed in order to investigate the delivery of the nucleoside diphosphates. First, in chemical hydrolysis, studies were conducted in PBS (25 mmol, pH 7.3). These studies proved that the stability of the compounds was generally high (above 100 h) and was dependent on the length of the alkyl moiety covalently linked to the 4-hydroxybenzyl alcohol used as the masking group – the more lipophilic the acyl residue to higher the chemical stability. In addition to the intermediate mono-masked nucleoside diphosphate always (with the exception of the diacetoxybenzyl-NDPs), some amounts of the nucleoside monophosphate were detected as well. In general, the cleavage of the second masking group present in the intermediate always proceeded much slower than the first step, which is evidenced by the accumulation of intermediates 13 during the course of the reaction. This was caused by the formation of a second charge on the pyrophosphate moiety in intermediates 13, which led to unfavorable interactions with the incoming nucleophile. Interestingly, it was unambiguously proven that no nucleoside monophosphate formation occurred starting from the intermediate. So, the additional charge at the β-phosphate group after the removal of the first acyloxybenzyl group seemed to prevent the unwanted side reaction at the pyrophosphate unit.
In addition to the stability in PBS, the cleavage of prodrugs 10 was also investigated in T-lymphocyte CEM cell extracts. Selected examples were also studied in human serum (HS), RPMI-1640 culture medium containing 10% heat-inactivated fetal calf serum (RPMI/FCS) and in FCS. Half-lives of prodrugs 10 were found to be in the range of 1–21 h. Thus, the cleavage was 10- to 67-fold faster in cell extracts as compared to the chemical hydrolysis, and this was due to a marked contribution by enzymatic cleavage. The formation and the cleavage of intermediates 13 were also observed. Again, the preferred formation of d4TDP from DiPPro-d4TDPs was observed by HPLC. It was impossible to quantify the amount of d4TDP formed because this compound was still subjected to additional enzymatic degradation by phosphatases from the cell extracts. As observed in the chemical hydrolysis, the rates decreased with the increase in the length of the aliphatic chains. Generally, half-lives determined in 20% HS in PBS, pH 6.8, were considerably higher as those determined in cell extracts.
In summary, a remarkable acceleration for the cleavage of the masking group took place in CEM cell extracts. For example, more than 95% of d4TDP was released within a few minutes from the prodrug bearing two acetoxybenzyl moieties. As in the chemical hydrolysis, the corresponding intermediate was hydrolyzed markedly slower than the parent prodrug (t1/2 ∼4 h vs. ∼10 min). Compared to the chemical half-lives, the cleavage of the first acyloxybenzyl-group was 500-fold faster and the second cleavage was 3000-fold faster in the cell extracts. The strong acceleration and the formation of d4TDP clearly point to an enzymatically catalyzed cleavage of the prodrug.
Finally, d4TDP-containing DiPPro-compounds were tested for their anti-HIV activity in wild-type CEM/0 and mutant TK-deficient CEM/TK− cell cultures. It should be mentioned that TMPK-deficient cells are non-existent because TMPK is essential for providing thymidine triphosphate necessary for DNA synthesis. Antiviral activity in TK-deficient cells is a proof that the d4TDP prodrug entered the cell by diffusion. As expected, the parent d4T used as a reference compound was only very poorly phosphorylated in this cell line. Most of the tested compounds proved to be equipotent or slightly less potent compared to the parent d4T against HIV-1 and HIV-2 in wild-type CEM/0 cells. In contrast to the wild-type CEM cells, the compounds bearing short acyl groups lost activity in the mutant TK-deficient CEM/TK− cell line. The loss in antiviral activity is a result of their low lipophilicity combined with a quick extracellular hydrolysis of some of these compounds in RPMI-1640/FCS incubation media used for this cell assay. The undesired extracellular cleavage of the masking groups led to nucleoside diphosphates. These nucleotides will then be dephosphorylated to give finally the parent nucleoside analogue. This explains the almost identical activity of the prodrugs and parent nucleoside in the wild-type CEM cells. However, antiviral activity in the TK-deficient cells is an indication that the NDP-prodrug entered the cell and released the nucleoside diphosphate. All compounds bearing acyl residues R ≥ C6H13 have shown very potent anti-HIV activities in CEM/TK− cells and thus proving their ability to pass through cellular membranes and the subsequent release of the phosphorylated d4T metabolites. As an example, the DiPPro-d4TDP bearing two decanoyl groups in the mask showed the strong potential of the DiPPro-concept because it was found to be 1570-fold more antivirally active in TK-deficient CEM cells and showed also a strong increase in antiviral activity in HIV-infected wild-type CEM/0 cells as compared to the parent d4T. Notably, none of the prodrugs showed significant cytotoxicity. Thus, the DiPPro-system is, to our knowledge, the most effective bio-reversible protection of nucleoside diphosphates reported so far.
Non-symmetric DiPPro-nucleoside diphosphate prodrugs
From the results summarized above, one can conclude that the DiPPro-approach is principally suited for the delivery of nucleoside diphosphates. However, an unfavorable aspect of the symmetric aliphatic DiPPro-compounds 10 (R1 = R2) was that they also led to some extent to the formation of the nucleoside monophosphates (NMP). The amount of NMP formed correlated with the lipophilicity and therefore with the stability of the DiPPro-compounds. Due to the high hydrolytic stability of the lipophilic masking units, a concurrent side reaction in which the water nucleophile is attacking the β-phosphate of the pyrophosphate moiety obviously played a noticeable role. However, as mentioned above, after removal of one masking group leading to the mono-masked intermediate (path A; Scheme 6), no further increase of the amount of NMP formed was observed most probably due to the additional charge on the β-phosphate, which then prevented a second reaction at this moiety.
Scheme 6.
Possible hydrolysis pathways of DiPPro-compounds 10 shown with d4T 1 as an example for a nucleoside analogue.
These observations led to the development of a second generation of DiPPro-compounds. Here, non-symmetric DiPPro-nucleotides 10 bearing two different acylesters were introduced into the benzylester units to the β-phosphate.26 The concept entailed the following: one masking group bearing a short alkyl chain carboxylic acid ester should be quickly cleaved by chemical and/or enzymatic means. The second masking moiety comprises a long alkyl residue carboxylic acid ester in order to add a sufficient level of lipophilicity to the prodrug. As a result, a quick conversion of these DiPPro-compounds into the mono-masked intermediate 13 was achievable, thus avoiding the side reaction, which led to the nucleoside monophosphate. The second mask entity was subsequently removed from the intermediate to form the target nucleoside diphosphate. With these non-symmetrical compounds, a highly selective conversion of the DiPPro-compounds into nucleoside analogue diphosphates was finally achieved.
As described above, we expected that the short chain masking group would be cleaved chemically faster to form an intermediate 13 bearing the second lipophilic and hydrolytically more stable masking group. Surprisingly, in chemical hydrolysis studies, although in different amounts in almost all cases, both intermediates were observed. From analyzing the intermediates obtained, the short alkanoyl ester moieties were hydrolyzed more quickly, while the long alkyl bearing moieties were, as anticipated, more stable. The half-lives of the non-symmetric DiPPro-compounds 10 in PBS, at pH 7.3 were found to be higher as compared to those of the symmetric DiPPro-NDPs.25 Steric hindrance or aggregation due to the second, more lipophilic moiety could be the reason for this increase in stability. The half-lives for the delivery of the intermediate were between 15 and 69 h. As was found for their symmetric counterparts, small amounts of the NMP were still formed. This is due to the surprisingly high stability to hydrolysis as compared to the symmetric DiPPro-compounds having short alkyl chains in the prodrug group. Nevertheless, the ratio of NDP to NMP in these chemically driven hydrolytic reactions was significantly higher for the non-symmetric compounds as was observed for the symmetrically modified counterparts with the longer lipophilic chains.
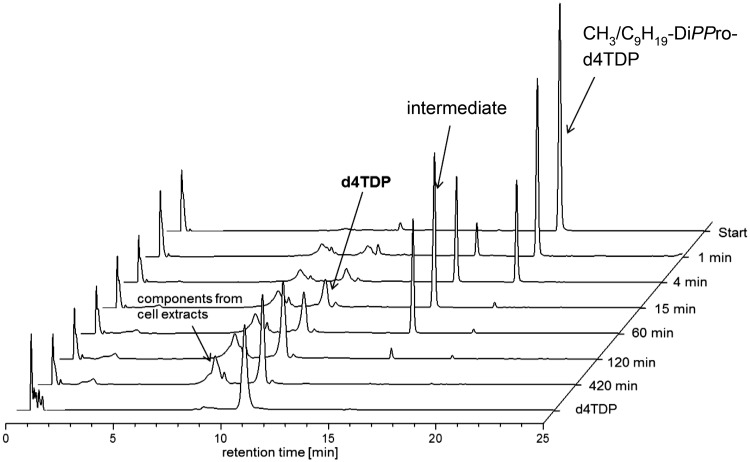
However, in cell extracts and in contrast to the chemical hydrolysis, the prodrugs were now selectively converted into nucleoside diphosphates within only 2 to 7 hours in CEM cell extracts (Figure 1).
Figure 1.
RP-18-HPL-chromatograms of the hydrolysis of CH3/C9H19-DiPPro-d4TDP in CEM/0 cell extracts.
As a model system, hydrolysis studies were also performed using pig liver esterase (PLE) in PBS, pH 7.3. The non-symmetric aliphatic DiPPro-d4TDPs bearing an R1 = CH3 or C4H9 in combination with long alkyl chains as the R2 residue were incubated with PLE to further investigate the influence of the chain length on the enzymatic cleavage. All compounds were rapidly hydrolyzed and exclusively delivered the nucleoside diphosphate d4TDP within a few minutes (0.21–0.27 min).
Finally, the non-symmetric DiPPro-nucleotides were compared to DiPPro-compounds comprising identical prodrug groups. Thus, the non-symmetric and the symmetric counterparts DiPPro-AZTDP derivatives were hydrolyzed in PBS, pH 7.3, and in CEM/0 cell extracts. The hydrolysis studies in PBS led to marked differences in the ratios of NDP and NMP that were formed. In all cases, the symmetric compounds 10 led to considerably more NMP and less NDP as compared to the non-symmetric DiPPro-compounds.26
Nucleoside triphosphate prodrugs: The TriPPPro-compounds
Despite the success of therapeutically very efficient nucleoside monophosphate prodrugs, e.g. the anti-HCV agent Sofosbuvir and the intracellular delivery of nucleoside diphosphates, the final goal of the research on pronucleotide systems has to be the selective intracellular delivery of nucleoside triphosphates of nucleoside analogues. The advantages are obvious: (a) the whole phosphorylation metabolism into the triphosphate is by-passed because the ultimately active triphosphate is delivered inside cells and (b) catabolic processes normally only take place at the nucleoside and the nucleoside monophosphate level will be prevented. Thus, e.g. deamination of adenosine or cytidine analogues can by excluded if triphosphates are delivered into cells. Despite these obvious advantages, almost no reports were known on the development of nucleoside triphosphate prodrugs. Of course, there are also challenges that have to be solved in the development of nucleoside triphosphate prodrugs: (a) the inherent lability of the triphosphate unit comprising reactive phosphate anhydride linkages, (b) the high negative charge that has to be compensated by lipophilic masking groups, (c) a possible unselective cleavage of these masking groups, and (d) the risk of low stability of the delivered triphosphate toward kinases/phosphatases leading to dephosphorylation and thus clearance of the bioactive metabolite. Therefore, it was common sense in the community that the design of nucleoside triphosphate prodrugs would be almost impossible: “Direct delivery of triphosphate or diphosphate forms of nucleoside analogs would be desirable but is impractical because of their instability during synthesis.”33 One of the main reasons was that it seemed impossible to synthesize such compounds and get them inside cells. Since a few years, my group was working intensively on the development of such prodrug systems, and today, we succeeded with the so-called TriPPPro-approach. We have unambiguously proven that such compounds not only can be prepared but they also are taken up into cells and deliver bioactive nucleoside triphosphates.
In this context, we disclosed recently a new, robust synthesis approach for this class of compounds, biophysical properties of the TriPPPro-derivatives, a polymerase chain reaction (PCR), antiviral activity data of a variety of approved as well as so-far non-active nucleoside analogues as well as a study to demonstrate the cellular uptake of the compounds and the intracellular delivery of the triphosphate metabolite using a fluorescent nucleoside. This study demonstrated the general applicability and potential of the approach.28,29
In our work, we have used two different routes for synthesis toward the TriPPPro-derivatives 11.28,29 One route is based on the phosphoramidite chemistry used already for the synthesis of the DiPPro-compounds (analogously to the route summarized in Scheme 5). The difference is that the P(III)-reagent comprising the two masking groups was reacted with a nucleoside diphosphate instead of a monophosphate in the earlier case. Analogous to the above-described convergent synthesis method, the target compounds were synthesized using a DCI-mediated coupling of the nucleoside diphosphate and an appropriate phosphoramidite bearing acyloxybenzyl masking groups within 1 min. However, although different protocols have been published for the synthesis of nucleoside diphosphates, this is often still a challenge. We applied our cycloSal-technology (see above) as a phosphate active ester method to couple a cycloSal-nucleoside monophosphate with an appropriate phosphate salt. By this nucleophilic reaction, the phosphate anhydride linkage was formed and the cycloSal-moiety was displaced.34–36 This approach has been successfully introduced by us for the synthesis of nucleoside triphosphates, nucleotide bioconjugates (e.g. carbohydrates), and phosphorylated oligonucleotides. The synthesis of the TriPPPro-nucleotides was achieved with yields between 25% and 70% by using this phosphoramidite chemistry.
In order to achieve a more efficient conversion of the parent nucleoside to the TriPPPro-compounds 11, an alternative route was developed again by formation of the phosphate anhydride linkage. The approach is based on coupling of a nucleoside monophosphate and P,P-bis-(4-acyloxybenzyl)pyrophosphate 16 as a phosphorus (V)-reagent (Scheme 7). In this synthesis, the linkage between the α- and the β-phosphate of the triphosphate prodrug was formed, while in the earlier approach, the linkage between the β- and the γ-phosphate was formed. The advantage of this route is that monophosphates are generally easier to prepare than nucleoside diphosphates. A second advantage of this second route is that after the formation of the O-P-O-linkage, no oxidation has to be done in contrast to the phosphoramidite chemistry. Thus, also nucleoside analogues or masking groups sensitive for oxidation can be used. In this second approach, first H-phosphonate 17 was prepared from diphenyl-H-phosphonate (DPP) and 4-(hydroxymethyl)phenylacylate 15. Next, compound 17 was converted into the phosphorochloridate with N-chlorosuccinimide (NCS)37 followed by a phosphorylation with (nBu)4N+-phosphate. Despite its high chemical reactivity, the crude product was purified by extraction which led to compound 16 in almost quantitative yield.
Scheme 7.
Synthesis of TriPPPro-prodrugs 11 using the H-phosphonate route.
Nucleoside monophosphates were synthesized according to the Sowa and Ouchi method30 or, again, by the cycloSal-method. The cycloSal-method was used in cases of acid labile purine nucleosides, e.g. ddG. CycloSal-triesters 9 were used to prepare nucleoside monophosphates by basic hydrolysis. The final coupling reaction was accomplished by modifying literature methods38 by stepwise activation of compound 16 with trifluoroacetic acid anhydride and 1-methylimidazole followed by addition of the nucleoside monophosphate (e.g. d4TMP 3) to give TriPPPro-nucleotides 11 (non-optimized yields of 30%–81%). Using this synthetic pathway, the overall yields were found to be at least as good as on the phosphoramidite route – but often better – and this method proceeded much faster and gave more reliable yields. Moreover, this method is more tolerable to the used solvents; even the addition of some drops of methanol to achieve a better solubility of the nucleoside monophosphate was tolerated.
Synthesis of mono-masked triphosphate intermediates
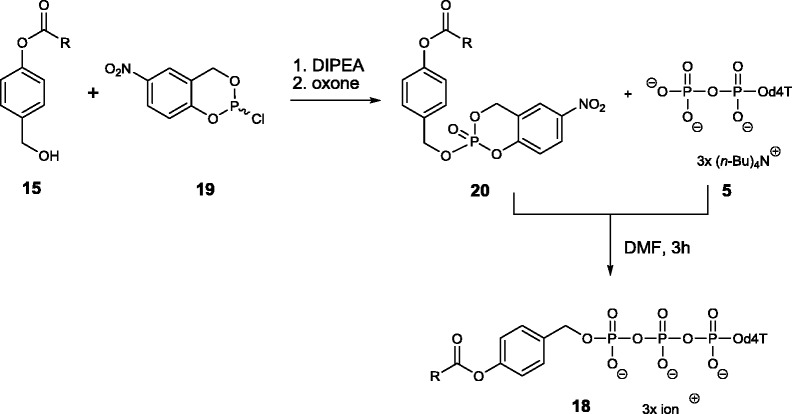
In addition to the TriPPPro-compounds 11, the mono-masked acyloxybenzyl-nucleoside triphosphate derivatives which are formed first in the hydrolysis were synthesized as well (Scheme 8) because they were needed as reference compounds for further studies. Several synthesis routes mainly based on DCC-activated coupling were published. In our studies on the DiPPro-prodrugs, we isolated these mono-esterified compounds by chemical hydrolysis under mild basic conditions.25 However, this procedure proved not very efficient in the case of the mono-masked triphosphate intermediates 18. Therefore, an efficient access to such compounds was developed. The mono-esterified nucleoside triphosphates were prepared starting from 4-acyloxybenzyl alcohols 15 and its conversion into the 5-nitro-cycloSal-triesters 20, which were purified by preparative TLC (up to 89% yield). Next, triesters 20 were reacted with a nucleoside diphosphate such as 5 which gave the mono-masked acyloxybenzyl-nucleoside triphosphates d4TTPs 18 in yields of 26%–50%.
Scheme 8.
Synthesis of the mono-masked-NTP compounds 18 again shown with d4T as the nucleoside analogue.
Properties of the TriPPPro-derivatives
As before, the hydrolysis properties of TriPPPro-compounds 11 were evaluated in different media. The chemical stability was determined in PBS (25 mM, pH 7.3), and the hydrolysis samples were analyzed by means of analytical RP18-HPLC. All prodrugs hydrolyzed via the single masked intermediate 18 and then released NTPs, e.g. d4TTP 7, which were identified by HPLC, NMR, and mass spectrometry. However, for purine derivatives, a few non-identified side reactions occurred. As observed for the DiPPro-compounds, the half-lives of the mono-masked intermediates 18 were much higher as compared to those of their precursors 11. Finally, intermediates 18 delivered nucleoside analogue triphosphates selectively.
As the DiPPro-system, the TriPPPro-concept was designed to be triggered by the action of (carboxy)esterases. In studies using PLE in PBS (pH 7.3), the removal of both prodrug groups occurred much faster as compared to the chemical hydrolysis. Moreover, as long as the enzymatic cleavage occurred quickly enough to yield intermediates 18, the nucleoside triphosphates were formed selectively.
To confirm the formation of the nucleoside triphosphate, two thymine-bearing TriPPPro-compounds were exposed to PLE. After complete consumption of TriPPPro-prodrugs 11 comprising d4T or thymidine39 and the corresponding intermediates 18, the solvents were removed. The remaining products were used in a primer extension assay using a 30mer template and a 25mer primer strand. Reverse transcriptase (RT) was used as the polymerase for the elongation of the primer. As positive control, first thymidine triphosphate was incubated and as can be seen on the polyacrylamide gel, RT incorporated the nucleotide opposite to its canonical base adenine in the template strand (see Figure 2). Next, we used the product obtained from the PLE hydrolysis of TriPPPro-TTP. As for the reference, the primer was elongated by one nucleotide – obviously TTP. Mixtures of all commercial nucleoside triphosphates or a mixture of three commercial triphosphates (dGTP, dATP, and dCTP) and the hydrolysis product led in both cases to a full extension of the primer. A second experiment was carried out with a TriPPPro-d4TTP derivative. Again the hydrolysis was achieved by addition of PLE. The product was added to the polymerase assay. Again the primer was extended by one nucleotide. However, when we mixed the PLE-hydrolysis product with any of the commercial triphosphates dGTP, dATP, and dCTP, no extension was observed. Thus, in the hydrolysis, d4TTP was formed which was used by RT as a substrate but acted as an immediate chain terminator after incorporation. Both experiments proved the formation of the corresponding nucleoside triphosphate from the TriPPPro-derivative.
In a second proof-of-principle experiment, the PLE hydrolysis products were used in a PCR assay using FIREPol DNA polymerase. In case of the hydrolysate of TriPPPro-d4TTP, no amplification of the DNA probe occurred. In contrast, formed TTP from its corresponding prodrug TriPPPro-TTP mixed with the three other 2′-deoxynucleoside triphosphates led to an amplification of the DNA sample.29
The incubation of TriPPPro-compounds with human CD4+ T-lymphocyte cell extracts led to a marked acceleration of the triphosphate formation. The half-lives of prodrugs 11 were in the range of 1–2.5 h independent on the nucleoside analogue. In addition to intermediates 18, the corresponding nucleoside analogue triphosphates were detected. However, we often observed the corresponding diphosphates as an additional product which was most likely the result of a dephosphorylation of the triphosphate by phosphatases/kinases present in the cell extracts.
The effectiveness of the TriPPPro-compounds 11 to act as inhibitors against HIV-1 and HIV-2 was determined in HIV-infected wild-type (CEM/0) and TK-deficient (CEM/TK−) cell cultures. The inhibition of the replication of HIV-1 and HIV-2 by prodrugs 11 was much higher or at least similar as compared to their parent nucleosides.28,29 The retention of the antiviral activity in the TK-deficient cells strongly point to a cell uptake of the TriPPPro-compounds bearing thymidine analogues into cells. In particular, the entirely inactive nucleoside analogues 3′-deoxy-3′-fluoro-5-chlorouridine29 and 3′-up-azido-uridine were converted into a highly potent compound after conversion into the corresponding TriPPPro-compound. The stability, hydrolysis process, and antiviral activity were significantly influenced by the chain length of the prodrug moieties.28,29
Surprisingly, the TriPPPro-compounds of 2′,3′-dideoxy-2′,3′-didehydrouridine (d4U) and 2′,3′-dideoxyuridine (ddU) showed no marked antiviral activity. Incubations of ddUTP in cell extracts showed an extremely fast dephosphorylation (t1/2 < 1 min) of the triphosphate to yield ddUDP and later ddUMP. Therefore, we speculate that in some cases, the formed triphosphates (although released from TriPPPro-compounds as proven by hydrolysis in PLE) were not retained in sufficient concentrations in cells to exhibit antiviral activity. This means, that the uridine analogues d4U and ddU seem to be inappropriate for antiviral therapy, although the triphosphates itself were found to be very potent inhibitors when tested in a biochemical assay using isolated RT. In contrast, other nucleoside analogues were converted into potent inhibitors against HIV by application of the TriPPPro-approach. Interestingly, 2′,3′-dideoxyinosine (ddI) triphosphate and abacavir (ABC) triphosphate released from the corresponding TriPPPro-compounds are to be considered as novel metabolites that are usually not formed from the corresponding parent nucleoside analogues ddI and ABC. Indeed, the eventual triphosphate metabolite intracellularly formed from ddI is 2′,3′-dideoxyadenosine triphosphate (ddATP) (but not ddITP, due to prior metabolic amination of ddIMP to ddAMP) and from ABC is carbovir-TP (not ABCTP due to prior deamination of ABCMP to carbovir-MP).40,41 Thus, the TriPPPro-technology also enables to deliver and discover novel antivirally active compounds that otherwise cannot be formed by the parent (or any other) nucleoside analogue.
Finally, in a first study investigating the cell uptake of TriPPPro-compounds 11 and to confirm again the intracellular delivery of the NTP metabolite from TriPPPro-compounds, a fluorescent 2′,3′-dideoxy-bicyclic nucleoside analogue triphosphate (ddBCNATP)-prodrug was incubated in T-lymphocytes for 60 or 180 min at 37 °C, respectively. The fluorescent ddBCNA was used because the analysis of the cell extract samples could be performed by fluorescence detection on the HPLC, which led to a marked increase in the sensitivity and avoided concomitant detection of interfering cellular components. This study proved that the fluorescent prodrug penetrated the cell membrane and delivered ddBCNA triphosphate intracellularly within 1 h as the main metabolite present in the extracts. As expected, ddBCNA triphosphate was also dephosphorylated by cellular enzymes. Interestingly, after 180 min, no triphosphate was detectable in the extracts but high amounts of ddBCNADP and small amounts of ddBCNAMP and the parent ddBCNA were detected instead. As mentioned above, this dephosphorylation has also been detected in CEM cell extract hydrolyses. As a control, studies with the parent compound ddBCNA revealed that the nucleoside was taken up by cells in relatively small amounts, and it was not phosphorylated to yield any of the three metabolites. As a conclusion, ddBCNATP observed in our experiment was clearly formed after the uptake of the TriPPPro-precursor into intact cells followed by hydrolysis of the prodrug.29
Summary and conclusion
In conclusion, our continuous work on the design of nucleotide prodrugs led finally to the development of a first example of a successful nucleoside di- and also a triphosphate prodrug system. The key for this development was that the reactive phosphate anhydride linkage(s) in the DiPPro- or TriPPPro-compounds were stabilized by the presence of negative charges. All attempts to mask all of the phosphate oxygen atoms led to completely uncharged di- or triphosphate moieties which were extremely reactive and proved to be unsuitable to be used in the pronucleotide sense. However, by keeping the charges, the lipophilicity needed for the uptake into cells has to be provided by the two lipophilic masking groups attached to the terminal phosphate group. The second key was not using a chemical triggering process for the delivery of the nucleotide any longer as in the cycloSal-compounds but to use an enzyme-catalyzed cleavage in the masking group without involving the phosphate groups. This avoided almost all possible side reactions associated with the phosphate esters or phosphate anhydride bonds. All this finally led to the successful formation of the bioactive di- or triphosphate inside the cell after cell uptake. The results summarized here clearly proved that the DiPPro- and the TriPPPro-approach allowed the intracellular delivery of nucleoside analogue di- or -triphosphates and the latter approach was used to convert inactive nucleoside analogues into powerful antivirally active metabolites. Thus, the TriPPPro-strategy offers high potential to be used in antiviral and antitumor therapies. In principle, the approach can be used for all those nucleoside analogues that lack efficient conversion into the triphosphate or which are not phosphorylated at all at one of the three individual phosphorylation steps. Also compounds that are quickly cleared due to catabolic processes may be rescued by this approach. In addition, this approach might be also suitable to address chemical biology questions by delivering triphosphorylated nucleotide probes into living cells.
Nevertheless, there are still some remaining tasks to be solved. One major issue are the surprising differences in stability of nucleoside analogue triphosphates. For example, it has been shown that the triphosphate of the Sofosbuvir nucleoside (a uracil nucleoside analogue) showed an extremely high intracellular stability of about 35 h, which was also made responsible for the significant and persistent anti-HCV effect of this triphosphate to inhibit NS5B-polymerase. In contrast, its cytosine analogue showed a half-life of just 5 h under the same experimental conditions.42 As mentioned above, we have shown that ddUTP or d4UTP (also uracil nucleoside analogues) were extremely quickly dephosphorylated in CEM cell extracts. Thus, if these compounds are not efficiently rephosphorylated and so restoring the triphosphate pool, the compounds are useless as antiviral agents.
Another important issue in the development of such charged, lipophilic triphosphate derivatives is still the membrane passage. So far, we just performed the cell assay summarized above. However, we have neither data on the uptake mechanism nor data on the quantity of compound which is taken up by cells. So, which portion of the added compound is actually responsible for the antiviral activity observed?
Another issue can be the dependence of the masking groups of the compounds to be initially cleaved by enzymes. Is the differentiation between the blood stability and the intracellular stability sufficient to allow a successful application of these compounds in vivo? Maybe it is possible to introduce a different trigger for the masking group which might be more efficient?
Work along this line is currently underway in our laboratories.
Acknowledgements
I would like to acknowledge all the contributions made by my hard-working coworkers. The list of names would be too long to name all of them here but they are all coauthors of our original papers published during the last decade. Without their enthusiasm and creativity, these projects would never had reached this level.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported continuously by Universität Hamburg and Deutsche Forschungsgemeinschaft (DFG; Me1161/3–1; -4–1; -9–1; -13–1 and 14–1).
References
- 1.Jordheim LP, Durantel D, Zoulim F, et al. ▪. Nat Rev Drug Discov 2013; 12: 447–464. [DOI] [PubMed] [Google Scholar]
- 2.Chilar T andRay AS.. ▪. Antiviral Res 2010; 85: 39–58. [DOI] [PubMed] [Google Scholar]
- 3.El Safadi Y Vivet-Boudou V andMarquet R.. ▪. Appl Microbiol Biotechnol 2007; 75: 723–737. [DOI] [PubMed] [Google Scholar]
- 4.Burton JR andEverson GT.. ▪. Clin Liver Dis 2009; 13: 453–465. [DOI] [PubMed] [Google Scholar]
- 5.Balzarini J Herdewijn P andDe Clercq E.. J Biol Chem 1989; 264: 6127–6133. [PubMed] [Google Scholar]
- 6.Balzarini J, Pauwels R, Baba M, et al. ▪. Biochem Pharmacol 1988; 37: 2065–2068. [DOI] [PubMed] [Google Scholar]
- 7.Freeman S andRoss KC.. ▪. Prog Med Chem 1997; 34: 112–142. [Google Scholar]
- 8.Van Rompay AR Johansson M andKarlsson A.. ▪. Pharmacol Ther 2000; 87: 189–198. [DOI] [PubMed] [Google Scholar]
- 9.Ho HT andHitchcock MJ.. Antimicrob Agents Chemother 1987; 33: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balzarini J, Pauwels R, Baba M, et al. ▪. Biochem Pharmacol 1988; 37: 2065–2068. [DOI] [PubMed] [Google Scholar]
- 11.Furman PA, Clair MHS, Weinhold K, et al. ▪. Proc Natl Acad Sci USA 1986; 83: 8333–8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertenbreiter F Balzarini J andMeier C.. ▪. Chem Med Chem 2015; 10: 94–106. [DOI] [PubMed] [Google Scholar]
- 13.Wagner CR Iyer VV andMcIntee EJ.. ▪. Med Res Rev 2000; 20: 417–451. [DOI] [PubMed] [Google Scholar]
- 14.Pradere U, Garnier-Amblard EC, Coats SJ, et al. ▪. Chem Rev 2014; 114: 9154–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erion MD, Reddy KR, Boyer SH, et al. ▪. J Am Chem Soc 2004; 126: 5154–5163. [DOI] [PubMed] [Google Scholar]
- 16.Cahard D, McGuigan C, Balzarini J. ▪. Mini Rev Med Chem 2004; 4: 371–381. [DOI] [PubMed] [Google Scholar]
- 17.Farquhar D, Srivastva DN, Kuttesch NJ, et al. ▪. J Pharm Sci 1983; 72: 324–325. [DOI] [PubMed] [Google Scholar]
- 18.Puech F, Gosselin G, Lefebvre I, et al. ▪. Antiviral Res 1993; 22: 155–174. [DOI] [PubMed] [Google Scholar]
- 19.Meier C. ▪. Eur J Org Chem 2006; 2006: 1081–1102. [Google Scholar]
- 20.Jessen HJ Balzarini J andMeier C.. ▪. J Med Chem 2008; 51: 6592–6598. [DOI] [PubMed] [Google Scholar]
- 21.Gisch N Balzarini J andMeier C.. ▪. J Med Chem 2009; 52: 3464–3473. [DOI] [PubMed] [Google Scholar]
- 22.Bonnaffé B, Dupraz B, Ughetto-Monfrin J, et al. ▪. J Org Chem 1996; 61: 895–902. [Google Scholar]
- 23.Kreimeyer A, Ughetto-Monfrin J, Namane A, et al. ▪. Tetrahedron Lett 1996; 37: 8739–8742. [Google Scholar]
- 24.Jessen HJ, Schulz T, Balzarini J, et al. ▪. Angew Chem Int Ed Engl 2008; 47: 8719–8722. Angew Chem 2008; 120: 8847–8850. [DOI] [PubMed]
- 25.Schulz T, Balzarini J, Meier C. ▪. Chem Med Chem 2014; 9: 762–775. [DOI] [PubMed] [Google Scholar]
- 26.Weinschenk L, Schols D, Balzarini J, et al. ▪. J Med Chem 2015; 58: 6114–6130. [DOI] [PubMed] [Google Scholar]
- 27.Meier C, Jessen HJ, Schulz T, et al. ▪. Curr Med Chem 2015; 22: 3933–3950. [DOI] [PubMed] [Google Scholar]
- 28.Gollnest T, Dinis de Oliveira T, Schols D, et al. ▪. Nat Commun 2015; 6: 8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gollnest T, Dinis de Oliveira T, Rath A, et al. Angew Chem Int Ed Engl 2016; 55: 5255–5258. [DOI] [PubMed] [Google Scholar]
- 30.Sowa T andOuchi S.. ▪. BCSJ 1975; 48: 2084–2090. [Google Scholar]
- 31.The BAB-concept has been applied to nucleoside monophosphates: ; a) Freeman S.; Ross, K. C. Prog. Med. Chem 1997, 34, 111-147; ; b) Meier, C.; Muus, U.; Renze, J.; Naesens, L.; De Clercq, E.; Balzarini, J. Antiviral Chem. Chemother 2002, 13, 101-114. c) Routledge, A.; Walker, I.; Freeman, S.; Hay, A.; Mahmood, N. Nucleosides & Nucleotides 1995, 14, 1545-58.
- 32.Weinschenk L, Gollnest T, Schols D, et al. ▪. Chem Med Chem 2015; 10: 891–900. [DOI] [PubMed] [Google Scholar]
- 33.Tan X andChu K.. ▪. Adv Drug Deliv Rev 1999; 39: 117–151. [DOI] [PubMed] [Google Scholar]
- 34.Warnecke S andMeier C.. ▪. J Org Chem 2009; 74: 3024–3030. [DOI] [PubMed] [Google Scholar]
- 35.Tonn VC andMeier C.. ▪. Chem Eur J 2011; 17: 9832–9842. [DOI] [PubMed] [Google Scholar]
- 36.Sarac I andMeier C.. ▪. Chem Eur J 2015; 21: 16421–16426. [DOI] [PubMed] [Google Scholar]
- 37.Kumar V andKaushik MP.. ▪. Synlett 2007; 19: 2937–2951. [Google Scholar]
- 38.Mohamady S andTaylor SD.. ▪. J Org Chem 2011; 76: 6344–6349. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Gao Y, Wen X, et al. ▪. Asian J Pharm Sci 2014; 9: 65–74. [Google Scholar]
- 40.Balzarini J. ▪. Pharm World Sci 1994; 16: 113–126. [DOI] [PubMed] [Google Scholar]
- 41.Faletto MB, Miller WH, Garvey EP, et al. ▪. Antimicrob Agents Chemother 1997; 41: 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami E, Niu C, Bao H, et al. ▪. Antimicrob Agents Chemother 2008; 52: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]