Abstract
Background
Zika virus is an emerging crisis as infection is implicated in severe neurological disorders—Guillain–Barré syndrome and fetal microcephaly. There are currently no treatment options available for Zika virus infection. This virus is part of the flavivirus genus and closely related to Dengue Fever Virus, West Nile Virus, and Japanese Encephalitis Virus. Like other flaviviruses, the Zika virus genome encodes three structural proteins (capsid, precursor membrane, and envelope) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Currently, no structural information exists on these viral proteins to facilitate vaccine design and rational drug discovery.
Methods
Structures for all Zika virus viral proteins were predicted using experimental templates available from closely related viruses using the online SwissModel server. These homology models were compared to drug targets from other viruses using Visual Molecular Dynamics Multiseq software. Sequential alignment of all Zika virus polyproteins was performed using Clustal Omega to identify mutations in specific viral proteins implicated in pathogenesis.
Results
The precursor membrane, envelope, and NS1 proteins are unique to Zika virus highlighting possible challenges in vaccine design. Sequential differences between Zika virus strains occur at critical positions on precursor membrane, envelope, NS2A, NS3, NS4B, and NS5 as potential loci for differential pathogenesis. Druggable pockets in Dengue Fever Virus and West Nile Virus NS3 and NS5 are retained in predicted Zika virus structures.
Conclusions
Lead candidates for Zika virus can likely be established using NS3 and NS5 inhibitors from other flaviviruses, and the structures presented can provide opportunities for Zika virus intervention strategies.
Keywords: Zika virus, flavivirus, homology modeling, vaccine design, drug discovery
Introduction
Due to recent outbreaks in the Americas, Zika virus (ZIKV) was recently classified as a public health emergency of international concern by the World Health Organization.1 The virus is spread primarily by the Aedes mosquito vector present in Africa, South Asia, South America, Central America, and the Southern United States. Though ZIKV infection typically presents with mild symptoms of fever, rashes, headache, and joint pain, it is associated with severe neurological disorders, although the evidence is not conclusive.2 Guillain–Barré syndrome is an autoimmune disease characterized by muscle weakness from an immune-damaged nervous system, and a recent spike in this disease in Brazil coincides with ZIKV outbreak. Furthermore, evidence suggests that early pregnancy maternal ZIKV infection results in congenital defects, fetal microcephaly, and miscarriage. ZIKV is broadly categorized into the East/West African and Asian/Brazilian strains based on similarity and clustering, and the Asian/Brazilian strains are implicated in severe neurological symptoms. At this time, there is no approved therapy for ZIKV infection necessitating pursuit of novel intervention strategies for prevention and treatment.
ZIKV is a positive-sense single-stranded RNA virus and is a member of the Flaviviridae family Flavivirus genus. Other similar viruses in this genus include Dengue Fever Virus (DENV), Japanese Encephalitis Virus (JEV), Murray Valley Encephalitis Virus (MVEV), St. Louis Encephalitis Virus, West Nile Virus (WNV), and Yellow Fever Virus (YFV). Based on understanding of other flaviviruses, the ∼11,000 base-pair ZIKV genome encodes a ∼3500 amino-acid polyprotein. Host and viral processing cleaves the polyprotein into three structural proteins (capsid, precursor membrane (prM), and envelope (Env)) and seven nonstructural proteins (nonstructured protein 1, 2A, 2B, 3, 4A, 4B, and 5). Understanding the sequential and structural composition of these viral proteins would provide insight into the pathogenesis of the disease and illuminate targets for drug discovery. At the time of publication, only one cryo-EM structure of the ZIKV envelope exists,3 but no experimental structures are available for other crucial ZIKV viral proteins.
Here, we predict the structures of ZIKV proteins based on homology to other flaviviruses. Unique from other studies,4 the sequences of different ZIKV strains are compared in the context of the viral proteins. These data facilitate discussion on ZIKV structural conservation in the context of pathogenesis, vaccine development, and drug discovery.
Methods
The polyprotein sequences for ZIKV were obtained from GenBank, and the supplementary information provides the names and accession numbers analyzed in this study. ZIKV polyprotein sequences were aligned using Clustal Omega,5 and sites of viral and host processing were obtained from GenBank ZIKV entry (NC_012532). The pair-wise Clustal Emboss algorithm6 was used to compare ZIKV and DENV strain NGC for the NS2A scheme, and the same approach compared ZIKV and JEV in NS4B topology. The HeliQuest server7 was used to predict amphipathic helices in ZIKV NS2A and NS4B schemes.
Homology models for the ZIKV proteins were constructed by submission of individual viral proteins from the Polynesia 2013 sequence (GenBank KJ776791) to SwissModel server.8 This sequence was selected as it was analogous to recent ZIKV fetal isolates. Homology models for ZIKV proteins were constructed using only structural templates of high coverage and sequential identity. The resulting models were compared to existing crystal structures using Visual Molecular Dynamics MultiSeq tool and colored by sequence entropy.9
Results
Structural ZIKV proteins
Of the 11 viral-encoded proteins (Figure 1), 3D homology models were created for six (cap, prM, Env, NS1, NS3, and NS5). Topology models for NS2A and NS4B were prepared by alignment to DENV and JEV template sequences. Sequential differences and structural comparisons are presented for the prM, Env, NS1, NS3, NS4B, and NS5 proteins, and the supplementary information contains discussion on the ZIKV Cap, NS2A, and NS4A as these proteins are not yet drug or vaccine targets.
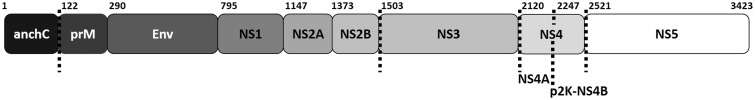
Figure 1.
ZIKV viral protein positions along the polyprotein. The three structural ZIKV proteins are the anchored capsid protein (anchC), the precursor membrane protein (prM) and the envelope glycoprotein (Env). The non-structural proteins are numbered starting from the N-terminus (NS1-5). Dashed lines indicate sites of NS3 cleavage, and the numbers denote the amino acids along the polyprotein separating each viral protein.
prM and Env proteins
A recent triumph in vaccinology is the development of ChimeriVax technology.10 This approach utilizes attenuated YFV (YFV-17D) substituted with the prM and Env proteins for the virus of interest as a vaccine candidate. ChimeriVax technology was used to create YFV vaccine, horse WNV vaccine, human JEV vaccine, and tetravalent human DENV vaccine currently in trials. With this technology in mind, we considered the premature membrane and Env proteins of ZIKV together.
prM protein
A drop in pH during maturation induces conformational change in the viral membrane proteins leading to prM cleavage.11 The ZIKV prM protein is 168 residues long with residues 1–93 composing the precursor region responsible for protection against premature cleavage. The ZIKV prM protein is 42% identical to that found in the DENV-2 prM-Env crystal (PDBID 3C5X),12 and this structure was used as a template for the ZIKV prM model. The prM residues at the Env interface are highly conserved between DENV and ZIKV (Figure 2). Nine of 93 residues differ between the DENV and ZIKV prM proteins on the surface of the protein: T5R, K19A, K21E, K26P, M33K, E43H, K52E, R57D, T81H. These results indicate that the contacts between the prM and Env are preserved between DENV and ZIKV, but protection and pH-induced conformational change of the prM may be unique to ZIKV. Furthermore, notable mutations between ZIKV strains occur in the protective prM region: I3V, S17N, K21E, A26P, V31M, H35Y, and V36I. The homology model predicts that these mutations occur on the exposed surface of the prM protein. As such, these differences between ZIKV strains may affect Env maturation and presentation as it pertains to the ChimeriVax technology.
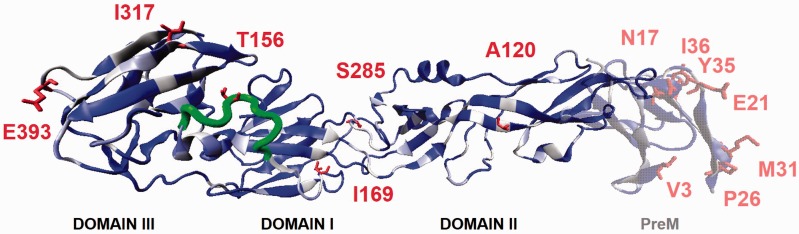
Figure 2.
Model of the ZIKV Env protein (solid) and prM (transparent) proteins showing the binding interface. The homology model of ZIKV preM (transparent) was built from DENV2 template (PDBID 3C5X), and the model of the ZIKV Env (opaque) utilized the DENV2 template (PDBID 1UZG). The three structural domains of the envelope protein are marked. Dark colored regions are structurally conserved residues between the model and the DENV templates while white residues are dissimilar. Highlighted are mutations between ZIKV strains. Shown in dark tubes is a 5-amino acid present between domain I and III in all Asian/Brazilian sequences but missing in some African strains.
Env glycoprotein
Env proteins exist as homodimers on the viral surface and facilitate adhesion and fusion to the host cell surface. Crystal structures show that flavivirus Env is composed of three β-sheet domains: a centralized amino terminal domain I, a finger-like dimerization domain II containing the fusion sequence, and a carboxyl-terminal immunoglobulin-like domain III containing the transmembrane region (Figure 2). The ZIKV Env protein is 504 residues in length, and the N-terminal residues 1–392 had high sequential identity to the DENV3 Env protein crystal structure (PDBID 1UZG, 58% identity).13
The resulting homology model shows that domain II is similar between DENV and ZIKV. The fusion sequence (100–108) is identical between DENV and ZIKV. The fusion peptide serves as the recognition epitope for the antiflavivirus D1-4G2-4-15 (4G2)14 and 2A10G6 antibodies.15 These observations suggest that both flaviviruses utilize the same molecular machinery for viral fusion that is recognized by existing antibodies. Domains I and III house significant differences between DENV and ZIKV. Domain III is responsible for host surface recognition and binding, and the exposed β-sheets are not conserved between DENV and ZIKV suggesting different viral recognition elements. The hinge region between domain I and II contains a druggable pocket observed in DENV Env crystal structures.16 Notable replacements surrounding this binding pocket are E49T, V130I, I270A, and loss of conserved structure leads to L277-R283, F279-L284, and T280-S285 substitutions between DENV and ZIKV. There is less than 50% identity between DENV and ZIKV in this region indicating that novel compounds must be discovered as Env-binding entry inhibitors for ZIKV.
Eleven mutations occur in the ZIKV Env protein out of 502 residues. The T120A substitution occurs in domain II, V169I and F285S are predicted between domains I and II, and V317I and D393E present on the surface of domain III. Not present in the homology model are biochemically similar mutations (V437A, F438L, V473M, T487M, and M495L) in membrane-spanning regions. Previous studies highlighted a five amino acid insertion in the Env protein related to the YAP epidemic not present in other African ZIKV strains.17,18 Other ZIKV genomes also lose amino acids between residues 152 and 162 (Supplementary Materials).1 The homology model predicts this insertion spans domains I and III presumably affecting host surface recognition. Within this insertion is the I156T mutation that occurs between ZIKV strains, and this is predicted to modulate N-linked glycosylation (Asn-X-Thr) for N154. A recent cryo-EM structure of the ZIKV biological assembly provides the experimentally determined coordinates of the Env protein (PDBID 5IRE),3 and the homology model places the ZIKV residues at the exact same position. This structure also highlights the 10-residue insertion identified by homology modeling, including the glycosylation site, supporting the importance of these observations. This insertion may reflect changes in host surface protein targets between the ZIKV strains; however, deletion of glycosylation sites happens in extensive passages in mice brains and mosquito cells as observed for WNV.19 Recent studies have shown that ZIKV can productively infect human cortical neural progenitor cells,20 which differentially express surface proteins with mosquito homologs, such as GLAST,21 suggesting a route of fetal neurotropism and mother-to-child transmission mediated by strain-specific Env mutants. As the Env and prM proteins play deciding roles in cell discrimination, infectivity, and pathogenesis, the mutations discussed here should be evaluated in future ZIKV sequences.
Nonstructural ZIKV proteins
Nonstructural protein 1 (NS1)
The NS1 plays multiple roles in flavivirus infection and is required for viral replication (for excellent review see Muller and Young (2013)).22 At the early stages, glycosylated NS1 is associated with the RNA replication complex though the specific role is unclear. At later stages of infection, NS1 is secreted in proteoliposomes to modulate host immune responses, and secreted NS1 is a potential biomarker for flavivirus infections.
The ZIKV NS1 protein is 56% identical to the WNV crystal that served as a structural template (PDBID 4O6D).23 This template consists of three domains: a β-roll containing a dimerization interface, an α/β wing domain, and a central continuous β-sheet “ladder” at the C-terminus (Figure 3). The sequential identity between ZIKV and WNV localizes to the β-roll and internal cores of the wing and ladder domains. Dissimilar residues present at the edge of the β-sheet “ladder” far from the β roll, notably within residues 310–330 responsible for generating cross-reactive antibodies for host proteins.24 The exposed residues of the α-helix in the wing are also not conserved between WNV and ZIKV. Like WNV and DENV, ZIKV contains the 116KXWG119 core motif at the tip of the wing domain (unresolved in the structure). Linear epitopes containing this sequence have been previously reported to generate polyclonal antibodies that cross-react with proteins expressed by human umbilical vein endothelial cells.25
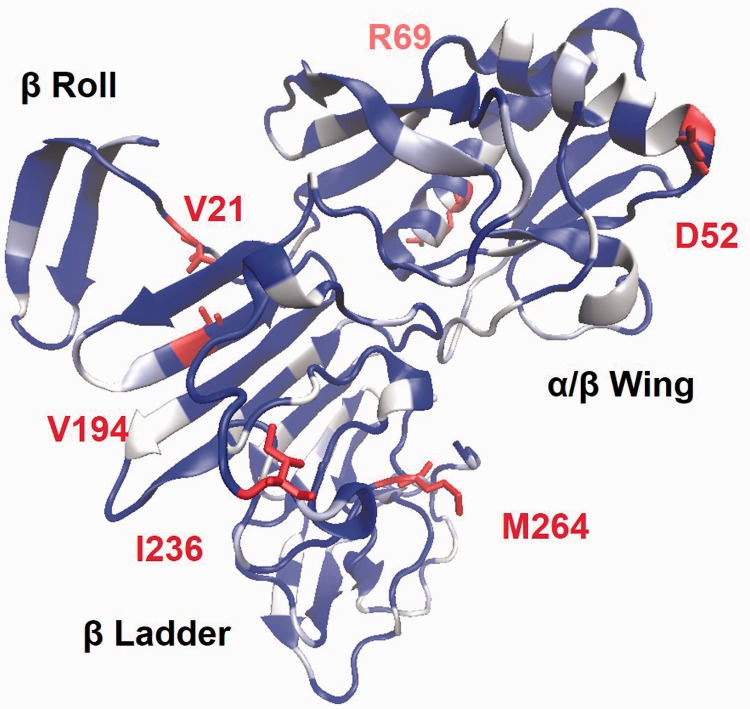
Figure 3.
Structural model of ZIKV NS1 protein. The homology model was constructed using a WNV template (PDBID 4O6D) with indicated structural domains. Dark regions are structurally conserved between the ZIKV model and the WNV template while white residues are dissimilar. Highlighted are residues that are dissimilar between ZIKV strains.
Comparison between ZIKV strains reveals only similar mutations (E52D, K69R, A194V, V236I, and V264M) in the β ladder and wing domains far from oligomeric interfaces. Eighty C-terminal residues are responsible for platelet dysfunction and pathogenesis in DENV.26 The ZIKV NS1 C-terminus is 67.1% identical to the DENV-2 region, and this sequence is conserved across all ZIKV strains. These results point to NS1-mediated pathogenesis that somewhat unique to ZIKV yet retained across all strains.
Nonstructural proteins 2B and 3 (NS2B and NS3)
The NS3 performs two vital functions for flavivirus replication: the N-terminal subunit (residues 1–170) contains the viral protease and the C-terminal subunit (180–617) holds the helicase function tethered by a short linker region (170–179) (for superb review see Luo et al.).27 The NS3 protease subunit requires NS2B as a cofactor. Five homology models were considered for ZIKV NS2B-NS3 from DENV, WNV, and MVEV crystal structures. All models predicted the same alignment and positioning of ZIKV NS3 residues (data not shown), and the MVEV template (PDBID 2WV9)28 offered the most sequential coverage (18–616aa) while retaining high identity (66%). The MVEV template lacks residues 68–130 of NS2B and 1–18 of NS3, but structures for these regions were predicted in the supplemental ZIKV homology model. The resulting full-length ZIKV NS3 homology model exhibited high similarity to the MVEV template, namely in the active site and core residues for both subunits (Figure 4(a)). Additionally, the NS2B cofactor for ZIKV is 48% identical and 75% similar to that from the MVEV template (MVEV: 1ATDMWLERAADVSWEAGAAIT21, ZIKV: 48SVDMYIERAGDITSEKDAEVT68).
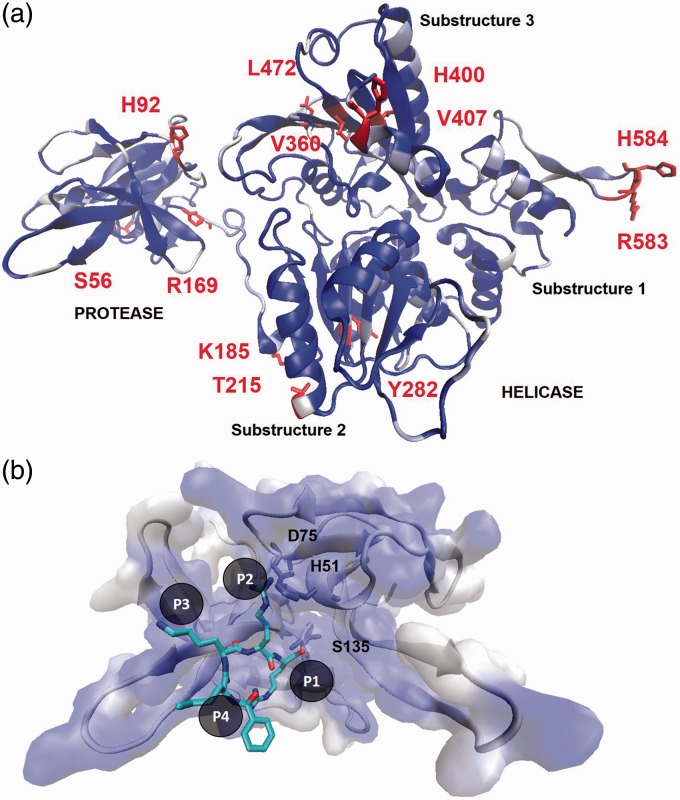
Figure 4.
Predicted structure of ZIKV NS2B–NS3 complex. (a) The full-length ZIKV NS3 homology model with indicated protease and helicase domains. The homology model of the DENV NS2B–NS3 protein was built with the MVEV template (PDBID 2WV9). Dark regions are structurally conserved residues between the model and the MVEV template while white residues are dissimilar. Highlighted are residues that are dissimilar between the African and Asian strains of ZIKV. (b) Comparison of the ZIKV NS3 protease homology model to a DENV-2 NS3 protease crystal structure (PDBID 3U1I) bound to peptidomimetic inhibitor (colored as element). Catalytic residues are indicated and identical binding pocket residues are colored as dark surface.
The protease subunit is responsible for posttranslational cleavage at sites between anchC–prM, NS2B–NS3, NS3–NS4A, NS4A–NS4B, and NS4B–NS5. Due to the essential enzymatic function, the flavivirus NS3 protease is the focus of drug discovery efforts for DENV and WNV. Like FDA-approved HIV-1 and hepatitis C (HCV) protease inhibitors, compounds targeting DENV and WNV protease bind tightly to the active site preventing polyprotein processing. The ZIKV NS3 model retains the serine-protease catalytic triad of H51, D75, and S135 (Figure 4(b)). The model active site possesses four subpockets to accommodate residues at cleavage sites (P1, P2, P3, and P4). The P2 and P3 sites recognize positively charge residues LYS and ARG, and the P1 site binds small or polar substrate residues. Alignment of the ZIKV homology model to the DENV-2 and WNV protease structures shows exact conservation of residues in these subpockets. The high degree of active site identity strongly supports that protease inhibitors for DENV and WNV will also inhibit ZIKV. Conversely, the HCV NS3 is sequentially dissimilar from the ZIKV NS3 model, namely around the active site (SI Figure 1). This observation suggests that, unlike the promise of the DENV and WNV agents, HCV protease inhibitors are unlikely to potently inhibit ZIKV. Comparison of ZIKV strain sequences shows that mutations are typically similar replacements and occur on the surface of the NS3 protease subunit far from the active site (A56S, L92H, K169R, A174T, K215T, N282Y, and R185K). It is then reasonable to expect that NS3 protease inhibitors will be effective for all ZIKV strains.
The NS3 helicase domain can be divided into three substructures. Substructures 1 and 2 are essential for RNA binding and ATP hydrolysis while substructure 3 contains the single-stranded RNA-binding tunnel (Figure 4(a)). Comparing the ZIKV and MVEV structures, the most variation occurs in substructure 3 yet the putative RNA tunnel remains conserved facing the interior of the protein. The ZIKV strains provide nine mutations in the NS3 helicase occurring in each substructure. The mutations in substructure 1 (K583R and Y584H) arise in a disordered, solvent-exposed loop. In substructure 2, the R185K, K215T, and N282Y substitutions also present at the surface far removed from the enzymatic machinery. The mutations N400H and M472L in substructure 3 are near the RNA-binding tunnel. However, these substitutions maintain the same chemical nature and are not expected to greatly affect the behavior of the NS3 helicase including host factor interactions.29 These results demonstrate that the NS3 helicase is highly conserved among flaviviruses with no major variations between ZIKV strains.
Nonstructural protein 4B (NS4B)
The NS4B and p2K proteins anchor the replication complex, interact with multiple host proteins, and evade immune response (for review see Zmurko et al.).30 A topological map for flavivirus NS4B consists of two perimembrane domains (pTM1 and pTM2), three transmembrane domains (TM3, TM4, and TM5), and a cytosolic domain.31 Alignment of the ZIKV sequence to JEV provides the loci for each of the topological domains (SI Figure 2). The N-terminal pTM1 and pTM2 are implicated in interferon antagonism, and these domains are 46% identical and 69% similar between JEV and ZIKV. This result supports that, like other flaviviruses, ZIKV antagonizes interferon signaling using these domains. The cytosolic loop serves as a core for three roles: binding to NS4A, binding to NS3, and suppressing RNAi. The ZIKV cytosolic loop is 50% identical to JEV with a highly conserved N-terminal region suggesting retention of these three roles. Lastly, the TM4 and TM5 regions are associated with oligomerization. These domains are similar to JEV (46% identity and 70% similarity) indicating that ZIKV NS4B likely undergoes dimerization at this site. As NS4B is prone to mutation, this protein serves as a “hotspot” for adaptive modifications attributed to phenotypes observed from other viruses. For example, the T108 residue observed in ZIKV enhances viral RNA replication for DENV,32 and I113 is a Vero cell adaptation mutation acquired in YFV.33 Interestingly, two residues in ZIKV are related to WNV and YFV phenotypes with abrogated neurovirulence (S102 and S204)34 in contrast to the neurological disorders associated with ZIKV infection. The sequences for the five topological domains are exactly conserved among ZIKV strains aside from one mutation in pTM1 and three in TM4. These observations support that the NS4B functions identified in other flaviviruses are retained among all ZIKV strains.
Several compounds have been discovered that inhibit flavivirus replication by modulating NS4B; NITD-618, CCG-3394, and SDM25N.35–38 The residues required for antiviral activity of these compounds are not conserved in ZIKV (Q128, H104, and P164). These results indicate that novel NS4B inhibitors should be pursued for ZIKV.
Nonstructural protein 5 (NS5)
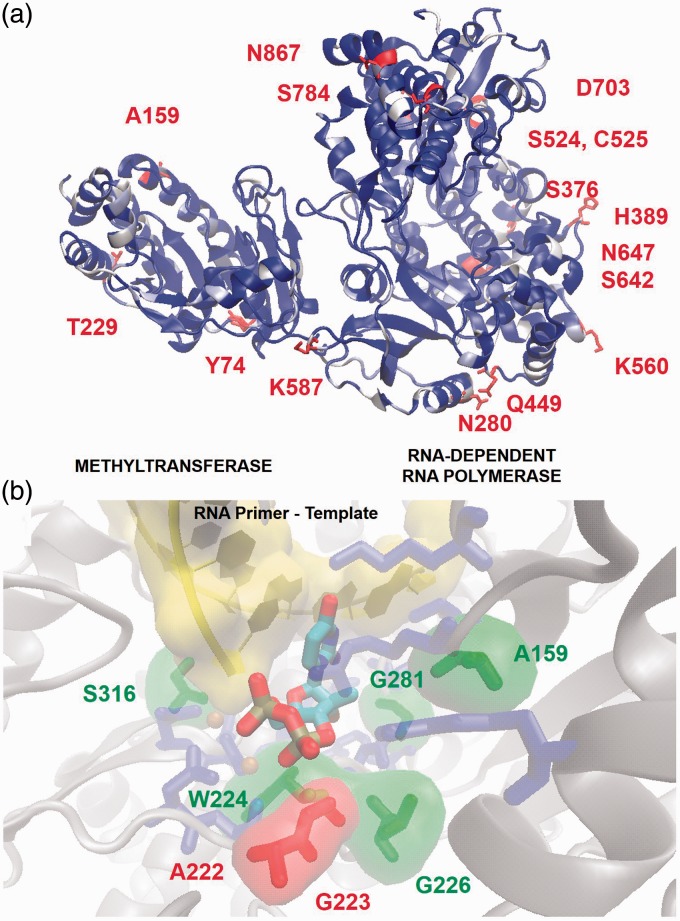
The NS5 protein is the largest encoded in the viral genome spanning 903 residues composed of two subunits essential for replication. The methyltransferase (MTase) occupies the N-terminal 270 residues and is responsible for viral RNA modification (for excellent review on DENV NS5 see Lim et al.).39 The RNA-dependent RNA polymerase (RDRP) domain synthesizes RNA from the viral template, and the interface between these domains regulates both functions. A JEV template (PDBID 4K6M, 68% identical)40 (Figure 5(a)) was chosen over other crystals because it is full-length protein, the sequence at the interdomain interface for ZIKV is nearest to JEV (SI Figure 3) and this structure provides the loop between β-sheet 3 and α-helix 16 in the RDRP active unresolved in other crystals.
Figure 5.
Molecular model of ZIKV NS5 protein. (a) Homology model of full-length ZIKV NS5 derived from JEV structural template (PDBID 4K6M) showing the MTase and RDRP subunits. The ribbons are colored by sequence entropy between ZIKV and the JEV template; dark regions are conserved and white areas are dissimilar. Highlighted residues mutate between ZIKV strains. (b) Comparison of the ZIKV RDRP active site to the HCV(RNA): 2′-fluoro-2′-C-methyl-UTP crystal (PDBID 4WTG). The protein backbone is shown as gray cartoons, the RNA primer template is labeled as cartoons, and the incoming 2′-fluoro-2′-C-methyl-UTP is shown in opaque tubes. Unlabeled dark residues are conserved between HCV and ZIKV, and highlighted residues are biochemically similar and dissimilar substitutions between the viruses.
MTase
The MTase subunit serves two enzymatic functions, GTP-transferase (GTase) and RNA N-7 MTase, to process viral RNA. In order to perform these functions, the MTase possesses binding pockets for GTP and S-adenosine methionine (SAM) substrates flanking a central RNA-binding groove. The ZIKV MTase model is virtually identical to the JEV template. Indeed, the residues in contact with the SAM and GTP substrates are exact for ZIKV, DENV, and JEV (SI Figure 4). Furthermore, the active sites are preserved across all ZIKV strains with minor substitutions at the surface of the subunit (H74Y, T159A, and I229T). The few reported viral MTase inhibitors exhibited low cell permeability, off-target activity, and poor drug-likeness.41–43 Despite this, these compounds are likely potent inhibitors of ZIKV NS5 MTase and may serve as lead candidates in optimization studies.
RDRP
Flavivirus RDRP features a well-characterized right-handed motif consisting of finger, palm, and thumb domains. The hand motif “grips” the nucleic primer templates during 5′ to 3′ polymerization of the RNA primer. The details of flavivirus RDRP structure are available in excellent reviews.44 Comparison of the ZIKV NS5 RDRP to the template JEV shows that the two proteins are highly homologous in all three domains (Figure 5(a)).
Viral RDRP proteins are well established and attractive drug targets. Two strategies are used to inhibit this enzyme. The first strategy involves nonnucleotide inhibitors that allosterically prevent the protein from adopting active conformations. The second strategy is the discovery of nucleotide/nucleoside RDRP inhibitors that bind to the active site and chain terminate the polymerization reaction. To aid in the design of nucleotide analogs, the DENV, JEV, and ZIKV NS5 model were aligned to the HCV-RNA primer/template crystal structure with 2′-fluoro-2′-C-methyl-UTP (the active metabolite of Sofosbuvir) in the active site (Figure 5(b)).45 This analysis revealed that, with the exception of L159A, the active site residues for JEV, DENV, and predicted for ZIKV are identical (SI Table 1). Furthermore, the HCV RDRP active site shares many residues in common with ZIKV around the 2′-OH and nucleobase recognition elements. Minor residue replacements occur between ZIKV and HCV for residues involved in long-distance backbone interactions with the sugar (A159, G226, and G281). The S316 substitution is near the primer nucleotide and plays a role in 3′OH positioning for polymerization. This residue is similar to the C316 observed in HCV suggesting similar orientation of the primer RNA chain in the active site. ZIKV replaces the F224 from HCV with W224, and this residue retains hydrophobic contact with the 4′CH. Major substitutions are observed near the phosphate chain. The A222 and G223 for ZIKV are biochemically dissimilar to the R222 and H223 residues for HCV. These residues orient the β,γ-phosphates, and these changes suggest enzymatic differences between the viruses. Aside from this, all nucleotide recognition elements are intact suggesting that HCV nucleotide analogs may also inhibit ZIKV, but at modulated potency arising from these minor differences.
The substitutions between African and Asian strains occur mostly on the surface of the NS5 protein. The K/R280N, H449Q, and G587K ZIKV mutations occur in the finger region. Two mutations are found in the thumb domain (A784S and D867N). Most substitutions for ZIKV appear on the surface of the palm domain. Flavivirus NS5 modulates host unfolded protein response (UPR) and antagonizes interferon signaling by JAK/STAT inhibition.46 The African and Asian/Brazilian strains may exhibit differential UPR and JAK/STAT interference due to these substitutions in the NS5 palm domain.
Discussion
This study presents and analyzes models of ZIKV proteins to provide insights on the structural biology of this virus as it relates to drug discovery. ZIKV proteins are sequentially similar to viral proteins from other flaviviruses (DENV, MVEV, WNV, and JEV) offering reasonable structural templates. ZIKV diverges from other flaviviruses in the prM, Env, and NS1 proteins suggesting sources of differential pathogenesis. ZIKV shares sequential homology, and presumably structural similarity, with other flaviviruses in the NS3 and NS5 drug-binding pockets. It follows that NS3 and NS5 modulators from DENV, WNV, and JEV should be considered as candidate inhibitors for ZIKV.
The ZIKV African and Asian/Brazilian sequences are > 95% identical, yet only the Asian/Brazilian strains appear to be neurotropic leading to neurological disease. Mutations arise between ZIKV strains in the prM, Env, NS1, NS3, and NS5 proteins—key players in pathogenesis and infectivity. These results direct future work in determining the molecular source of adverse outcomes from ZIKV infection. Taken together, the results presented here aid in the discovery of ZIKV antiviral agents that could be used to prevent and suppress the emerging epidemic and confront the public health crisis.
Supplementary Material
Note
Alignment of all ZIKV polyproteins and all homology models are provided in Supplementary Materials.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by funding from the NIH funded Emory Center for AIDS Research (P30-AI-050408).
References
- 1.Kindhauser MK, Allen T, Frank V, et al. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 2016, pp. 171082, , http://icmr.nic.in/zika/publications/Zika%20the%20origin%20and%20spread%20of%20a%20mosquitoborne%20virus.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucey DR, Gostin LO. The emerging Zika pandemic: enhancing preparedness. JAMA 2016; 315: 865–866. [DOI] [PubMed] [Google Scholar]
- 3.Sirohi D, Chen Z, Sun L, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016; 352: 467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekins S, Liebler J, Neves BJ, et al. Illustrating and homology modeling the proteins of the Zika virus. F1000Research 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWilliam H, Li W, Uludag M, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res 2013; 41: W597–W600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautier R, Douguet D, Antonny B, et al. HELIQUEST: a web server to screen sequences with specific α-helical properties. Bioinformatics 2008; 24: 2101–2102. [DOI] [PubMed] [Google Scholar]
- 8.Biasini M, Bienert S, Waterhouse A, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 2014; 1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph 1996; 14: 33–38. [DOI] [PubMed] [Google Scholar]
- 10.Guy B, Guirakhoo F, Barban V, et al. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010; 28: 632–649. [DOI] [PubMed] [Google Scholar]
- 11.Randolph VB, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 1990; 174: 450–458. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Lok S-M, Yu I-M, et al. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science 2008; 319: 1830–1834. [DOI] [PubMed] [Google Scholar]
- 13.Modis Y, Ogata S, Clements D, et al. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 2005; 79: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henchal EA, Gentry MK, McCown JM, et al. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg 1982; 31: 830–836. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y-Q, Dai J-X, Ji G-H, et al. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein. PLoS One 2011; 6: e16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kampmann T, Yennamalli R, Campbell P, et al. In silico screening of small molecule libraries using the dengue virus envelope E protein has identified compounds with antiviral activity against multiple flaviviruses. Antiviral Res 2009; 84: 234–241. [DOI] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14: 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6: e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers TJ, Halevy M, Nestorowicz A, et al. West Nile virus envelope proteins: nucleotide sequence analysis of strains differing in mouse neuroinvasiveness. J Gen Virol 1998; 79: 2375–2380. [DOI] [PubMed] [Google Scholar]
- 20.Tang H, Hammack C, Ogden SC, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016; 18.5: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson MB, Wang PP, Atabay KD, et al. Single-cell analysis reveals transcriptional heterogeneity of neural progenitors in human cortex. Nat Neurosci 2015; 18: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 2013; 98: 192–208. [DOI] [PubMed] [Google Scholar]
- 23.Akey DL, Brown WC, Dutta S, et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 2014; 343: 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H-J, Lin C-F, Lei H-Y, et al. Proteomic analysis of endothelial cell autoantigens recognized by anti-dengue virus nonstructural protein 1 antibodies. Exp Biol Med 2009; 234: 63–73. [DOI] [PubMed] [Google Scholar]
- 25.Liu I-J, Chiu C-Y, Chen Y-C, et al. Molecular mimicry of human endothelial cell antigen by autoantibodies to nonstructural protein 1 of dengue virus. J Biol Chem 2011; 286: 9726–9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M-C, Lin C-F, Lei H-Y, et al. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J Immunol 2009; 183: 1797–1803. [DOI] [PubMed] [Google Scholar]
- 27.Luo D, Vasudevan SG, Lescar J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antiviral Res 2015; 118: 148–158. [DOI] [PubMed] [Google Scholar]
- 28.Assenberg R, Mastrangelo E, Walter TS, et al. Crystal structure of a novel conformational state of the flavivirus NS3 protein: implications for polyprotein processing and viral replication. J Virol 2009; 83: 12895–12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khadka S, Vangeloff AD, Zhang C, et al. A physical interaction network of dengue virus and human proteins. Mol Cell Proteomics 2011; 10 M111. 012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zmurko J, Neyts J, Dallmeier K, et al. chameleon and jack-in-the-box roles in viral replication and pathogenesis, and a molecular target for antiviral intervention. Rev Med Virol 2015; 25: 205–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller S, Sparacio S, Bartenschlager R. Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. J Biol Chem 2006; 281: 8854–8863. [DOI] [PubMed] [Google Scholar]
- 32.Pletnev AG, Putnak R, Speicher J, et al. West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc Natl Acad Sci 2002; 99: 3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beasley DW, Morin M, Lamb AR, et al. Adaptation of yellow fever virus 17D to Vero cells is associated with mutations in structural and non-structural protein genes. Virus Res 2013; 176: 280–284. [DOI] [PubMed] [Google Scholar]
- 34.Wicker JA, Whiteman MC, Beasley DW, et al. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology 2006; 349: 245–253. [DOI] [PubMed] [Google Scholar]
- 35.Zou G, Puig-Basagoiti F, Zhang B, et al. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology 2009; 384: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie X, Wang Q-Y, Xu HY, et al. Inhibition of dengue virus by targeting viral NS4B protein. J Virol 2011; 85: 11183–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patkar CG, Larsen M, Owston M, et al. Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob Agents Chemother 2009; 53: 4103–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Cleef KW, Overheul GJ, Thomassen MC, et al. Identification of a new dengue virus inhibitor that targets the viral NS4B protein and restricts genomic RNA replication. Antiviral Res 2013; 99: 165–171. [DOI] [PubMed] [Google Scholar]
- 39.Lim SP, Noble CG, Shi P-Y. The dengue virus NS5 protein as a target for drug discovery. Antiviral Res 2015; 119: 57–67. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Y, Soh TS, Zheng J, et al. A crystal structure of the dengue virus NS5 protein reveals a novel inter-domain interface essential for protein flexibility and virus replication. PLoS Pathogens 2015; 11: e1004682–e1004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim SP, Sonntag LS, Noble C, et al. Small molecule inhibitors that selectively block dengue virus methyltransferase. J Biol Chem 2011; 286: 6233–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung KY, Dong H, Chao AT, et al. Higher catalytic efficiency of N-7-methylation is responsible for processive N-7 and 2′-O methyltransferase activity in dengue virus. Virology 2010; 402: 52–60. [DOI] [PubMed] [Google Scholar]
- 43.Brecher M, Chen H, Liu B, et al. Novel broad spectrum inhibitors targeting the flavivirus methyltransferase. PLoS One 2015; 10: e0130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malet H, Massé N, Selisko B, et al. The flavivirus polymerase as a target for drug discovery. Antiviral Res 2008; 80: 23–35. [DOI] [PubMed] [Google Scholar]
- 45.Appleby TC, Perry JK, Murakami E, et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015; 347: 771–775. [DOI] [PubMed] [Google Scholar]
- 46.Ye J, Zhu B, Fu ZF, et al. Immune evasion strategies of flaviviruses. Vaccine 2013; 31: 461–471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.