Abstract
This report details two novel RAB3GAP1 mutations causing Warburg Micro syndrome, a rare autosomal recessive disorder characterized by multiple organ abnormalities involving the ocular, nervous, and endocrine systems. Two Italian sisters were referred to our department for the assessment of congenital bilateral cataracts. They also presented with microphthalmia, postnatal microcephaly, severe developmental delay, and hypotony. Perinatal investigations were negative for any toxins or infectious diseases during pregnancy, including toxoplasmosis, rubella, cytomegalovirus, and herpes virus. Genetic tests were performed on samples from probands and their parents, targeting a total of 114 genes. After sequence analysis of RAB3GAP1, two heterozygous changes were identified in both sisters: C.519G>A, p.(Trp173Ter) and c.2486T>A, p.(Leu829Ter). The identified mutations have not previously been described in the literature, but they affect critical regions of the gene, suggesting a legitimate causal relationship between the genetic alterations and the clinical features of the patients.
Keywords: Congenital cataracts, gene mutation, microphthalmia, RAB3GAP1, Warburg micro syndrome
INTRODUCTION
Warburg Micro syndrome is a rare recessive genetic disorder.[1] Affected patients generally present congenital bilateral cataracts, small atonic pupils, microphthalmia, microcornea, postnatal microcephaly, and severe developmental delay. To date, four genes have been associated with Warburg Micro syndrome: RAB3GAP1,[2] RAB3GAP2,[3] RAB18,[4] and TBC1D20.[5] These genes encode Ras-associated binding (RAB) proteins and regulators, which are involved in vesicle trafficking, axonal transport, synaptic transmission, and autophagy. We report two cases of Warburg Micro syndrome in siblings in which we identified a compound heterozygous mutation of RAB3GAP1 that has not been previously described in the literature.
CASE REPORT
The first child of a nonconsanguineous couple, born at full term after a normal pregnancy, was referred to us because of congenital cataracts and microphthalmia. She underwent cataract surgeries at 1 and 3 months of age. Postoperative strabismus and esotropia of the left eye were treated with contact lens and occlusion. She developed global developmental delay and postnatal microcephaly. She had axial and limb hypotony, with poor straightening and vertical control of the head. Brain magnetic resonance imaging showed enlarged lateral and third ventricles and hypoplasia of the corpus callosum. The child underwent visual and physical rehabilitation showing mild improvements. She is now 21 months old. She can maintain a sitting position and pick up objects. Her inferior limbs are hypertonic [Figure 1a]. Her sister, who is a year younger, was also born with congenital bilateral cataracts and shares the same phenotype [Figure 1b].
Figure 1.
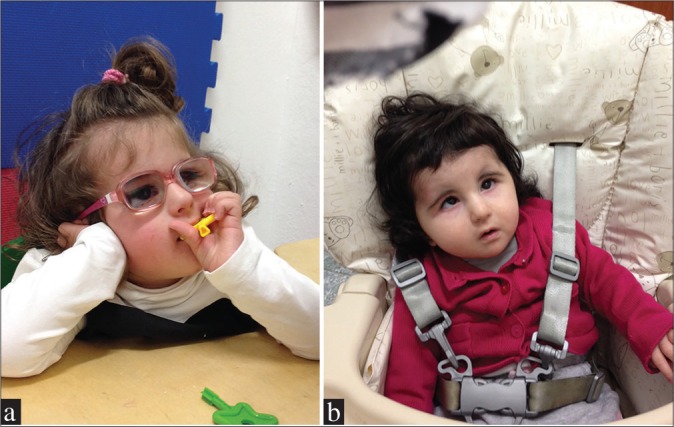
Two sisters affected by Warburg Micro syndrome. (a) The older one has developmental delay and postnatal microcephaly. She underwent bilateral cataract surgery. At the age of 21 months, she was able to maintain the sitting position and to pick up objects. (b) The younger one affected by Warburg Micro syndrome was born with bilateral congenital cataracts. She shares the same genotype and phenotype with her older sister
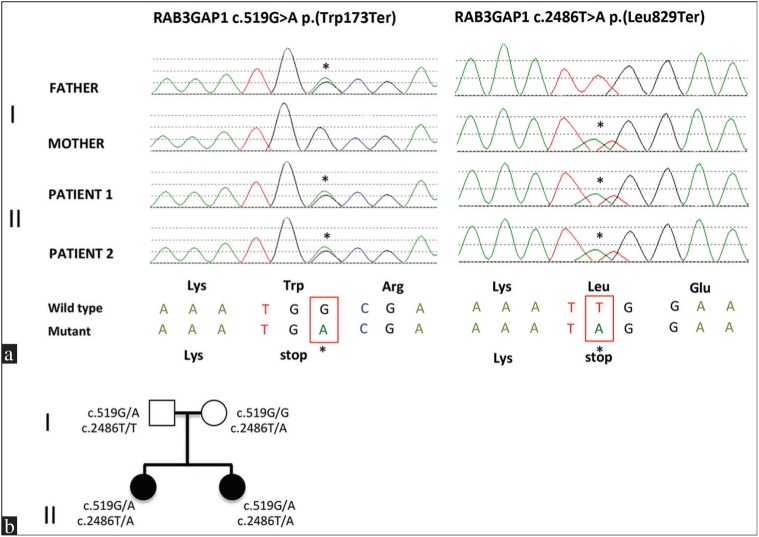
The Ethical Review Board of the University of Pisa, Italy, approved this study. After obtaining written informed consent, genetic analysis was performed on blood samples from probands and their parents. A total of 114 genes [Supplementary Table S1] were targeted using the Agilent SureSelect Custom Target enrichment kit (Congenital Cataract v2) and sequenced on the HiSeq 2500 (Illumina, San Diego, CA, USA). Sequence data were mapped with the Genome Analysis ToolkitLite-v2.0.39 with hg19 human genome as reference. Two heterozygous changes were identified in RAB3GAP1 and confirmed by bidirectional Sanger sequencing: C.519G>A, p.(Trp173Ter) and c.2486T>A, p.(Leu829Ter). The same mutations were present in both sisters. Genetic analysis of the parents demonstrated that they were healthy carriers for each of these mutations [Figure 2].
Figure 2.
Familiar pedigrees and mutations. (a) Electropherograms of patients and their unaffected parents. The altered bases are shown with asterisks and in red boxes. Green line = A; red line = T; black line = G; blue line = C. The sisters inherited the mutations on separate chromosomes, RAB3GAP1c.519>A, p.(Trp173Ter), from their father, and RAB3GAP1c.2486T>A, p.(Leu829Ter) from their mother (b) Family pedigree. Probands of first generation have heterozygous mutations on RAB3GAP1 gene and are unaffected. Probands of second generation inherited each of these mutations and are compound heterozygous mutants showing the pathologic phenotype. Black symbols = affected; white symbols = unaffected
DISCUSSION
When Warburg et al.first described the syndrome in 1993,[1] they used the term Micro Syndrome, inspired by the morphological traits of the affected patients: microcephaly, microphthalmia, microcornea, and micrognathia. To date, four different genes have been associated with Warburg Micro syndrome: RAB3GAP1, RAB3GAP2, RAB18, and TBC1D20. Mutations in any of these genes result in clinically indistinguishable phenotypes.
RABs are a large group of GTP-binding proteins involved in the spatiotemporal control of intracellular traffic among organelles and extracellular communication via synaptic vesicles and exocytosis and endocytosis of hormones. In particular, RABs control the cargo selection of vesicles for cytoskeletal transport. RABs can switch between two forms: active RABs are bound to GTP while inactive RABs are bound to GDP. Guanine nucleotide exchange factors (GEFs) promote the exchange of GTP for GDP while GTPase-activating proteins (GAPs) accelerate GTP hydrolysis. RABs cycle between a cytosolic and a membrane-bound form. GDP-dissociation inhibitors (GDIs) mediate the dissociation from the membrane into the cytosol while GDI-dissociation factors (GDFs) return the RAB proteins to the membrane.
Recent studies have clarified the roles of the four RABs mutated in Warburg Micro syndrome: RAB3GAP1, the catalytic subunit, forms a heterodimeric complex with RAB3GAP2 named RAB3GAP, which has GAP activity toward RAB3 isoforms, thereby regulating Ca2+-mediated exocytosis of hormones and neurotransmitters.[2] The RAB3GAP enzymatic complex also functions as a GEF for RAB18.[6] RAB18 is involved in lipolysis, lipogenesis, the assembly of secretory granules,[7] and trafficking between the endoplasmic reticulum (ER) and Golgi apparatus.[8] Loss of RAB18 leads to alterations of the neuronal cytoskeleton, causing accumulations of neurofilaments and microtubule proteins at synaptic terminals.[9] These findings may explain the neurological defects of individuals with Warburg Micro syndrome. These findings also highlight the neurodegenerative aspect of Warburg Micro syndrome. TBC1D20 is an ER protein that functions as a GAP for RAB1 and RAB2, promoting traffic between the ER and Golgi apparatus.[5] TBC1D20 also has modest GAP activity toward RAB18 in vitro.[10] In summary, Warburg Micro syndrome can be caused directly by loss of RAB18, or indirectly through loss of the RAB18 regulators RAB3GAP or TBC1D20.
In our patients, we found two novel heterozygous changes in RAB3GAP1 (ref.sequence: NM_001172435): C.519G>A, p.(Trp173Ter) and c.2486T>A, p.(Leu829Ter). The alterations are both single nucleotide changes in the coding sequence (c.519G>A in exon 7 and c.2486T>A in exon 21, respectively). Methods to determine the degree of conservation showed that the altered nucleotides belong to conserved DNA elements, which suggests their functional relevance in the final protein product (c.519G>A: PhastCons = 1 and phyloP=5.15-5.15-3.14; c.2486T>A: PhastCons = 1 and phyloP=0.087-4.457-0.566). In silico analysis using the program Mutation Taster (www.mutationtaster.org) predicted that the mutations may be responsible for the appearance of premature stop codons and may modify the activity of donor splice sites. Moreover, these alterations are predicted to induce nonsense-mediated mRNA decay. Although molecular studies are necessary to understand the biological effects of these novel mutations in the RAB3GAP1 gene, altogether these data suggest a causal pathological relationship between these genetic alterations and the clinical presentation of our patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Warburg M, Sjö O, Fledelius HC, Pedersen SA. Autosomal recessive microcephaly, microcornea, congenital cataract, mental retardation, optic atrophy, and hypogenitalism. Micro syndrome. Am J Dis Child. 1993;147:1309–12. doi: 10.1001/archpedi.1993.02160360051017. [DOI] [PubMed] [Google Scholar]
- 2.Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, et al. Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet. 2005;37:221–3. doi: 10.1038/ng1517. [DOI] [PubMed] [Google Scholar]
- 3.Borck G, Wunram H, Steiert A, Volk AE, Körber F, Roters S, et al. A homozygous RAB3GAP2 mutation causes Warburg Micro syndrome. Hum Genet. 2011;129:45–50. doi: 10.1007/s00439-010-0896-2. [DOI] [PubMed] [Google Scholar]
- 4.Bem D, Yoshimura S, Nunes-Bastos R, Bond FC, Kurian MA, Rahman F, et al. Loss-of-function mutations in RAB18 cause Warburg Micro syndrome. Am J Hum Genet. 2011;88:499–507. doi: 10.1016/j.ajhg.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liegel RP, Handley MT, Ronchetti A, Brown S, Langemeyer L, Linford A, et al. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg Micro syndrome in humans. Am J Hum Genet. 2013;93:1001–14. doi: 10.1016/j.ajhg.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, et al. Rab18 and a rab18 GEF complex are required for normal ER structure. J Cell Biol. 2014;205:707–20. doi: 10.1083/jcb.201403026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez-Martinez R, Cruz-Garcia D, Duran-Prado M, Peinado JR, Castaño JP, Malagon MM, et al. Rab18 inhibits secretory activity in neuroendocrine cells by interacting with secretory granules. Traffic. 2007;8:867–82. doi: 10.1111/j.1600-0854.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 8.Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, et al. Rab18 and rab43 have key roles in ER-Golgi trafficking. J Cell Sci. 2008;121:2768–81. doi: 10.1242/jcs.021808. [DOI] [PubMed] [Google Scholar]
- 9.Carpanini SM, McKie L, Thomson D, Wright AK, Gordon SL, Roche SL, et al. A novel mouse model of Warburg Micro syndrome reveals roles for RAB18 in eye development and organisation of the neuronal cytoskeleton. Dis Model Mech. 2014;7:711–22. doi: 10.1242/dmm.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handley MT, Carpanini SM, Mali GR, Sidjanin DJ, Aligianis IA, Jackson IJ, et al. Warburg Micro syndrome is caused by RAB18 deficiency or dysregulation. Open Biol. 2015;5:150047. doi: 10.1098/rsob.150047. [DOI] [PMC free article] [PubMed] [Google Scholar]