Abstract
Background
Influenza is a highly contagious viral infection of the respiratory system. To attack two processes involved in flu pathogenesis—viral replication in the infected body and oxidative damages, we studied the combination effect of neuraminidase inhibitor oseltamivir and antioxidant α-tocopherol in experimental model of influenza.
Methods
After inoculation of albino mice with 10 MLD50 (50% mouse lethal dose) of influenza virus A/Aichi/2/68 (H3N2), oseltamivir was applied orally at three doses, 2.5 mg/kg, 1.25 mg/kg, and 0.625 mg/kg, for five days post infection. α-Tocopherol (120 mg/kg, in sunflower oil) was administered intraperitoneally. Three schemes of α-tocopherol five-day course were tested: onset five or two days before infection, or on the virus inoculation day.
Results
Strongly dose-dependent augmented antiviral effect of the combination α-tocopherol and 0.625 mg/kg oseltamivir was demonstrated when α-tocopherol was administered simultaneously with oseltamivir: a pronounced decrease in mortality rate (a 78% protection), and a lengthening of mean survival time by 3.2–4 days. Lung parameters showed a substantial decrease in infectious virus content (Δ logs = 3.8/4.1) and a marked diminishment of lung index and pathology. Combination α-tocopherol with 1.25 mg/kg oseltamivir manifested a marked protective effect, but the effect on lung parameters was less. The combination effect of α-tocopherol with 2.5 mg/kg oseltamivir did not surpass the monotherapeutic effect of oseltamivir. When α-tocopherol was applied in courses starting five or two days before infection, its combination with oseltamivir was ineffective.
Conclusions
Evidently, α-tocopherol could be considered as prospective component of influenza therapy in combination with oseltamivir.
Keywords: Drug combination, influenza, virus, infection, animal model
Introduction
Annual influenza epidemics are associated with complications and huge medical and economical losses. Influenza virus infection attacks the populations of all continents in periodic epidemics and pandemics and often manifests with a severe course, complications, and mortality. Currently, the World Health Organization recommends influenza vaccines as a main tool for prevention1 and effective anti-influenza chemotherapeutics for treatment. Antivirals for influenza are divided into two types, based on their modes of action: (i) the neuraminidase inhibitors oseltamivir, zanamivir, peramivir, and related compounds, and (ii) the protein M2 blockers rimantadine-HCl and amantadine-HCl. Although both types of agents have proven antiviral efficacy, the rate of drug resistance is constantly increasing, especially for M2 blockers.2
The pathogenesis of influenza virus infection is associated with two general processes in the body: (i) local lung damage due to viral replication in the columnar ciliary epithelium of bronchi and bronchioles, which leads to progressive damage of the alveolar cells, bronchopneumonia (viral or combined viral-bacterial), massive bronchitis (including bronchiolitis) as the major causes of death3; (ii) a dramatic inflammatory burst that induces among other processes an increase of reactive oxygen species generation, causing extensive damage to cellular membranes, predominantly in the small vessels, arterioles, and capillaries.4–7 In addition, extrapulmonary complications affect many organs and tissues, such as heart, brain, middle ear, liver and endocrine glands, and occasionally the stomach and kidneys.8–13
Currently, treatment of influenza is directed mainly at targeting the primary pathogenic consequences of virus infection through administration of specific antivirals. Therapy of indirect aspects of pathogenesis aimed at controlling inflammation and oxidative stress has received less attention.
Evidently, a more effective strategy for treatment is needed. Immunomodulators have been successful in treating disease, at least in mice models of influenza infection.14–16 Acting directly on downstream deleterious events of inflammation with antioxidative agents is also of significant importance for influenza therapy.
Among the antioxidants tested in influenza virus infections in mice,17–21 α-tocopherol (vitamin E) occupies the leading position because of its efficacy in preventing oxidative damage through its free-radical scavenging activity.18,21–27
However, researchers have shown that endogenic levels of vitamin E are significantly decreased in lung, liver, and blood plasma during the course of influenza infection.23,24,28 Animal and human studies have demonstrated a negative correlation between endogenous levels of vitamin E and pulmonary inflammation. Vitamin E supplementation has been associated with reducing the severity of symptoms of lung disease.21–23,29
An underappreciated approach to influenza therapy comprises combination administration regimens of specific viral replication inhibitors together with antioxidants. Therefore, investigations on the combination effects of specific anti-influenza chemotherapeutic agents and antioxidants are of special interest. Previously we demonstrated a favorable combination effect of the antioxidant 4-methyl-2,6-ditretbutylphenol (ionol) with M2-blocker rimantadine in mice infected with influenza virus A(H3N2) virus [A. S. Galabov et al. Antivir Res 1994; 23:S(I):140].
In the present work, we studied the combination effect of oseltamivir, which has proven high antiviral efficacy against influenza virus, and α-tocopherol (a component of vitamin E) known as a powerful antioxidant that decreases the effects of oxidative stress, in experimental infection with influenza virus A (H3N2) in albino mice.
Materials and methods
Compounds
Oseltamivir phosphate (the ethyl ester prodrug of oseltamivir) was purchased from Hoffmann-La Roche (Switzerland). The compound was diluted ex tempore in phosphate-buffered saline (PBS) for in vivo experiments.
α-Tocopherol (vitamin E), Sigma Aldrich, was dissolved in sunflower oil for in vivo testing.
Virus
Influenza virus A/Aichi/2/68 (H3N2) was obtained from the D. I. Ivanovsky Institute of Virology, Moscow (Russia), adapted to mice, and then propagated in 10-day-old chicken embryos through serial intra-allantoic passages.
Cells
Madine-Darby canine kidney (MDCK) cells were obtained from ATCC (Manassas, VA, USA) and grown in DMEM (Gibco BRL, Paisley, Scotland, UK) supplemented with 10% fetal bovine serum (Gibco BRL, Paisley, Scotland, UK), 3.7 mg/ml sodium bicarbonate, 10 mM HEPES buffer (AppliChem GmbH, Darmstadt, Germany), 100 IU/ml penicillin, 100 µg/ml streptomycin, and 50 µg/ml gentamicin, in a 5% CO2 incubator (Thermo Scientific 311, Thermo Fisher Scientific, USA).
Mice
White male mice of the ICR line with body weight 12–14 g, obtained from Slivnitza Animal Pharm (Bulgarian Academy of Sciences (BAS) Bulgaria), were placed in specially designed, well-ventilated acrylic cages, with free access to water and food, and maintained in the Animal House facility of the Stephan Angeloff Institute of Microbiology, BAS. During a three-day acclimatisation period (prior to experimental onset), they were observed for any signs of diseases and/or physical abnormalities. Animal husbandry and experiments were conducted in accordance with the guidelines of Bulgaria’s Directorate of Health Prevention and Humane Behaviour toward Animals.
General procedure and experimental groups for antiviral testing in mice
Mice were anesthetized by ether inhalation and were inoculated intranasally with 0.05 ml/mouse of diluted virus containing 10 MLD50. The following experimental groups were formed:
Scheme A
Group A1: α-Tocopherol (dissolved in vegetable oil) was administered individually intraperitoneally at a daily dose of 120 mg/kg for five days post infection. The first dose was administered 2 h before virus inoculation.
Groups A2-4: Oseltamivir (in PBS) at a daily dose (in two intakes) of 2.5 mg/kg (A2), of 1.25 mg/kg (A3), or 0.625 mg/kg (A4) was administered orally over a five-day course following virus inoculation, starting 4 h before infection.
Group A5-7: Combinations of α-tocopherol and oseltamivir—α-tocopherol 120 mg/kg/day intraperitonealy, for five days post virus inoculation, plus oseltamivir at a daily dose (in two intakes) of 2.5 mg/kg given orally over a five-day course following virus inoculation (A5), or plus oseltamivir 1.25 mg/kg (A6), or plus oseltamivir 0.625 mg/kg (A7). The first doses of oseltamivir and α-tocopherol were applied 4 and 2 h before virus inoculation, respectively.
Two placebo groups were set up: placebo vegetable oil (A8) and placebo PBS (A9).
Scheme B
Group B1: α-Tocopherol (dissolved in vegetable oil) was administered individually intraperitoneally once daily at a dose of 120 mg/kg for five days before virus inoculation.
Groups B2-4: Oseltamivir (in PBS) at a daily dose (in two intakes) of 2.5 mg/kg (BA2), of 1.25 mg/kg (B3), or 0.625 mg/kg (B4) was administered orally over a five-day course following virus inoculation.
Group B5-7: Combinations of α-tocopherol and oseltamivir—α-tocopherol 120 mg/kg/day intraperitonealy, for five days before virus inoculation, plus oseltamivir at a daily dose (in two intakes) of 2.5 mg/kg given orally in a five-day course following virus inoculation (B5), or plus oseltamivir 1.25 mg/kg (B6), or plus oseltamivir 0.625 mg/kg (B7).
Two placebo groups were set up: placebo vegetable oil (B8) and placebo PBS (B9).
Scheme C
A similar experimental scheme was formed in which α-tocopherol was given intraperitoneally, individually and in combination with oseltamivir in courses started two days before infection and continued until day 3 post infection. The oseltamivir course was analogous to that in Schemes A and B. Two placebo groups were also formed: vegetable oil- and saline (PBS)-injected animals.
Experimental groups consisted of 10–15 mice each. Two to three repetitions were performed. Parameters of antiviral activity such as protection index (PI), based on cumulative mortality rate, and the mean survival times (MST), were evaluated until day 14 post infection.
Determination of lung parameters: virus titer, lung index and lung pathology surface score
In parallel with the experiments described in the General procedure and experimental groups for antiviral testing in mice section, three infected mice per experimental group were sacrificed at various days of infection; body weight was measured, and lungs were removed and weighed. The lung index was calculated by multiplying the lung weight/body weight ratio by 100 (%). Lung pathology surface (plum-like lung consolidation) score was estimated on a range of 0 (normal lungs) to 4 (maximal hepatization) based on the percentage of damaged lung area with characteristic plum-like coloration.30
In addition, three mice from each experimental group were sacrificed on days 4 and 5 post infection. Under sterile conditions, the lungs were removed, washed three times with PBS, homogenized, and suspended in a total volume of 1 ml PBS (10% w/v suspension). After centrifugation at 2000 r/min for 10 min, the infectious virus titer of the supernatants was determined in MDCK cell monolayers in 96-well plastic microplates (Cellstar, Friekenhausen, Germany) following the end-point dilution design. Twenty-four-hour cell monolayers were inoculated with 100 µl of diluted virus per well (60-min adsorption at room temperature, four wells per dilution). Maintenance medium (200 µl per well) was DMEM containing 5% bovine fetal serum, 3 µg/ml trypsin (Gibco BRL, Paisley, Scotland, UK), 3.7 mg/ml sodium bicarbonate, 10 mM HEPES buffer (AppliChem GmbH, Darmstadt, Germany), and antibiotics. Infectious virus titers were presented in 50% cell culture infectious doses (CCID50)/0.1 ml by recording the viral cytopathic effect under light microscope following 48–72 h of incubation at 37℃.1
Statistical analysis
The MST was calculated as the period from the day of virus inoculation until the day before the animal’s death. Mortality was recorded until day 14. The PI was calculated using the equation PI = [(PC − 1) / PC] × 100, where PC is the protection coefficient: percentage mortality in placebo group/ percentage mortality in the drug-treated group. The number of survivors was compared by Fisher’s exact test. For comparisons of MST and body and lung weights, one-way ANOVA tests with Bonferroni’s post-test were used. Viral titers were compared by two-way ANOVA with Bonferroni’s post-test. The Kruskal–Wallis test, with Dunn’s multiple comparisons post-test, was used to compare estimated lung pathology surface scores. P < 0.05 was considered statistically significant. Standard errors (SE) were calculated for the parameters where applicable. Data were analyzed by Graph Pad Prism® (Version 5.03 for Windows, Graph Pad Software Inc., USA). The character of the combination effect was assessed by isobolographic analysis.31 In the special case of the present study in which one of the two drugs lacks efficacy in producing the effect, the linear isobole is a horizontal line whose intercept is the ED50 (50% effective dose) of the active agent.
Results
Testing the antiviral effect of the combination α-tocopherol and oseltamivir in experimental flu infection in mice
In our previous study in mice infected with influenza virus A (H3N2) using 2 MLD50, it was established that the effect of the α-tocopherol at intraperitoneal doses of 120 mg/kg once daily was equal of the effect of 240 mg/kg and markedly superior than the effect of 60 mg/kg. These data were based on the biochemical markers of oxidative damages in lung, liver, plasma, and stomach of infected animals, as well as on the protection index and lung markers.9,23,24 Thus, the α-tocopherol dose of 120 mg/kg was selected for our present experimental work in mice infected with 10 LMD50. In the present experimental design, which included combinations with oseltamivir, the daily dose of 120 mg/kg α-tocopherol manifested a superior effect over the dose of 240 mg/kg (data not illustrated).
Our current results show that the combination of α-tocopherol (120 mg/kg i.p., once daily) applied simultaneously with oseltamivir demonstrated a well-expressed therapeutic effect in experimental infection in mice with influenza virus A (H3N2) (Table 1, Scheme A). This effect was clearly shown at oseltamivir daily doses of 1.25 mg/kg and 0.625 mg/kg, which are 1/8 and 1/16, respectively, of the drug’s 10 mg/kg optimal treatment dose. Oseltamivir, applied individually, was inactive at the 0.625 mg/kg/day dose and demonstrated a moderate activity at the 1.25 mg/kg/day dose; however, the combination with α-tocopherol and 0.625 mg/kg oseltamivir produced a strong decrease in mortality rate and a protection effect of almost 76%. This combination effect could be characterized as augmentative. The protection effect of the combination 120 mg/kg/day α-tocopherol and 1.25 mg/kg/day oseltamivir (53.2/55.1%) was markedly increased compared to the monotherapeutic effect of oseltamivir at this dose (35.3%).
Table 1.
Combination effect of α-tocopherol and oseltamivir on influenza virus A (H3N2).
| Group N | Treatment, daily doses | n | PI ± SE (%) | MST ± SE | Δ MST |
|---|---|---|---|---|---|
| Scheme A. α-tocopherol 120 mg/kg/day intraperitonealy, five days since virus inoculation. | |||||
| 1A | α-tocopherol 120 mg/kg/day | 24 | 0.0 | 9.0 ± 0.6 | −1.1 |
| 2A | oseltamivir 2.5 mg/kg | 26 | 61.5 ± 11.4a | 12.6 ± 0.2 | +3.3 |
| 3A | oseltamivir 1.25 mg/kg | 23 | 35.3 ± 2.5a | 12.5 ± 0.6 | +3.2 |
| 4A | oseltamivir 0.625 mg/kg | 20 | 0.0 | 10.4 | +1.1 |
| 5A | α-toc 120 mg/kg + osel 2.5 mg/kg | 24 | 51.9 ± 0.1/52.8 ± 11.1a | 12.2 ± 0.4 | +2.1/2.9a |
| 6A | α-toc 120 mg/kg + osel 1.25 mg/kg | 23 | 53.2 ± 3.01/55.1 ± 9.3a | 12.4 ± 1.2 | +2.3/+3.1a |
| 7A | α-toc 120 mg/kg + osel 0.625 mg/kg | 22 | 76.0 ± 12.9/75.9 ± 10.5a | 13.3 ± 0.4 | +3.2/+4.0a |
| 8A | placebo vegetable oil | 36 | 0.0 | 10.1 ± 0.3 | – |
| 9A | placebo PBS | 41 | 0.0 | 9.3 ± 0.9 | – |
| Scheme B. α-tocopherol 120 mg/kg/day, intraperitonealy, five days before virus inoculation. | |||||
| 1A | α-tocopherol 120 mg/kg/day | 22 | 3.9 ± 3.8 | 10.0 ± 1.1 | +1.3 |
| 2A | oseltamivir 2.5 mg/kg | 26 | 61.5 ± 11.4 | 12.6 ± 0.2 | +3.3 |
| 3A | oseltamivir 1.25 mg/kg | 23 | 35.3 ± 2.5 | 12.5 ± 0.6 | +3.2 |
| 4A | oseltamivir 0.625 mg/kg | 20 | 0.0a | 10.4 | +1.1 |
| 5A | α-toc 120 mg/kg + osel 2.5 mg/kg | 24 | 70.3 ± 6.6/60.1 ± 0.5a | 12.6 ± 0.1 | +3.9/ + 3.3a |
| 6A | α-toc 120 mg/kg + osel 1.25 mg/kg | 26 | 23.1/0.0a | 11.0 | +2.3/+1.7a |
| 7A | α-toc 120 mg/kg + osel 0.625 mg/kg | 20 | 0.0/0.0a | 11.2 | +2.5/+1.9a |
| 8A | placebo vegetable oil | 33 | 0.0 | 8.7 ± 1.1 | – |
| 9A | placebo PBS | 41 | 0.0 | 9.3 ± 0.9 | – |
Note: Data are expressed as mean value ± SE. Influenza virus (H3N2) inoculation dose: 10 MLD50 intranasal. Albino mice ICR 12-14 g. Osletamivir (per os): five days treatment course since virus inoculation. PI% = [(PC − 1)/PC] × 100, where PC = lethality in placebo (%)/lethality in treated group (%).
Presented a significant statistical level of P < 0.01 vs group placebo PBS.
The prophylactic five-day course of α-tocopherol starting five days before virus inoculation combined with an oseltamivir course at a daily dose of 2.5 mg/kg did not change significantly the separate effect of the antiviral (Table 1, Scheme B). However, the influence of prophylactic application of α-tocopherol on the individual oseltamivir course at 1.25 mg/kg post virus inoculation was even unfavorable, a decrease of the oseltamivir protection effect been recorded. The courses with 0.625 mg/kg/day oseltamivir, applied with or without α-tocopherol, were inactive.
The combination of a five-day α-tocopherol course, starting 48 h before infection, and 0.625 mg/kg oseltamivir (five-day course applied days 1–5 post infection, i.e. Scheme C (described in the Chapter “Materials and methods”, in the subchapter “General procedure and experimental groups for antiviral testing in mice”)) showed better survival only, compared to placebo groups and monotherapy groups. The combination α-tocopherol and 1.25 mg/kg oseltamivir showed no significant effect (not illustrated).
Lung parameters of the combination effect of α-tocopherol and oseltamivir in experimental flu infection in mice
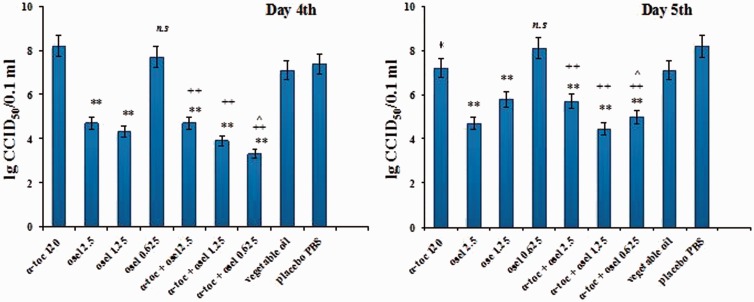
Determination of the virus titer in mouse lungs showed a marked antiviral effect of the simultaneously administered combination of α-tocopherol and oseltamivir (Scheme A) with a significant decrease in infectious virus content in the lungs (Figure 1) (Δ logs = 3.8 and 4.1, compared to placebo sunflower oil and placebo PBS, respectively) for the combination of α-tocopherol and 0.625 mg/kg oseltamivir. The augmentation of the action of the 0.625 mg/kg dose of oseltamivir by α-tocopherol is evident from the comparison with the lung titre for this dose in the absence of α-tocopherol (which was ineffective) and correlates with the protective index (Table 1, Scheme A). The titres for the combinations administered at 2-fold and 4-fold higher doses of oseltamivir were similar to oseltamivir’s monotherapeutic effects (Δ logs = 2.4/2.7 and 3.2/3.5) and showed no evidence of augmentation.
Figure 1.
Lung infection titers recorded on Day 4th and 5th after virus inoculation. Values are expressed as means lg CCID50/0.1 ml ±SEM. **P < 0.001 vs group placebo PBS; *P < 0.05 vs group placebo PBS; ++P < 0.001 vs group α-tocopherol 120 mg/kg; ^P < 0.05 vs group α-tocopherol +oseltamivir 2.5 mg/kg; n.s. Non significant vs group α-tocopherol 120 mg/kg.
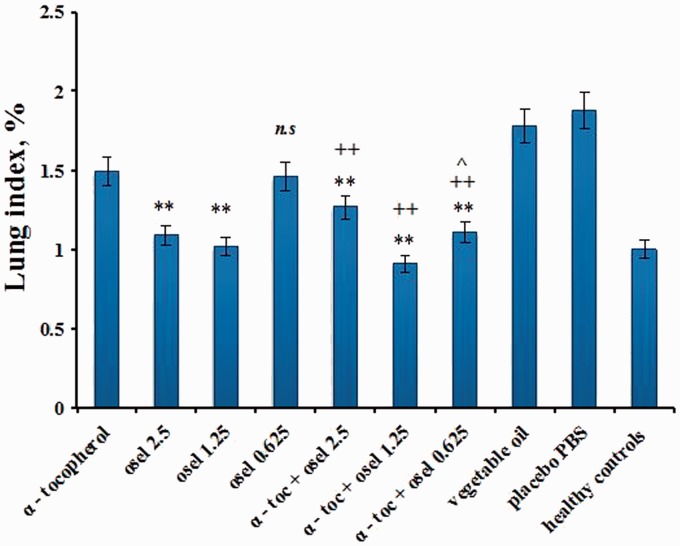
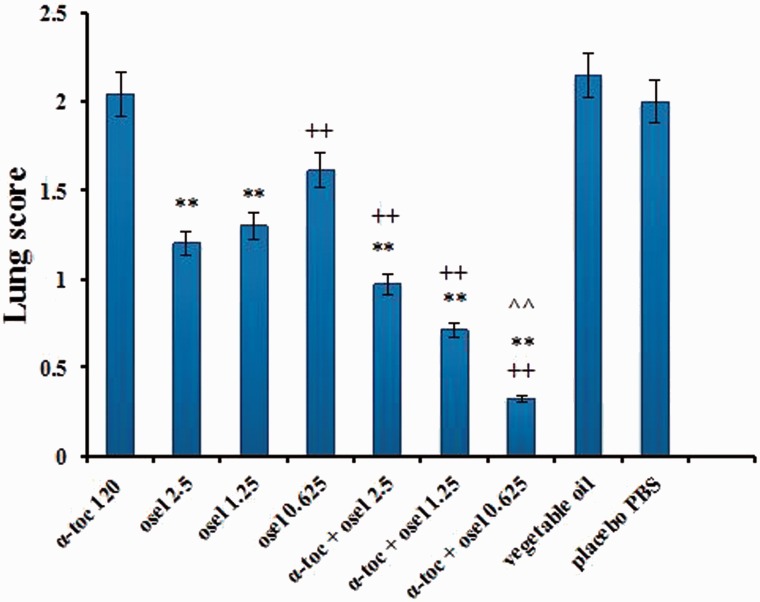
The effects of the various treatment courses on lung indices and lung pathology are presented in Figures 2 and 3, respectively. A markedly decreased lung index was found only for the combination of α-tocopherol and 0.625 mg/kg oseltamivir, as compared to the separate effect of this oseltamivir dose (Figure 2). As seen in Figure 3, the lung pathology was markedly diminished when the combination was applied at oseltamivir doses of 0.625 mg/kg/day and 1.25 mg/kg/day (the latter being optimal).
Figure 2.
Lung index recorded on Day 5th after virus inoculation. Values are expressed as mean lung index (%) ±SE. **P < 0.01 vs group placebo PBS; ++P < 0.001 vs group α-tocopherol 120 mg/kg; ^P < 0.05 vs group α-tocopherol + oseltamivir 2.5 mg/kg; n.s. Non significant vs group α-tocopherol 120 mg/kg.
Figure 3.
Lung pathology surface score on Day 5th after virus inoculation. Values are expressed as mean lung lesions ±SE. **P < 0.01 vs group placebo PBS; ++P < 0.001 vs group α-tocopherol 120 mg/kg; ^^P < 0.001 vs group α-tocopherol + oseltamivir 2.5 mg/kg.
Discussion
The present study convincingly demonstrates a strong beneficial “augmentative” effect of combining α-tocopherol (vitamin E) and oseltamivir in the treatment of experimental infection with influenza virus A/H3N2 in mice. The combination of agents that simultaneously suppress the two main processes in the pathogenesis of influenza-pulmonary lesion development (virus replication and membrane damage in small vessels and in tissues due to oxidative stress) is a prospective therapy for influenza.
The observed augmentation of antiviral activity, expressed by a marked protective effect for the survival of animals infected with influenza A virus, occurred when α-tocopherol was administered simultaneously with oseltamivir phosphate via a five-day course post virus inoculation. However, this effect was not seen when the α-tocopherol course started 120 or 48 h before viral inoculation. The absence of augmention in these cases may be due to the pharmacokinetics of vitamin E in the animal body.32,33
Our previous data, and most of the literature, showed that experimental influenza virus infection causes progressive damage of the alveolar cells and acute inflammatory reaction as well as development of massive bronchitis and probably pneumonia. These occur in parallel with a decrease in the level of endogenous lipid- and water-soluble antioxidants as well as compensatory changes in antioxidant enzyme activity.23,24,29,34
Lung disorders associated with influenza virus infection may be triggered by (i) a massive infiltration of leukocytes, mainly polymorphonuclear leukocytes, into the alveolar space after influenza virus inoculation; (ii) a decrease in partial oxygen pressure and the development of hypoxia; (iii) an increase in partial CO2 pressure and the development of metabolic acidosis; (iv) a release of cytokines, eicosanoids, and prostaglandin E2 and enhanced immune response; or (v) the development of oxidative stress.4,6,18,22
It is well known that antioxidants are able to prevent oxidative damage during the course of influenza virus infection.35,36 Among antioxidants, α-tocopherol merits special attention as a lipid-soluble antioxidant whose major function is to protect membrane polyunsaturated fatty acids against free radical attack (i.e. oxidation caused by reactive oxygen species) by terminating free radical chain reactions.37–39 It also affects inflammatory responses in different tissues, including the lung, not only via direct quenching of oxidative stress,21,22,25,40 but also through modulation of oxidative eicosanoid pathways and prostaglandin synthesis, inhibition of inflammatory mediators, and control of apoptotic lipid signaling.41 A stabilizing role for α-tocopherol in membrane phospholipids has also been reported.39 Several non-antioxidant functions of α-tocopherol may be essential for the maintenance of cell integrity and functions, such as its role as an antiphospholipase A2 agent that stabilizes membrane lipid bilayers against hydrolyzed and oxidized lipids.42
Our present results showed that α-tocopherol applied individually had no significant effect on the course of influenza A virus infection caused by 10 MLD50; the only beneficial result was a lower lung index value. This contrasts Hayek et al.’s data,22 which showed that six-week vitamin E supplementation decreased lung virus titers in mice subsequently infected with influenza virus A (H3N2). In our previous study,23,24 we found some protective effect of α-tocopherol at virus infection with 2 MLD50.
Our results also highlight the clear synergistic character of the antiviral effect of the combination α-tocopherol and 0.625 mg/kg oseltamivir administered simultaneously. This effect manifested a pronounced increase in the protection index, attaining 76% and a lengthening of MST by 3.2–4 days, and strongly influenced lung markers—a marked decrease of the lung infectious virus titer and of the lung index values, as well as a strong reduction in lung lesions. Oseltamivir at the same dose but applied separately did not manifest antiviral activity and only insignificantly reduced lung index and lesions.
The combination of α-tocopherol and oseltamivir at a dose of 1.25 mg/kg/day manifested a markedly lower effect than 0.625 mg/kg oseltamivir, evidenced by a decrease in the protection index and increased viral titres whereas lung index value paradoxically improved. When 2.5 mg/kg oseltamivir was combined with α-tocopherol, no marked differences between the combination and the individual effects of the antiviral were seen.
To explain the observed phenomenon of the strong oseltamivir inverse dose dependence of the combination’s antiviral effect, we considered that the interaction of these two agents is related to their specific mechanisms of action on the viral target structures in the lung. As mentioned previously, α-tocopherol, as a lipid-soluble substance, is a constituent of most cell membranes. There it forms complexes with cell membrane lipid components and protects membrane polyunsaturated fatty acids against free radical attack.37–49
Oseltamivir, for its part, mimics cellular neuraminic (sialic) acid, thus interfering in the release of new progeny virions.43,44 Convincing data suggest that the glycoprotein structures of influenza A virus, neuraminidase and hemagglutinin, are associated with lipid raft in the cell plasma membrane bilayer.45 Thus, they promote the formation of a lipid domain, which is incorporated into the influenza virus envelope during the budding process of viral progeny release from the host cell.45,46
Evidently, α-tocopherol and oseltamivir directed processes run in parallel, opposing the virus infection course on the cellular level. It is probable that the combination of their effects could have a bell-shaped dose–response curve. However, the lack of data on the combinations with lower doses of oseltamivir (<0.625 mg/kg) troubles the categorical answer of such suggestion. Besides, in the combination resultant effect, the influence of α-tocopherol on oseltamivir pharmacokinecs could not be excluded.
Administration routes are another significant side to our experimental design. Applied intraperitoneally, α-tocopherol is quickly absorbed and reaches the tissues earlier, including viral targets in the lung. Administered orally, oseltamivir metabolizes in the liver via drug-metabolizing enzyme systems of the cytochrome P-450 family—the phosphate prodrug is transformed into the antivirally active carboxylate form. According to Oo et al.,47 oseltamivir is metabolized to the carboxylate by the high-capacity carboxylesterase in the liver. After application in humans, the maximum concentration of oseltamivir in plasma quickly declines (half-life of 1–3 h), while the carboxylate levels decline more slowly (half-life of 6–10 h).48
Тhe described augmentation of oseltamivir activity was absent when α-tocopherol was applied for five days before virus inoculation, that is, before onset of the oseltamivir course.
Our results suggest that vitamin E has an important place as a component of the complex therapy of epidemic flu when administered simultaneously with chemotherapeutic agents such as neuraminidase inhibitors. Moreover, vitamin E, in addition to its membrane protective effect in the influenza virus target area, manifests pronounced activities as an antioxidant agent, a protein kinase C inhibitor, and a protector of lung tissue during inflammatory lung illnesses.21,36,40
In summary, vitamin E could be recommended as a reliable component in multitarget flu therapy. These data merit more detailed consideration, while keeping in mind their significance for the treatment of flu as an infection with broad health and social impacts.
Acknowledgements
The authors are grateful to Mrs. Kirilka Todorova for her excellent technical assistance and to Dr. Petya Stoyanova for supplying and housing the experimental animals.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. WHO report on global surveillance of epidemic-prone infectious diseases. WHO/CsR/ISR/2000.1, http://www.who.int/emc (2010).
- 2. World Health Organization. WHO guidelines for pharmacological management of pandemic influenza A (H1N1)2009 and other influenza viruses. Part I. Recommendations. From WHO website. (accessed 4 Feb 2014).
- 3.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Ann Rev Pathol 2008; 3: 499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr 1997; 127: S962–S965. [DOI] [PubMed] [Google Scholar]
- 5.Kuiken T, Riteau B, Fouchier RA, et al. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol 2012; 2: 276–286. [DOI] [PubMed] [Google Scholar]
- 6.Yamada Y, Limmon GV, Zheng D, et al. Major shifts in the spatiotemporal distribution of lung antioxidant enzymes during influenza pneumonia. PLoS ONE 2012; 7: e31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berri F, Lê VB, Jandrot-Perrus M, et al. Switch from protective to adverse inflammation during influenza: viral determinants and hemostasis are caught as culprits. Cell Mol Life Sci 2013; 71: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetverikova LK, Inozemtseva LI. Role of lipid peroxidation in the pathogenesis of influenza and search for antiviral protective agents (in Russian). Vest Ross Akad Med Nauk 1996; 3: 37–40. [PubMed] [Google Scholar]
- 9.Mileva MM, Marazova KA, Galabov AS. Gastric mucosal lesions in influenza virus infected mice: role of gastric lipid peroxidation. Meth & Find in Exper Biol & Med 2003; 25: 521–524. [DOI] [PubMed] [Google Scholar]
- 10.Brydon EWA, Morris SJ, Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev 2005; 29: 837–850. [DOI] [PubMed] [Google Scholar]
- 11.Sood MM, Rigatto C, Zarychanski R, et al. Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian Province. Am J Kidney Dis 2010; 55: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Short KR, Diavatopoulos DA, Thornton R, et al. Influenza virus induces bacterial and nonbacterial otitis media. J Inf Dis 2011; 204: 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papic N, Pangercic A, Vargovic B, et al. Liver involvement during influenza infection. Influenza Resp Viruses 2012; 6: 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berri F, Rimmelzwaan GF, Hansss M, et al. Plasminogen controls inflammation and pathogenesis of influenza virus infections via fibrinolysis. PLoS Pathog 2013; 9: e1003229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoufache K, Berri F, Nacken W, et al. PAR1 contributes to influenza A virus pathogenicity in mice. J Clin Infest 2013; 123: 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lê VB, Schneider JG, Boergeling Y, et al. Platelet activation and aggregation promote lung inflammation and influenza virus 1 pathogenesis. Am J Respir Crit Care Med 2015; 191: 804–819. [DOI] [PubMed] [Google Scholar]
- 17.Sidwell RW, Moss VR, Huffman JH, et al. Inhibition of virus infections of mice by the combination of aerolsolized MnSOD and ribavirin. Antivir Res 1994; 23: 141. [Google Scholar]
- 18.Han S, Meydani S. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J Infect Dis 2000; 182: S74–S80. [DOI] [PubMed] [Google Scholar]
- 19.Mileva MM, Bakalova RB, Zlateva GA, et al. Еffect of rutin and quercetin and the “Oxidative stress” induced by influenza virus infection in mice alveolocytes. In: Proceedings of the second symposium of the International Society of Rare Sugars, Kagawa, 27–29 May 2004, pp.64–69. Takamatsu, Japan: Kagawa University Press, 2005.
- 20.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm & Allergy – Drug Targets 2009; 8: 2–10. [DOI] [PubMed] [Google Scholar]
- 21.Meydani SN, Barklund MP, Liu S, et al. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990; 52: 557–563. [DOI] [PubMed] [Google Scholar]
- 22.Hayek MG, Taylor SF, Bender BS, et al. Vitamin E supplementation decreases lung virus titers in mice infected with influenza. J Infect Dis 1997; 176: 273–276. [DOI] [PubMed] [Google Scholar]
- 23.Mileva M, Tancheva L, Bakalova R, et al. Effect of vitamin E on lipid peroxidation and liver monooxygenase activity in experimental influenza virus infection. Toxicol Lett 2000; 114: 39–45. [DOI] [PubMed] [Google Scholar]
- 24.Mileva MM, Bakalova RA, Tancheva L, et al. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp Immunol Microbiol Infect Dis 2002; 25: 1–11. [DOI] [PubMed] [Google Scholar]
- 25.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress and inflammation. Annu Rev Nutr 2005; 25: 151–174. [DOI] [PubMed] [Google Scholar]
- 26.Niki E, Traber MG. A history of vitamin E. Ann Nut Metab 2012; 61: 207–212. [DOI] [PubMed] [Google Scholar]
- 27.Blaner WS. Vitamin E: enigmatic one!. J Lipid Res 2013; 54: 2293–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tölle A, Schlame M, Charlier N, et al. Vitamin E differentially regulates the expression of peroxiredoxin-1 and -6 in alveolar type II cells. Free Rad Biol Med 2005; 381: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 29.Nencioni L, Sgarbanti R, Amatore D, et al. Intracellular redox signaling as therapeutic target for novel antiviral strategy. Curr Pharm Des 2011; 17: 3898–3904. [DOI] [PubMed] [Google Scholar]
- 30.Sidwell RW, Dixon GJ, Sellers SM, et al. In vivo antiviral properties of biologically active compounds. II. Studies with influenza and vaccinia viruses. Appl Microbiol 1968; 16: 370–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer 2011; 2: 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko K, Kiyose C, Ueda T, et al. Studies on the metabolism of α-tocopherol stereiisomers in rats using [5-methyl-14C]SRR- and RRR-α-tocopherol. J Lipd Res 2000; 41: 357–367. [PubMed] [Google Scholar]
- 33.Traber MG, Leoard SW, Bobe G, et al. α-Tocopherol disappearance rates from plasma depend on lipid concentrations: studies using deuterium-labeled collard greens in younger and older adults. Am J Clin Nutr 2015; 101: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nencioni L, Iuvara A, Aquilano K, et al. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J 2003; 17: 758–760. [DOI] [PubMed] [Google Scholar]
- 35.Kumar P, Khanna M, Srivastava V, et al. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res 2005; 3: 449–459. [DOI] [PubMed] [Google Scholar]
- 36.Schock BC, Van der Vliet A, Corbacho AM, et al. Enhanced inflammatory responses in alpha-tocopherol transfer protein null mice. Arch Biochem Biophys 2004; 423: 162–169. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Quinn PJ. Vitamin E and its function in membranes. Progr Lipid Res 1999; 38: 309–336. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Quinn PJ. The location and function of vitamin E in membranes (Review). Mol Membr Biol 2000; 17: 143–156. [DOI] [PubMed] [Google Scholar]
- 39.Sutken E, Inal M, Ozdemir F. Effects of vitamin E and gemfibrozil on lipid profiles, lipid peroxidation and antioxidant status in the elderly and young hyperlipidemic subjects. Saudi Med J 2006; 27: 453–459. [PubMed] [Google Scholar]
- 40.Rocksen D, Ekstrand-Hammarstrom B, Johansson L, et al. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol 2003; 28: 199–207. [DOI] [PubMed] [Google Scholar]
- 41.Royer MC, Lemaire-Ewing S, Desrumaux C, et al. 7-Ketocholesterol incorporation into sphingolipid/cholesterol-enriched (lipid raft) domains is impaired by vitamin E. A specific role for α-tocopherol with consequences on cell dead. J Biol Chem 2009; 284: 15826–15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagan VE. Tocopherol stabilizers membrane against phospholipases A, free fatty acids and lysophospholipids. Ann N Y Acad Sci 1989; 570: 121–135. [DOI] [PubMed] [Google Scholar]
- 43.Garman E, Laver G. The structure, function, and inhibition of influenza virus neuraminidase. In: Fischer W. (ed). Viral membranes, protein structure, function, and drug design, New York, NY: Kluwer Academic/Plenum Publishers, 2005, pp. 247–267. [Google Scholar]
- 44.Shtyrya YA, Mochalova LV, Bovin NV. Influenza virus neuraminidase: structure and function. Acta Naturae 2009; 1: 26–32. [PMC free article] [PubMed] [Google Scholar]
- 45.Ohkura T, Momose F, Ichikawa R, et al. Influenza A virus hemagglutinin and neuraminidase mutually accelerate their apical targeting through clustering of lipid rafts. J Virol 2014; 88: 10039–10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheiffele P, Rietveld A, Wilk T, et al. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem 1999; 274: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 47.Oo C, Barrett J, Dorr A, et al. Lack of pharmacokinetic interaction between the oral anti-influenza prodrug oseltamivir and aspirin. Antimicrob Agents Chemother 2002; 46: 1993–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet 1999; 37: 471–484. [DOI] [PubMed] [Google Scholar]