Short abstract
Background
Previously, we established a reporter cell line for human cytomegalovirus and screened anti-human cytomegalovirus compounds using the cell line. In this study, we characterized one of the identified compounds, 2,4-diamino-6–(4-methoxyphenyl)pyrimidine (coded as 35C10).
Methods
50% Effective concentrations (EC50s) and 50% cytotoxic concentrations (CC50s) of 35C10 and its derivatives in human fibroblasts were determined by X-gal staining of the cells infected with human cytomegalovirus Towne strain expressing β-galactosidase.
Results
EC50 and CC50 of 35C10 were 4.3 µM and >200 µM, respectively. Among several 35C10 derivatives, only one lacking 4-amino group of pyrimidine showed a similar EC50. 35C10 weakly inhibited murine cytomegalovirus, herpes simplex virus type 1, and varicella-zoster virus. A “time of addition” experiment suggested that 35C10 inhibited an early phase of the infection. Although 35C10 did not inhibit viral attachment to the cells nor the delivery of viral DNA to the nuclei, it decreased the number of infected cells expressing immediate-early 1 and 2 (IE1/IE2) proteins. 35C10 also inhibited the activation of a promoter for TRL4 in the reporter cells upon human cytomegalovirus infection, but not in the same reporter cells transfected with a plasmid expressing IE2.
Conclusion
Our findings suggest that 35C10 is a novel compound that inhibits IE gene expression in human cytomegalovirus-infected cells.
Keywords: Cytomegalovirus, compounds, gene expression, BrdU
Introduction
Human cytomegalovirus (HCMV) is the most common cause of congenital virus infection and is associated with significant morbidity in immunocompromised individuals, including transplant patients and other immunosuppressed patients.1 Although currently available drugs, such as ganciclovir (GCV), are effective in the treatment of HCMV-associated diseases, their side effects, such as neutropenia and thrombocytopenia in the case of GCV, and the development of resistant strains during long-term usage, have necessitated a search for alternative compounds.2–4 Although several new types of therapeutic compounds have been investigated, with some of them subsequently evaluated in phase 2 and 3 clinical trials, no alternative to GCV except for letermovir has been established to date.5–9
To screen and evaluate novel antiviral compounds more easily, we previously established a reporter cell line that produces luciferase upon HCMV infection.10 Using the cell line, we screened a library of 9600 diverse compounds and identified several compounds that showed anti-HCMV activity. Among the identified compounds, 1–(3,5-dichloro-4-pyridyl)piperidine-4-carboxamide (coded as DPPC) was previously characterized as a unique compound that targets the very early phase of HCMV infection, probably by disrupting a pathway that is important after viral entry but before immediate-early (IE) gene expression. In this study, we characterized another compound, 2,4-diamino-6–(4-methoxyphenyl)pyrimidine (coded as 35C10) and found that this compound inhibited IE gene expression.
Materials and methods
Cells and viruses
Human embryonic lung fibroblasts(HEL, kindly provided by I. Kosugi), NIH3T3 (ATCC CRL-1658), U4C (the HCMV reporter cell line established from U373MG cells),10 and HEK293 (ATCC CRL-1573) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (ThermoFisher Scientific). Vero (ATCC CCL-81) cells were grown in DMEM supplemented with 5% FBS and the same antibiotics, and guinea pig lung fibroblasts (GPL, ATCC CCL-158) were grown in F12 medium (ThermoFisher Scientific) supplemented with 10% FBS. A telomerase-immortalized human fibroblast cell line, hTERT-BJ1 (Invitrogen, Carlsbad, SF, USA), was grown in DMEM:199 (4:1) supplemented with 10% FBS and infected with HCMV Towne or with β-galactosidase-expressing Towne strain RC256.11 Murine CMV (MCMV) Smith strain, herpes simplex virus type 1 (HSV-1) F strain and varicella-zoster virus (VZV) vaccine Oka (V-Oka) strain were also used for the evaluation of the compounds.
Chemicals
35C10 is a compound identified during the screening of 9600 random compounds purchased from Maybridge (Fisher Scientific, Pittsburgh, PA, USA). Its derivatives were selected from a chemical database listing more than nine million commercially available compounds (ChemCupid, Namiki Shoji Co., Japan), purchased from the suppliers indicated in Figure 1(d) through Namiki Shoji Co. and dissolved in dimethyl sulfoxide (DMSO) (Wako, Japan).
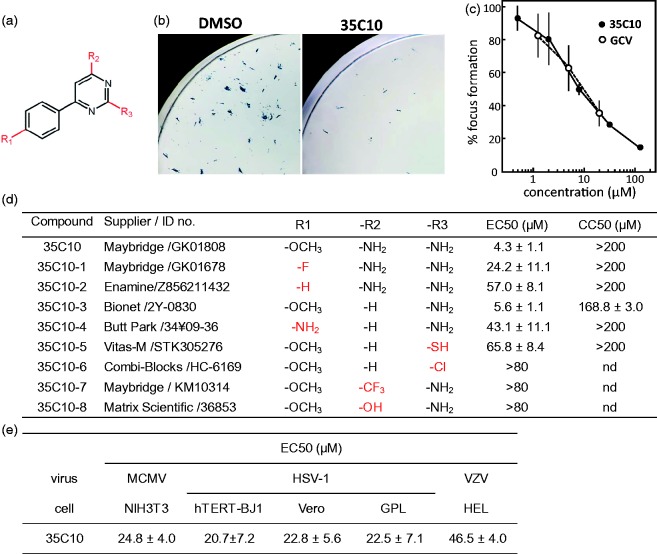
Figure 1.
Antiviral activities of 35C10 and its derivatives. (a) Chemical structure of 35C10 derivatives. (b) Examples of HCMV RC256-infected foci detection by X-gal staining. The images obtained six days after infection of hTERT-BJ1 cells with 150 PFU of RC256 per well in 24-well plates in the presence of DMSO (vehicle) and 10 µM 35C10 are shown. (c) Dose response curves of 35C10 (closed circles) and GCV (open circles) for the focus formation are shown. (d) EC50s against HCMV RC256 and CC50s of 35C10 and its derivatives in hTERT-BJ1 cells. The suppliers and ID numbers of the compounds are also listed. (e) EC50s of 35C10 against MCMV, HSV-1 and VZV in the indicated cells.
X-gal staining of HCMV RC256-infected cells and immunological assays
Immunostaining of HCMV-infected cells, immunofluorescent assay (IFA) and immunoblotting using anti-HCMV IE1/2 monoclonal antibody Mab810 (MerckMillipore, Billerica, MA, USA) as well as X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) staining were performed as described previously.10,12
Preparation and detection of BrdU-labeled HCMV
Bromo-deoxyuridine (BrdU)-labeled HCMV was prepared as described previously13 with some modifications. Briefly, hTERT-BJ1 cells were infected with HCMV RC256 at a multiplicity of infection (MOI) of 0.4. BrdU (Sigma-Aldrich Corp., St. Louis, MO, USA) was added to the culture at a final concentration of 10 µM at two days after infection. The cells were cultured for three days, and the culture medium was then replaced with fresh medium containing 10 µM BrdU. After culturing for one additional day, culture supernatants were harvested, and virus particles were precipitated through a 20% sucrose cushion by ultracentrifugation at 70,000g for 2 h, and then suspended in a small volume of phosphate-buffered saline (PBS). hTERT-BJ1 cells were infected with the BrdU-labeled viruses in eight-well chamber slides (Nalge Nunc, Penfield, NY, USA), 6 h later the culture supernatants were removed, and the cells were rinsed several times with PBS. After fixation of the cells on a slide glass with acetone for 5 min, the cells were treated with 4 M HCl for 10 min, rinsed with PBS, and reacted with anti-BrdU antibody (3D4, Becton Dickinson, Franklin Lakes, NJ, USA) for 1 h, with FITC-conjugated anti-mouse IgG (Dako, Agilent, Santa Clara, CA, USA) for 30 min, and finally with DAPI for 10 min.
Reporter cell assay
Subconfluent U4C cells in 96-well plates were infected with HCMV or transfected with pcDNA-IE2 using FuGENE HD (Promega, Madision, WI, USA). After the indicated number of hours, their luciferase activities were measured by chemiluminescence assay (One-Glo luciferase assay system; Promega) followed by measurement of relative light units (RLU) with a luminometer (GloMax; Promega).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 5 (Graph pad). Statistical significance between conditions was calculated using the Student’s t-test, and values of p < 0.05 were considered significant.
Results
Antiviral activities of 35C10 and its derivatives against HCMV
In this study, we characterized one of the compounds identified in our previous study,10 2,4-diamino-6–(4-methoxyphenyl)pyrimidine (coded as 35C10) (Figure 1(a)). X-gal staining of cells infected with HCMV RC256, β-galactosidase-expressing Towne strain, in the presence of 35C10, demonstrated the decrease in the number of X-gal-positive foci at six days after infection (Figure 1(b)). In a focus reduction assay using X-gal staining, 35C10 against HCMV RC256 showed a dose response similar to GCV (Figure 1(c)). In the assay, the 50% effective concentration (EC50) of 35C10 against HCMV RC256 in hTERT-BJ1 cells was 4.3 ± 1.1 µM, and the 50% cytotoxic concentration (CC50) of 35C10 was >200 µM (Figure 1(d)).
The EC50s and CC50s of eight commercially available derivatives of 35C10 were also obtained (Figure 1(d)). Only 35C10–3, which lacks an amino group at the R2 position, showed an EC50 similar to that of 35C10. Further, substitutions of the methoxy group at the R1 position and of the amino group at the R3 position either decreased or abolished the anti-HCMV activity.
Antiviral activities of 35C10 against other herpesviruses
35C10 inhibited not only HCMV but also MCMV and HSV-1. The EC50s against MCMV in NIH3T3 cells and against HSV-1 in Vero and GPL cells were around 20–25 µM. In addition, 35C10 weakly inhibited VZV in HEL cells (Figure 1(e)). 35C10–3 was also effective against MCMV and HSV-1 at 40 µM (data not shown).
Inhibition of 35C10 at very early phase of infection
hTERT-BJ1 cells were infected with HCMV RC256, and the effect of “time of addition” of 35C10 on viral growth was analyzed (Figure 2(a)). Since the focus reduction assay allows multiple infection cycles, we used concentration (20 µM) of 35C10 higher than EC50 to see the difference in antiviral effects caused by the delay of chemical treatment in the first cycle. 35C10 was more effective when it was applied at the earlier phase (<4 h) of infection. However, in a preliminary experiment done prior to the experiment shown in Figure 2(a), pretreatment of the cells for 2 h prior to infection did not increase the inhibitory effect of 35C10 (data not shown). Importantly, the addition of 35C10 at 4 h or at 6 h after infection partially inhibited HCMV infection, suggesting that 35C10 inhibits infection soon after viral entry.
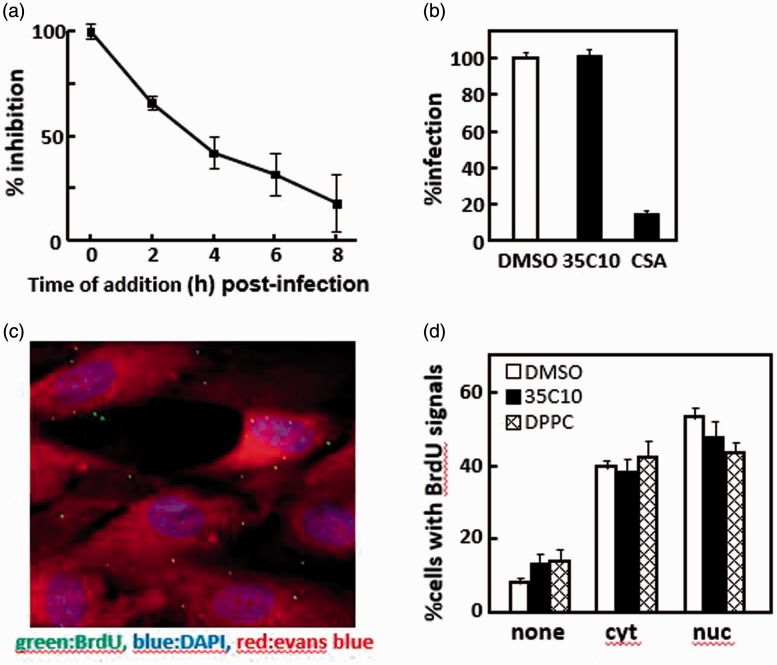
Figure 2.
Identification of infection phase targeted by 35C10. (a) hTERT-BJ1 cells were infected with 100 PFU of RC256 per well in 24-well plates, and 2 h later the inocula were replaced with fresh medium. 35C10 (final concentration 20 μM) was added to the cultures at the indicated time points. Seventy-two hours later, the infected cells were detected by X-gal staining. Inhibitory effects of the addition of 35C10 at the indicated time points are shown as % inhibition, based on the inhibitory effect at 0 h as 100% standard. The means and standard deviations of triplicated wells are shown. (b) In the presence of DMSO, 40 μM 35C10, and 1 mg/ml chondoroitin sulfate A (CSA), respectively, hTERT-BJ1 cells were infected with ∼500 PFU of RC256 per well at 4°C. Two hours later, the inocula were removed, pre-warmed fresh culture medium without any inhibitors were added, and the cells were cultured at 37°C for 72 h. HCMV infection was detected by X-gal staining. The number of X-gal-positive foci infected in the presence of DMSO was used as 100% standard. Means and standard deviations of triplicated wells are shown. (c, d) hTERT-BJ1 cells were infected with BrdU-labeled HCMV RC256 at an MOI of 1 in the presence of DMSO, 40 μM 35C10, and 40 μM DPPC, respectively, and 1 h later the inoculum was replaced with fresh medium containing each inhibitor. The cells were cultured for an additional 5 h, and then analyzed by IFA with anti-BrdU antibody. The cells were counterstained with DAPI (blue) and Evans blue (red). An example of one of the obtained images is shown (c). The cells were classified into one of three categories: with no BrdU signals (none), with signals only in the cytoplasm (cyt), and with signals both in the cytoplasm and nuclei (nuc). Thirty images containing 8–20 cells per image were analyzed and the relative ratios of the cells in each of the three categories for each set of conditions are shown (d).
No inhibition of viral attachment to cells by 35C10
To confirm that 35C10 has no effect on viral attachment to cells, hTERT-BJ1 cells were infected with RC256 at 4°C for 2 h in the presence of chondroitin sulfate A (CSA), a well-known inhibitor of virion attachment14 or 35C10. As shown in Figure 2(b), CSA but not 35C10 inhibited infection (p < 0.001), suggesting that 35C10 has no inhibitory effect on viral attachment.
No inhibition of any process from penetration to nuclear delivery of viral DNA by 35C10
To examine whether 35C10 inhibits any process from viral penetration to the nuclear delivery of viral DNA, BrdU-labeled infectious HCMV stocks were prepared, and the cells were infected with the stock at an MOI of 1 in the presence of DMSO, 35C10, or DPPC. The localization of BrdU-labeled viral DNA in more than 400 cells in the presence of each compound was determined using anti-BrdU antibody. Thirty images containing 8–20 cells (e.g. Figure 2(c)) were analyzed for each set of conditions, and each cell was classified into one of three categories: cells with no BrdU signals, those with signals only in the cytoplasm (cyt), and those with signals both in the cytoplasm and nuclei (nuc). As shown in Figure 2(d), 85–90% of the cells were infected with the BrdU-labeled virus and about 60% of the infected cells contained at least one viral genome in the nuclei. However, there was no difference in the efficiency of the nuclear delivery of viral DNA, indicating that both 35C10 and DPPC have no effect on any process from viral penetration to the nuclear delivery of viral DNA.
Inhibition of IE1/IE2 gene expression by 35C10
Next, we analyzed the expression of IE gene products, IE1 and IE2, by IFA using a monoclonal antibody against IE1 and IE2. As shown in Figure 3(a) and (b), the number of IE1/IE2-positive cells treated with 35C10 was about a half that of the cells treated with DMSO, which was a statistically significant decrease (p < 0.001). In an immunoblotting analysis using the same monoclonal antibody, the amounts of IE1 and IE2 in the cells treated with 35C10 was also less than half those in the cells treated with DMSO (Figure 3(c)). Since infection was done at a low MOI, the IE1 signals were weak, as expected.10
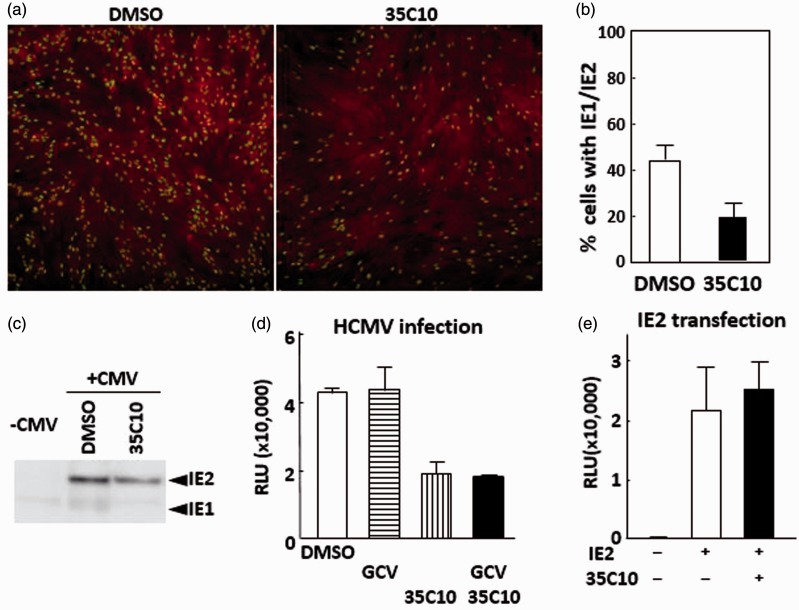
Figure 3.
Inhibition of IE gene expression by 35C10. (a, b) hTERT-BJ1 cells were infected with RC256 at an MOI of 0.4 in the presence of DMSO or of 40 µM 35C10, and 24 h later, the cells were fixed and analyzed for expression of IE1/2 in IFA. Several picture images were taken for the cells treated with DMSO and with 35C10, respectively. Examples of the images are shown in (a). Total cell numbers and IE1/2-positive cell numbers in each image were counted, and the percentages of IE1/2-positive cells in each image were obtained. More than 1000 cells were present in eight picture images for each condition. Means and SDs of the percentage of IE1/2-positive cells in each image are shown in panel b. (c) Detection of IE2 in an immunoblotting analysis using lysates of cells infected with RC256 at an MOI of 0.3 in the absence and presence of 35C10. (d) HCMV reporter U4C cells were infected with RC256 at an MOI of 0.4, cultured for 16 h in the presence of DMSO, 20 µM GCV, 40 µM 35C10, or 20 µM GCV plus 40 µM 35C10. Means and SDs of RLUs obtained from triplicated wells are shown. (e) HEK293 cells in 96-well plates were transfected with 100 ng of pcDNA-IE2, a plasmid encoding IE2, along with 100 ng of pGL-CMV112p, a reporter plasmid encoding luciferase gene under the control of the HCMV early gene UL112 promoter, in the presence of DMSO or 40 µM 35C10, cultured for 42 h, and luciferase activities of the cells were measured. Means and SDs of RLUs obtained from triplicated wells are shown.
Inhibition of IE2-dependent transactivation in HCMV-infected cells
To examine whether the observed decrease of IE1/IE2 in the infected cells by 35C10 is sufficient to account for the inhibition of viral growth, the inhibition of IE2-dependent activation of the TRL4 early gene promoter by 35C10 was measured in the HCMV reporter cells. As shown in Figure 3(d), 35C10 decreased luciferase activity, i.e. IE2-dependent early gene promoter activation, significantly, already at 12 h after infection. To confirm that the inhibition was not due to decreased replication of viral DNA, in other words, a decrease in the amount of template DNA, the same experiment was performed in the presence of GCV. As shown in Figure 3(d), 35C10 was effective even in the presence of GCV (p < 0.01), suggesting that 35C10 decreases the IE2 product or its capacity for transactivation in the infected cells.
No inhibition of IE2-dependent transactivation in cells transfected transiently with IE2
To confirm that the observed decrease in the infected reporter cells was not due to inhibition of IE2-dependent transactivation itself by 35C10, HEK293 cells were transfected with a plasmid encoding IE2 and a reporter plasmid pGL-CMV112p. As shown in Figure 3(e), 35C10 did not decrease the luciferase activity in the context of transient transfection, suggesting that the compound does not target the transactivation function or degradation of IE2.
Discussion
In this study, we demonstrated that 35C10 inhibited infection of α- and β-herpesviruses and decreased HCMV IE1/IE2 expression, but did not affect any process from viral attachment to the nuclear delivery of viral DNA. We reported previously that DPPC inhibited a process from penetration to IE gene expression,10 but here we demonstrated that it had no effect on the nuclear delivery of viral DNA, suggesting that 35C10 has an inhibitory mechanism similar to DPPC. It is likely that 35C10 inhibits IE gene expression. As the IE gene products play a key role in the subsequent processes for efficient viral growth, including early gene expression, viral DNA replication and structural assembly, and as inhibitors of IE gene expression have targets that obviously differ from those of nucleoside analogs, identification and characterization of such inhibitors may be useful for the future development of novel anti-HCMV drugs.
A few anti-HCMV compounds that inhibit the expression of IE proteins have been reported; for example, inhibitors against cyclin-dependent kinases (CDKs), such as roscovitine, inhibit infection of herpesviruses, including HCMV.15 Screening of CDK inhibitors that have greater specificity for HCMV infection and lower cellular toxicity led to the identification of CDK7 inhibitor LDC4297 and CDK9 inhibitor FIT-039.16,17 OG-L002, a LSD1 inhibitor that catalyzes the demethylation of lysine 9 (K9) site of histone H3, OSMI-1, which inhibits O-GlcNAc transferase, and SP600125, a JNK inhibitor, also inhibit HCMV IE gene expression.18–20
Preliminary structure–activity relationship analysis in this study suggests that flexibility of substituents is in the order of R1 > R2, R3. The methoxy group at the R1 position showed the highest activity, although this activity was dependent on R2 and R3. It seems that the strength of the negative elements (F > OCH3>NH2≫H), electron-donating (OCH3, NH2, F) and bulkiness (OCH3 > NH2 > F > H), but not the basic moiety (NH2), at the R1 position helped the activity. However, the bulky trifluoromethyl and acidic hydroxyl groups at the R2 seem to abolish the activity. Therefore, further studies are required to identify better candidates.
In conclusion, 35C10 is a novel compound that appears to inhibit IE gene expression. As different mechanisms for the inhibition of IE gene expression have been reported for some compounds and as there is no structural similarity between 35C10 and these compounds, further studies are required to elucidate the precise inhibitory mechanism of 35C10.
Acknowledgements
We thank Yoshiko Fukui and Mihoko Tsuda for their technical assistance.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research C (25460578) to NI.
References
- 1.Pass RF. Cytomegalovirus In: Knipe DM andHowley PM (eds) Fields virology. Philadelphia, PA: Lippincott Williams & Wilkins, 2001, pp.2675–2705. [Google Scholar]
- 2.Chou S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr Opin Infect Dis 2015; 28: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Göhring K Hamprecht K andJahn G.. Antiviral drug- and multidrug resistance in cytomegalovirus infected SCT patients. Comput Struct Biotechnol J 2015; 13: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert C andBoivin G.. New reporter cell line to evaluate the sequential emergence of multiple human cytomegalovirus mutations during in vitro drug exposure. Antimicrob Agents Chemother 2005; 49: 4860–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mercorelli B, Sinigalia E, Loregian A, et al. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol 2008; 18: 177–210. [DOI] [PubMed] [Google Scholar]
- 6.Prichard MN andKern ER.. The search for new therapies for human cytomegalovirus infections. Virus Res 2011; 157: 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemaly RF, Ullmann AJ, Stoelben S, et al. Protocol – Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med 2014; 370: 1781–1789. [DOI] [PubMed] [Google Scholar]
- 8.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med 2013; 13369: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 9.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 2011; 11: 284–292. [DOI] [PubMed] [Google Scholar]
- 10.Fukui Y, Shindoh K, Yamamoto Y, et al. Establishment of a cell-based assay for screening of compounds inhibiting very early events in the cytomegalovirus replication cycle and characterization of a compound identified using the assay. Antimicrob Agents Chemother 2008; 52: 2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaete RR andMocarski ES.. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A 1987; 84: 7213–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GQ, Suzutani T, Yamamoto Y, et al. Generation of a reporter cell line for detection of infectious varicella-zoster virus and its application to antiviral studies. Antimicrob Agents Chemother 2006; 50: 3142–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenke K andFortunato EA.. Bromodeoxyuridine-labeled viral particles as a tool for visualization of the immediate-early events of human cytomegalovirus infection. J Virol 2004; 78: 7818–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Caro A, Perola E, Bartolini B, et al. Fractions of chemically oversulphated galactosaminoglycan sulphates inhibit three enveloped viruses: human immunodeficiency virus type 1, herpes simplex virus type 1 and human cytomegalovirus. Antivir Chem Chemother 1999; 10: 33–38. [DOI] [PubMed] [Google Scholar]
- 15.Schang LM. Cyclin-dependent kinases as cellular targets for antiviral drugs. J Antimicrob Chemother 2002; 50: 779–792. [DOI] [PubMed] [Google Scholar]
- 16.Hutterer C, Eickhoff J, Milbradt J, et al. A novel CDK7 inhibitor of the pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrob Agents Chemother 2015; 59: 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Onogi H, Kii I, et al. CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J Clin Invest 2014; 124: 3479–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Quenelle D, Vogel JL, et al. A novel selective LSD1/KDM1A inhibitor epigenetically blocks herpes simplex virus lytic replication and reactivation from latency. MBio 2013; 4: e00558–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelova M, Ortiz-Meoz RF, Walker S, et al. Inhibition of O-linked N-acetylglucosamine transferase reduces replication of herpes simplex virus and human cytomegalovirus. J Virol 2015; 89: 8474–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Niu X, Qian Z, et al. The c-Jun N-terminal kinase inhibitor SP600125 inhibits human cytomegalovirus replication. J Med Virol 2015; 87: 2135–2144. [DOI] [PubMed] [Google Scholar]