Short abstract
Indolylarylsulfones are a potent class of human immunodeficiency virus type 1 non-nucleoside reverse transcriptase inhibitors. In this review, the structure activity relationship (SAR) studies to improve the profile of sulfone L-737,126 discovered by Merck AG have been analysed with focus on introduction of the 3′,5′-dimethyl groups at the 3-phenylsulfonyl moiety, the 2-hydroxyethyl tail at the indole-2-carboxamide nitrogen, coupling of the carboxamide nitrogen with one or two glycinamide and alaninamide units, a fluorine atom at position 4 of the indole ring and correlation between configuration of the asymmetric centre and linker length. IAS derivatives look like promising drug candidates for the treatment of AIDS and related infections in combination with other antiretroviral agents.
Keywords: Human immunodeficiency virus type 1, non-nucleoside reverse transcriptase inhibitor, indolylarylsulfone
Introduction
Human immunodeficiency virus type 1 (HIV-1) is the causative agent of HIV infection and acquired immunodeficiency syndrome (AIDS). HIV remains a major global public health issue, having claimed more than 35 million lives so far. AIDS and HIV-related infection caused some 1.1 million deaths in 2015 with more than two million HIV newly infected people.1 Current combination antiretroviral therapies (cART) combine drugs targeting different steps of the HIV life cycle: combination of three or four antiretroviral drugs has proven to inhibit effectively the infection in HIV-1-infected people adhering to a cART regimen.1 One cART pill a day remarkably reduces the risk of acquiring the infection in pre- and post-exposure prophylaxis to HIV uninfected people.2 Thus far, there are no safe and effective vaccines available for HIV. A National Institutes of Health funded study suggested that HIV preventive vaccination could reduce the number of HIV-infected people by 36% globally over a period of 15 years.3
The approved anti-HIV-1 drugs can be viewed as falling in five main classes: nucleoside reverse transcriptase inhibitors (NRTIs) and nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors which prevent the maturation step, viral entry inhibitors (fusion inhibitors and co-receptor antagonists) and integration inhibitors.4–8 These antiretroviral drugs are taken singly or combined into multidrug pills.9 The cART regimens have proven to be effective inhibitors of the HIV replication and prevent the breakthrough of the infection in early stages of the disease. After starting treatment with cART, the levels of plasma viraemia fall below the limit of detection within 24 weeks and remain for at least six months in most treated patients.10,11 Despite its great initial effectiveness, long-term cART treatments cause the development of drug resistance12 and cross resistance among compounds of the same class,13–15 toxicological problem and adverse side reaction, with associated compliance failure to the prescribed cART regimens.16 There is still a need of new antiretroviral drugs with improved resistance profiles and better tolerability.
Reverse transcriptase (RT) is a heterodimeric enzyme which is composed of two subunits, p66 and p51.17,18 The RT catalyses the transformation of the RNA retroviral genome into proviral DNA19 The catalytic core of the RT is placed in the p66 subunit and resembles a right hand with fingers, palm, thumb and connection subdomains. The p51 subunit does not exhibit any catalytic activity and shows structural capacity only.20,21 The NNRTIs behave as non-competitive allosteric inhibitors at the non-nucleoside binding site (NNBS) located a 10 Å distance from the catalytic site in the p66 palm subdomain.22,23
On 21 June 1996, the U.S. Food and Drug Administration (FDA) approved the first HIV-1 NNRTI, nevirapine ([1], NVP) (Viramune, Boehringer Ingelheim), for use in combination with NRTIs in HIV-1-infected adults. On 4 April 1997, delavirdine mesylate ([2], DLV) (Rescriptor, Pharmacia & Upjohn) and on 17 September 1998 efavirenz ([3], EFV) (Sustiva, DuPont) were approved for the treatment of HIV-1 infection. Etravirine ([4], ETR) (TMC-125, Intelence, Tibotec) and rilpivirine ([5], RPV) (Edurant, Tibotec) were approved by the FDA on 18 January 2008 and 20 May 2011, respectively, for treatment in drug combination of HIV-1-infected people for whom NNRTI-based therapies have failed (Chart 1).
Chart 1.
HIV-1 NNRTIs in clinical practice.
First sulfone HIV-1 NNRTIs
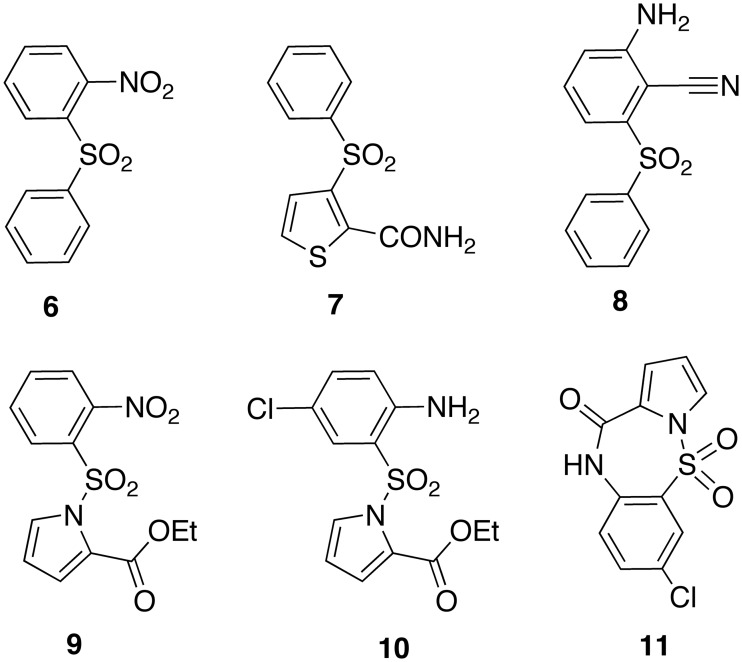
In 1993, McMahon et al.24 at the National Cancer Institute (NCI) of Bethesda started a drug-screening programme to identify new synthetic compounds and natural products with anti-HIV-1 activity. This study led to the selection of 2-nitrophenyl phenyl sulfone ([6], NPPS) (Chart 2), a diarylsulfone which showed appreciable anti-HIV-1 activity at micromolar concentration. Studies on diarylsulfone congeners led to the discovery of potent classes, such as 2-carboxamido-3-arylsulfonylthiophene [7],25 2-amino-6-arylsulfonylbenzonitrile [8]26 and diarylsulfone27 derivatives.
Chart 2.
Diarylsulfones [6]–[8], pyrrylarylsulfone [9] and [10], and pyrrolo[1,2-b][1,2,5]benzothiadiazepine [11].
At the time of the diarylsulfone project at the NCI, new pyrryl aryl sulfones (PASs) structurally related to NPPS, for example 2-nitro-PAS [9], were synthesized. First-generation PASs were characterized by a nitro group at position 2 of the benzene ring and an ethoxycarbonyl group at position 2 of the pyrrole nucleus.28 An important progress in PAS development was achieved by replacing the 2-nitrobenzene group with a 4-chloroanilino moiety to obtain 2-amino-PASs, for example [10], with potent antiviral activity at micromolar concentrations.29–32 The 4-chloroaniline was also a key pharmacophore for the antiretroviral activity of pyrrolo[1,2-b][1,2,5]benzothiadiazepines (PBTDs, for example [11]).32–34 PBTD NNRTIs, structurally related to NVP, were obtained by intramolecular cyclization of PASs.33
Indolylarylsulfone (IAS) HIV-1 NNRTIs
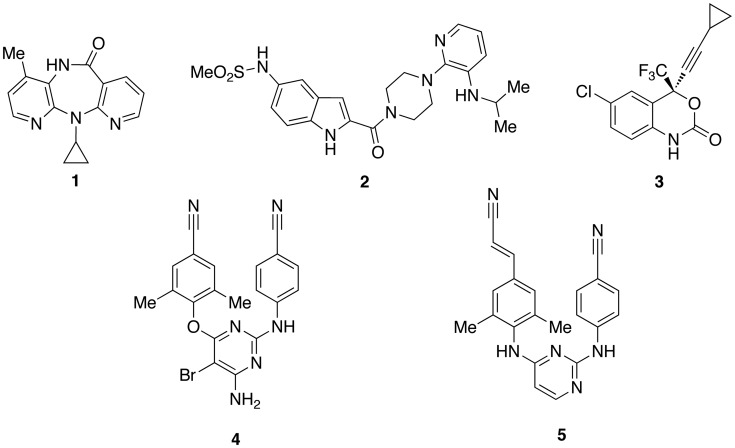
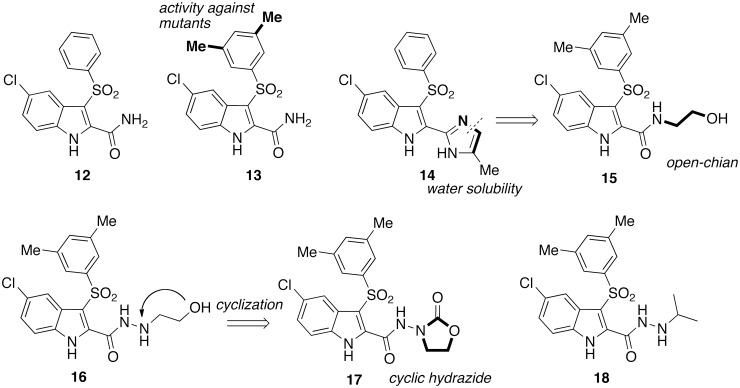
In 1993, Merck Research Laboratories reported the discovery of 5-chloro-3-(benzenesulfonyl)indole-2-carboxyamide (L-737,126; [12]) that showed potent and selective inhibition of HIV-1 WTIIIB strain with EC50 of 1 nM.35–37 Carboxamide [12] proved to be highly potent as inhibitor of the HIV-1 WT strain (EC50 = 1 nM).38 Introduction of a methyl group at position 2′, 3′ or 4′ of the 3-phenylsulfonyl ring led to IAS derivatives less cytotoxic than [12]. IAS derivatives bearing the 2′,4′- or 3′,5′-dimethyl [13] substitution pattern at the 3-phenylsulfonyl group were more cytotoxic than the monomethyl derivatives. Most importantly, the two methyl groups at positions 3′ and 5′ of the 3-phenylsulfonyl moiety proved to be a key structural requirement for an effective inhibition of the HIV-1 mutant strains38 (Chart 3). IAS derivative [13] exhibited potent activity against the HIV-1 WT (EC50 = 4 nM) and the HIV-1 mutant strains Y181C (EC50 = 30 nM), K103N-Y181C (EC50 = 650 nM) in MT-4 cells (MTT method) and EFVR (EC90 = 80 nM) in 8166 cell (p24 method) (EFVR: EFV-resistant HIV-1 strain carrying K103R, V179D and P225H mutations; EFV EC90 = 1800 nM). These studies highlighted the role of the 3′,5′-dimethyls against the drug-resistant HIV-1 mutant strains. In summary, compound [13] was comparable with [12] and EFV against HIV-1 WT and Y181C mutant, but it was more potent than [12] against the HIV-1 K103N-Y181C mutant, and superior to [12] and EFV against the EFVR mutant strain.
Chart 3.
IAS carboxamides [12], [13] and [15]; carboxamide bioisostere [14] and carbohydrazides [16]–[18].
Hydroxyethyl derivatives
Efforts to improve water solubility and bioavailability of [12] led Merck to synthesize a series of bioisosteres by replacing the 2-carboxamide with a nitrogen-containing heterocycle.39 Interestingly, among these new compounds 3-(benzenesulfonyl)-5-chloro-2-(5-methylimidazol-2-yl)indole [14] was found to be 11-fold superior to [12] against the HIV-1 K103N mutant strain. Molecular modelling studies were performed to gain insight of the binding mode of [12] into the NNBS of 14 RTs. A 3D quantitative structure activity relationship (SAR) model was obtained using a training set of 70 IAS derivatives.40 The structure of the diarylsulfone 739W9424 in complex with the HIV-1 RT (PDB code 1jlq) was used to model the training set.27 These findings prompted the synthesis of IAS derivatives bearing a 2-hydroxyethyl tail at the indole-2-carboxamide or indole-2-carboxydrazide nitrogen.41,42 Carboxamide [15] and carboxhydrazide [16] were the most potent inhibitors of the HIV-1 WTIIIB strain (EC50 = 1 nM) in MT-4 cells (MTT method). IAS [15] was superior to [12] against the HIV-1 Y181C and K103N-Y181C mutants and was more potent than [12] and EFV against the EFVR mutant strain. IASs [15] and [16] were as potent as EFV against the HIV-1 WT RT, superior to EFV against the HIV-1 K103N mutant, but slightly inferior as inhibitor of the HIV-1 KY181I mutation (i.e. comparable with the Y181C in terms of drug resistance).
N′-carbohydrazide derivatives
The training set of the 3D quantitative SAR model40 was enlarged from 70 to 101 compounds to improve the predictive capability.43 Docking simulations and 3D quantitative SAR models led to design new potent carbohydrazide derivatives. IASs [17] and [18] showed strong inhibition of the HIV-1 WTIIIB strain in MT-4 cells with EC50 of 3 and 0.7 nM, respectively, and high selectivity indexes. Compound [17] inhibited the HIV-1 K103N-Y181C double mutant with EC50 = 900 nM. Compounds [17] and [18] were evaluated against the primary isolates HIV-1 WTIIB, HIV-112 and HIV-AB1 carrying K103N–V108I–M184V and L100I–V108I mutations, respectively, from two HIV-1-Ab seropositive patients who developed treatment failure after an initial response to the cART therapy. In lymphocytes compound [18] inhibited the primary isolates with subnano- (IIIB) or low nanomolar (112, AB1) EC50 values; in macrophages, [17] inhibited the HIV-1 IIIBBa-L with EC50 of 2 nM and showed selectivity index >10.000.
IAS containing peptide units
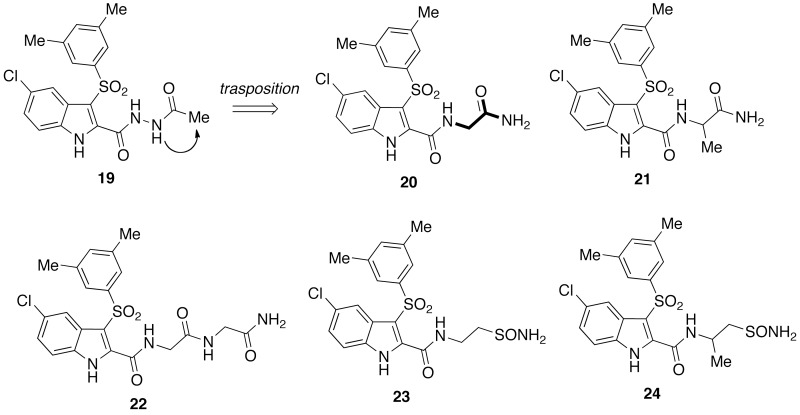
Efforts to improve the interaction of IAS NNRTIs with the NNBS of the HIV-1 RT prompted the design of IAS derivatives bearing 1–3 glycine/alanine unit(s) at the 2-carboxamide nitrogen. The chemical manipulations included, for example, transposition of N-acetylamino to 2-acetamido, or replacement of semicarbazide with glycine carbohydrazide (Chart 4).44 IAS derivatives bearing the glycine [20], alanine [21] or glycine–glycine [22] unit strongly inhibited the HIV-1 WTIIIB strain with EC50s of 3, 6 and 0.7 nM, respectively. As inhibitors of the HIV-1 Y181C mutant strain, IASs [21] and [22] showed EC50 of 10 and 5 nM, respectively. IASs [20] and [22] inhibited the HIV-1 K103N–Y181C double mutant with EC50s of 800 and 700 nM. IASs [20]–[22] inhibited the EFV-resistant HIV-1 K103R–V179D–P225H strain with EC50s of 100, 40 and 100 nM, respectively38 (Chart 4).
Chart 4.
IAS acetylhydrazide [19] and IASs containing peptide units [20]–[24].
These results prompted the synthesis of new IAS derivatives bearing natural and unnatural amino acid units at the indole-2-carboxamide, for example [23] and [24].45 As inhibitors of the HIV-1 WTIIIB strain in CEM cells the new IASs yielded nanomolar EC50 concentrations ([23]: EC50 = 1.4 nM; [24]: EC50 = 2.3 nM) and were comparable with EFV. The new compounds inhibited the Coxsackie B4 viruses with EC50s of 2–9 µm. These agents showed the potential as new drugs to treat both the HIV-1 and Coxsackie B4 infection.45
IASs containing two halogen atoms
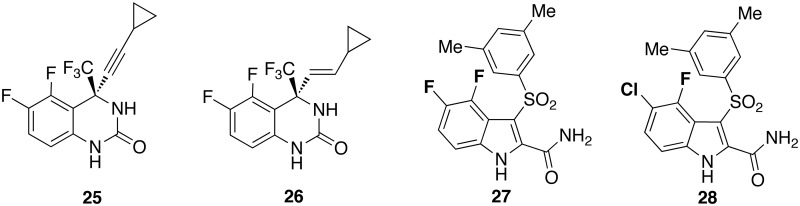
The HIV-1 K103N mutant strain is the most frequent mutation in >90% EFV-treated patients whose viral load rebounded after an initial response to the drug. The K103 mutation is frequently followed by the HIV-1 K103N–V108N and K103N–P225H double mutations.46 Moreover, drug expedited clearance may result as a consequence of upregulation of P450 liver isoenzyme CYP3A4.47 Efforts to improve the activity of EFV48 against the drug-resistant mutants led DuPont Pharmaceuticals to synthesize quinazolinone analogues with two halogen atoms49,50 (Chart 5). Compounds with the halogen atoms at positions 5 and 6 of the quinazolinone ring, for example [25] and [26], were superior to the corresponding 5- and 6-mono-halogenated counterparts, partly due to the weaker binding with the plasma proteins.51
Chart 5.
Di-halo IASs [27] and [28] correlated to quinazolinones [25] and [26].
New IAS derivatives bearing two halogen atoms at positions 4–7 of the indole ring were synthesized based on these findings.52 Introduction of chlorine and fluorine atoms at positions 4 and 5 of the indole provided highly potent HIV-1 NNRTIs. The di-halo IASs [27] and [28] exhibited potent inhibition of HIV-1 WTIIIB strain in MT-4 cells with EC50s of 1.0 and 0.5 nM, respectively. As inhibitor of the HIV-1 Y181C and K103N–Y181C mutant strains, [28] was comparable with EFV. Compounds [27] and [28] effectively inhibited the primary isolates 112 and the AB1 strains in lymphocytes and the HIV-1 WTIIIB Ba-L strain in macrophages (p24 method).
Di-halo-IASs showed selectivity for the enzyme–substrate complex because of a different dissociation rate of the drug from the enzymatic form along the reaction pathway.53 By comparing the HIV-1 WT RT and drug-resistant K103N-, L100I- and Y181I-mutated RTs, IAS derivatives were characterized by highly dynamic interaction with the viral RT.53,54 A greater flexibility of IAS [28] to form stable interactions with the amino acid residues of the NNBS of the RT may correlate with the potent anti-HIV-1 activity.55
IASs with a third ring
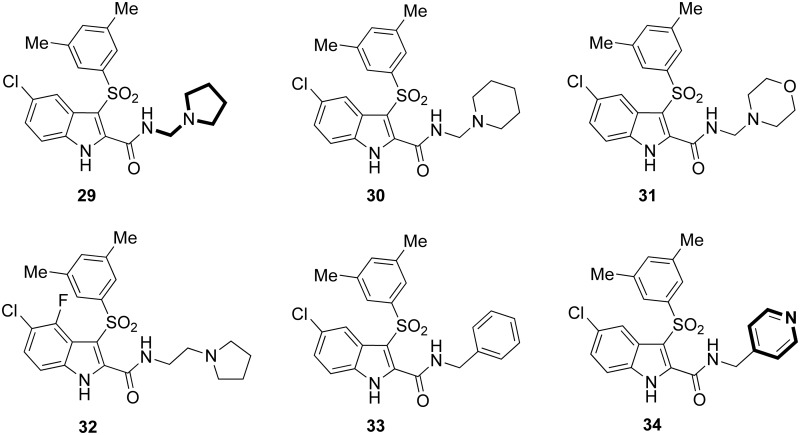
HIV-1 NNRTIs containing three aromatic rings, for example diaryltriazine (Janssen, NJ, USA),56 dipyridodiazepinone (Boehringer Ingelheim, CT, USA)57,58 and pyrrolidin-1-ylsulfone (Merck, PA, USA)59 derivatives, showed potent and broad spectrum anti-HIV-1 activity. Previous docking studies of IAS derivatives containing a short peptide unit44,45 highlighted that a tail at the 2-carboxamide nitrogen could form effective binding interactions inside the NNBS of the RT surrounded by the R172, I180, V179 and E138:B, and T139:B amino acid residues. These computational studies served as basis for the design of new IAS derivatives characterized by the presence of a pyrrolidine/piperidine/morpholine heterocyclic ring linked to the carboxamide nitrogen through a short 1–2 carbon spacer, for example [29]–[32] (Chart 6).60 The new IAS bearing the third nucleus showed potent inhibition of the HIV-1 WTIIIB strain in CEM cells with EC50 values at nanomolar concentrations ([29]: EC50 = 3.3 nM; [30]: EC50 = 1.3 nM; [31]: EC50 = 1.9 nM; [32]: EC50 = 6.5 nM). As inhibitors of the HIV-1 L100I and K103N mutant strains, [31] (EC50 = 8.0 and 11 nM) and [32] (EC50 = 11 and 15 nM) were superior to EFV (EC50 = 22 and 130 nM). Dissociation rates showed that compounds [29] and [30] formed stronger binding interactions with the L100I- and K103N-mutated enzymes than the WT enzyme.61 IAS derivatives [29], [31] and [32] inhibited the HIV-1 clade A in peripheral blood mononuclear cells (PBMCs) with EC50 values of 0.1, 0.1 and 2.1 nM, respectively.
Chart 6. IASs [29]–[34] containing a third cyclic ring.
Benzyl derivative [33] showed appreciable inhibition of the HIV-1 WTIIIB strain in CEM cells and the HIV-1 mutant strains in MT-4 cells. These findings prompted the synthesis of new IAS derivatives containing nitrogen heterocycles at the 2-carboxamide nitrogen.62 Among them, compound [34] showed consistent inhibition of the HIV-1 WTNL4-3 strain (EC50 = 2.0 nM) in MT-4 cells, the HIV-1 K103N (EC50 = 8.8 nM), Y181C (EC50 = 2.2 nM) and Y188L (EC50 = 22 nM) mutant strains and the HIV-1 IRLL98 multidrug-resistant strain (EC50 = 1 nM) containing the K101Q, Y181C and G190A mutations conferring resistance to NVP, DLV and EFV.63 Compound [34] was more active against the IRLL98 mutant strain than the WT NL4-3 strain, and it proved to be a potent inhibitor of HIV-1 clades A, B, C, D, A/E, F and G in PBMCs in the higher picomolar range, except clade G. Compound [34] inhibited the HIV-1 K103N RT, the major mutation emerging in EFV-treated patients,46 with EC50 of 45 nM, and exhibited EC50 of 11 nM against the HIV-1 L100I RT. These new IASs shared a typical feature of ETR, a state-of-art HIV-1 NNRTI, that is the presence of a pendant (third) aromatic ring.55 These results underline the potential of IAS [34] as a new agent for the treatment of EFV-treated patients who show the L100I and K103N mutations.
Focus on chirality of IASs
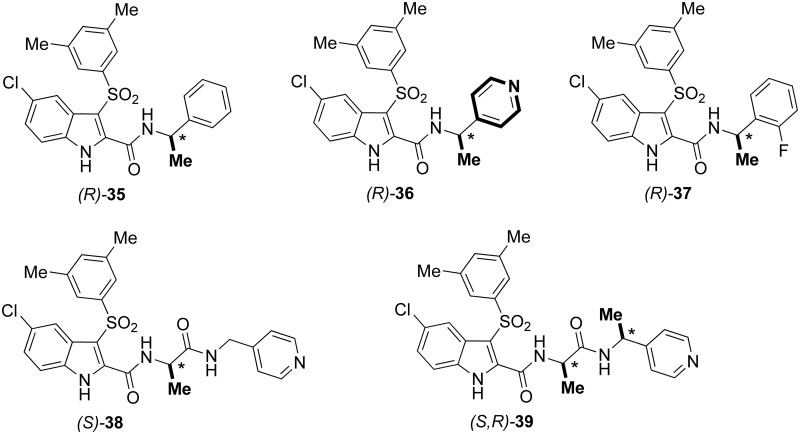
Chirality considerably affects the pharmacological profile of the drugs due to the high specific interaction of the ligand to the recognition site. Accordingly, the enantioselectivity plays an important role for the binding of antiviral agents to HIV-1 RT.64 New IAS HIV-1 NNRTIs were synthesized to evaluate unexplored substitutions of the benzyl/phenylethyl group linked at the indole-2-carboxamide.65 In recent studies, the enantiomers of IASs bearing chiral centres demonstrated significant differences in terms of antiretroviral activity. Several IAS derivatives were superior to NVP and EFV against the HIV-1 NL4-3 WT strain and inhibited the HIV-1 K103N mutant strain at nanomolar concentration. Some derivatives were superior to EFV against the HIV-1 Y181C and L100I mutant strains. The racemate (R,S)-[35] was separated into the enantiomers (R)-[35] and (S)-[35] by HPLC on the cellulose derived coated Chiralcel OD chiral stationary phase (CSP) using the binary mixture n-hexane–ethanol 1:1 as a mobile phase at both analytical and semipreparative level. Assignment of the absolute configuration of (R)-[35] and (S)-[35] was achieved by (i) synthesis of the enantiomer (S)-[35] starting from the amine of known stereochemistry (S)-(−)-α-methylbenzylamine, and (ii) comparison of the enantiomeric peaks of (R)-[35] and (S)-[35] obtained from the enantioseparation under the same enantioselective HPLC conditions. Against the NL4-3 HIV-1 strain, the enantiomers (R)-[35] and (S)-[35] showed small differences of activity. In contrast, (R)-[35] was found to be significantly more potent than (S)-[35] against the HIV-1 mutant strains ((R)-[35] EC50, (S)/(R) ratio: K103N (4.3 nM, 30-fold), Y181C (86 nM, 40-fold), Y188L (193 nM, >189-fold) and K103N-Y181C (1670 nM, >22-fold)) (Chart 7).
Chart 7. Chiral IASs [35]–[39].
Docking studies of (R)-[35] and (S)-[35] in the HIV-1 WT RT gave similar results. However, into the HIV-1 K103N-mutated RT the methyl group of the (R)-[35] pointed towards the entrance channel of the NNRTI NNBS, while the corresponding group of the (S)-[35] pointed towards the bottom of the cleft, leaving the binding pocket more exposed to the aqueous environment. The difference in the observed biological activity of (R)-[35] and (S)-[35] could be due to a different binding kinetics rather than affinity.66 (R)-[35] was able to seal the binding pocket, while (S)-[35] left the site accessible to water with a consequent negative impact on the binding kinetic of this inhibitor.
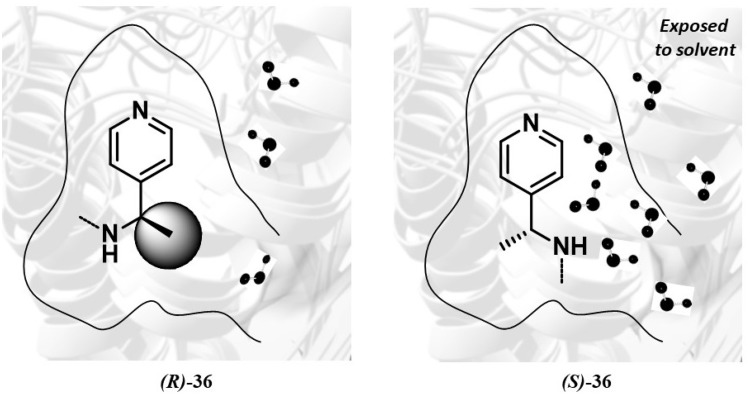
Further step of this research project was the synthesis of IAS derivatives carrying a heterocyclic tail at the indole-2-carboxamide nitrogen.67 Several new IASs inhibited the HIV-1 WT and mutant strains in MT-4 cells with EC50 values less than 1.0 nM. Replacement of the phenyl group of [35] with a pyridinyl ring to obtain [36] resulted in a general improvement of antiviral activity. Racemate [36] was found to be three-fold more potent than [35] as inhibitor of the NL4-3 WT strain (EC50 = 0.2 nM,). Against the K103N (EC50 = 9.4 nM), Y181C (EC50 = 87 nM) and K103N-Y181C (EC50 = 1111 nM) mutant strains, IAS [36] was, respectively, three, eight and three times superior to [35].
The racemate (R,S)-[36] was separated into the enantiomers (R)-[36] and (S)-[36] by enantioselective HPLC. The CD spectra of enantiomers (R)-[36] and (S)-[36] were compared with those of (R)-[35] and (S)-[35].65 Similar to the enantiomers of [35], (R)-[36] and (S)-[36] (EC50 = 0.2 nM) were equipotent against the HIV-1 WTNL4-3 strain; on the contrary, (R)-[36] showed higher inhibition than (S)-[36] of the HIV-1 mutant strains ((R)-[36] EC50, (S)/(R) ratio: K103N (0.2 nM, 22-fold), Y181C (2.1 nM, 61-fold) and K103N-Y181C (150 nM, 27-fold)) in the cellular assay. Compound (R)-[36] was superior to NVP, EFV and AZT reference drugs, except AZT, against the K103N–Y181C mutant strain. In addition, compounds (S)-[36] and (R)-[36] were evaluated against various HIV-1 group M clinical isolates in PBMC. The antiviral activity was potent and consistent and varied only between 0.7 and 5.2 nM when evaluated against all different virus isolates. Compound (R)-[36] showed excellent plasma and metabolic stability and did not behave as a prodrug. It showed good membrane permeability and low water solubility.67 Shifting the 4-nitrogen of the pyridine to either position 3 or 2 resulted in a decreased anti-HIV-1 activity against the mutant strains.
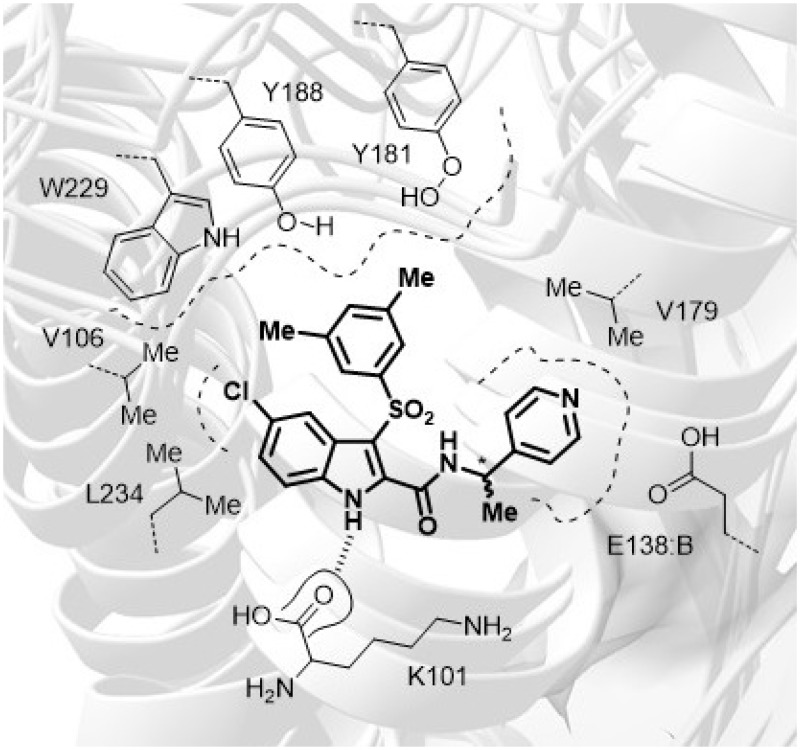
From docking simulations in the HIV-1 WT RT, the PLANTS proposed binding mode of (R,S)-[36] was consistent with the previously reported binding poses for the IAS family41–45,52,55,60,62,65 featuring these pharmacophoric interactions: (i) the indole NH established a H-bond with the K101 carbonyl oxygen; (ii) the chlorine atom fitted into a hydrophobic cavity surrounded by V106 and L234; (iii) the 3′,5′-dimethylphenyl moiety laid in the aromatic cleft formed by the side chains of Y181, Y188 and W229 residues establishing a network of hydrophobic interactions; (iv) the pyridyl moiety formed hydrophobic interactions with the side chains of V179 and E138:B (Figure 1).
Figure 1.
Sketch of amino acid residues within 5 Å of IAS (R,S)-[36] bounded in the NNBS of the HIV-1 WT RT.
The (R)-[36] and (S)-[36] enantiomers showed some significant differences in their binding modes: the methyl group of (R)-[36] pointed towards the cleft created by the K103N mutation, sealing the binding pocket and reducing the solvent-accessible surface, the corresponding group of (S)-[36] left the pocket more exposed to solvent, in agreement with the previously reported mechanism of resistance to the HIV-1 K103N mutation based on a binding kinetic effect.66,68 Molecular dynamics simulations showed that trajectory analyses of both K103N RT/((R)-[36] and K103N RT/(S)-[36] complexes were stable during the whole simulation time. Solvent-accessible surface area69 performed on the entire binding site of the receptor in complex with (S)-[36] (235.64 Å2) was greater than the corresponding one for the (R)-[36], (210.20 Å2). (S)-[36] showed a number of water molecules surrounding the methyl cleft, 3.5 times greater than the number of solvent molecules observed for the (R) enantiomer. These results were taken into account to explain the different biological activity observed between (R)-[36] and (S)-[36] enantiomers (Figure 2).
Figure 2.
Sketch the 1-(pyridin-4-yl)ethyl group of derivatives (R)-[36] and (S)-[36] into the NNBS of the K103N RT. The image shows a different shape and position of the methyl group.
A new series of chiral IASs showed potent inhibition of the HIV-1 WT NL4-3 strain and of the HIV-1 K103N, Y181C, Y188L and K103N-Y181C HIV-1 mutant strains.70 Six racemic mixtures were separated at the semipreparative level by enantioselective HPLC into their pure enantiomers. The (R)-enantiomers of IAS derivatives bearing the chiral α-methylbenzyl were superior to the (S)-counterparts. IAS (R)-[37] inhibited the HIV-1 WT strain with EC50 of 0.7 nM, and K103N (EC50 = 0.7 nM), Y181C (EC50 = 0.7 nM), Y188L (EC50 = 165 nM) and K103N–Y181C (EC50 = 2486 nM) in the cellular assay. In previous studies, the antiretroviral activity of IAS [21] appeared only weakly affected by the chirality of the alanine unit.45 On the contrary, coupling of alanine with pyridin-4-ylmethanamine provided IAS derivatives with marked stereospecific activity: (S)-[38] was superior to the corresponding (R)-enantiomer, and (S,R)-[39] and (S,S)-[39] were superior to the corresponding (R,S)-[39] and (R,R)-[39] enantiomers. IASs (R)-[38] and (S)-[38] were equally active against the HIV-1 WTNL4-3 with EC50 = 0.7 nM. Otherwise, (S)-[38] was really more potent than (R)-[38] against the HIV-1 mutant strains (EC50, (S)/(R) ratio) K103N (0.7 nM, 162-fold), Y181C (0.7 nM, 258-fold), Y188L (666 nM, >35-fold) and K103N–Y181C (857 nM, eight-fold) in MT-4 cells. The cellular data correlated with the enzymatic results. The diastereomeric mixture [39] was separated into the four stereoisomers (R,S)-[39], (S,R)-[39], (R,R)-[39] and (S,S)-[39]. IASs (S,R)-[39] and (R,S)-[39] showed marked differences against the HIV strains (EC50, (S)/(R) ratio) WTNL4-3 (0.6 nM, three-fold), K103N (0.6 nM, 340-fold), Y181C (0.6 nM, 1113-fold), Y188L (742 nM, 38-fold) and K103N–Y181C (1261 nM, 25-fold). The biological results highlighted a correlation between configuration of the asymmetric centre and linker length: The (R)-enantiomers were superior to the (S)-counterparts when associated to a short linker unit, and the (S)-enantiomers were more potent than the (R)-enantiomers in the presence of a long linker.
Despite the significant increase of life expectancy in HIV-infected people,71 cART treatments cause neurological problems to nearly half of HIV cases.72 The neurocognitive damage often accelerates during cART treatment73 and continues even after the peripheral viral infection has ceased.74 Compound (R,S)-[38] protected hippocampal neuronal cells from the excitotoxic insult, while EFV did not contrast the neurotoxic effect of glutamate. The new IASs showed improved resistance profile against the mutant HIV-1 strains and reduced neurotoxic effects.
Indole-3-sulfonamides, Merck & Co., Inc
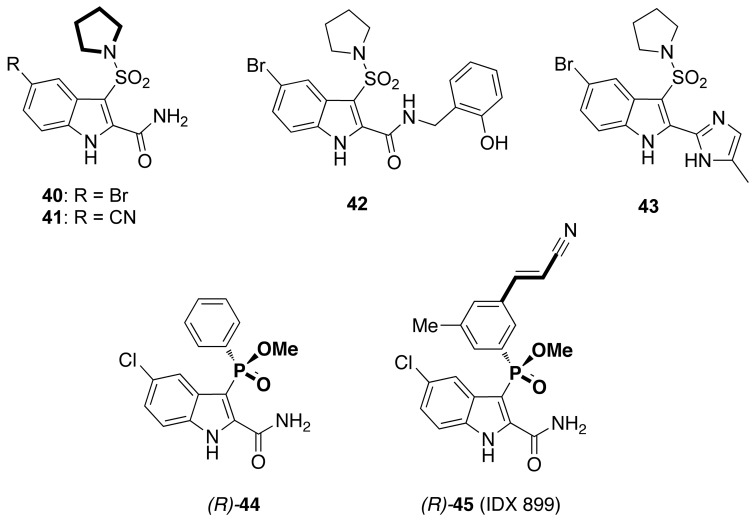
Fifteen years after the discovery of compound [12],35 Merck & Co., Inc. reported the synthesis of a series of indolylsulfonamides bearing a linear or cyclic alkylamine linked at the sulfone group at position 3 of the indole.59 Preliminary docking studies suggested that a 3-alkylsulfonamide and a halogen atom at position 5 of the indole could increase the activity against the HIV-1 Y181C mutant strain. Both secondary and tertiary sulfonamides inhibited the HIV-1 RT with IC50 values at nanomolar concentration. In HIV-1 WT-infected cells, only pyrrolidine [40], [41] and piperidine tertiary sulfonamides showed inhibitory concentrations (Spread CIC95) comparable to the enzymatic assay. X-ray co-crystal structure of [40] bound to HIV-1 WT RT revealed that it assumed a ‘butterfly-like’ orientation, similarly to [12] and other NNRTIs.55 However, [40] and [41] showed weak inhibition of the HIV-1 K103N and Y181C mutant strains. Efforts to improve the activity against the mutant strains led to modification of the 2-carboxmide function, as previously reported by Young et al.,39 with introduction of a benzyl group at the carboxamide nitrogen (e.g. [42]), or replacement of the carboxamide with an imidazole ring (e.g. [43]). Compounds [42] and [43] showed inhibition of the HIV-1 K103N and Y181C mutants comparable to the WT in both enzymatic and cellular assays (Chart 8).
Chart 8. Indolylsulfonamides [40]–[43] and API [44] and [45].
Arylphosphoindoles, Idenix Laboratories
Idenix Laboratories synthesized bioisosteres of the IAS derivatives by replacing the 3-sulfonyl bridging group with a phosphinic acid methyl ester one.75 Arylphosphoindole (API) derivative [44] inhibited the HIV-1 WT and the K103N, Y181C mutant strains at low nanomolar concentration. The racemic mixture [44] was separated into the corresponding enantiomers by supercritical fluid chromatography. In MT-4-infected cells, the enantiomer (R)-[44] inhibited the HIV-1 WT with EC50 of 0.1 nM and the HIV-1 mutant strains K103N, Y181C and K103N-Y81C with EC50 values of 1.2, 3.6 and 137.4 nM, respectively. The stereochemistry of (R)-[44] was assigned on the basis of binding energy calculation (−23 kcal/mol) and was confirmed by X-ray crystallography diffraction.
Idenix introduced the typical Z-cyanovinyl arm of RPV at position 3′ of the 3-phenyl phosphinic group to obtain HIV-1 NNRTIs with broad spectrum of activity against the drug-resistant mutant strains. The enantiomer (R)-[45] (IDX 899) showed effective inhibition of the HIV-1 WT and of the HIV-1 mutant strains resistant to other NNRTIs, reduced HIV-1 RNA levels and exhibited barrier to resistance superior to EFV. (R)-[45] was selected as highly potent second-generation NNRTI drug candidate and was evaluated in a phase II clinical trial. In February 2009 it was licensed to GlaxoSmithKline and its name was changed to fosdevirine/GSK2248761.76 In phase I clinical trials increased CD4+ cell counts in treatment-naïve patients infected with HIV-177,78 (Chart 8).
Synthetic aspects
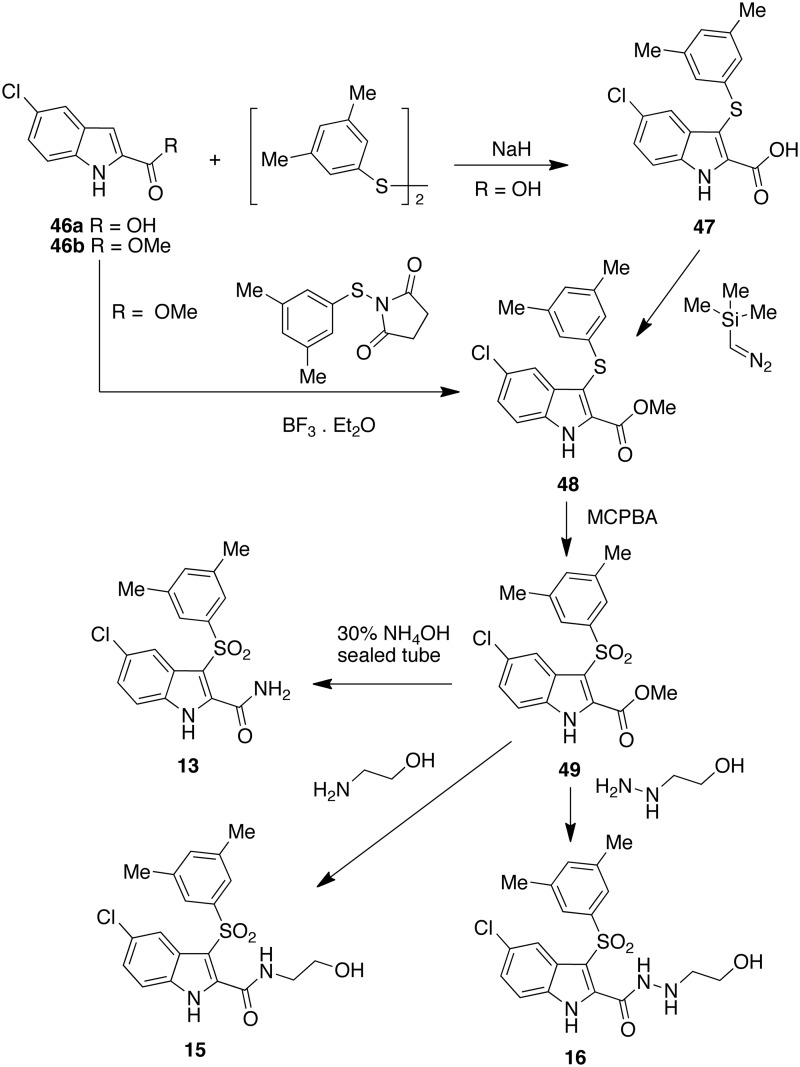
Compound [13] was synthesized starting from acid [46a] which transformed into the 3-arylthio intermediate [47] by Atkinson reaction79 with an appropriate arylthiodisulfide in the presence of sodium hydride and then transformed in the corresponding methyl ester [48] with trimethylsilyl diazomethane. Alternatively, [48] was obtained from ester [46b] and N-(3,5-dimethylphenylthio)succinimide in the presence of boron trifluoride diethyl etherate. Compound [48] was oxidized to sulfone [49] with 3-chloroperoxybenzoic acid. Finally, [49] was heated with ammonium hydroxide in closed vessel to give [13]. Heating of [49] in ethanolamine or 2-hydrazinoethanol furnished IAS [15] or [16], respectively (Scheme 1).
Scheme 1.
Synthesis of IASs [13], [15] and [16].
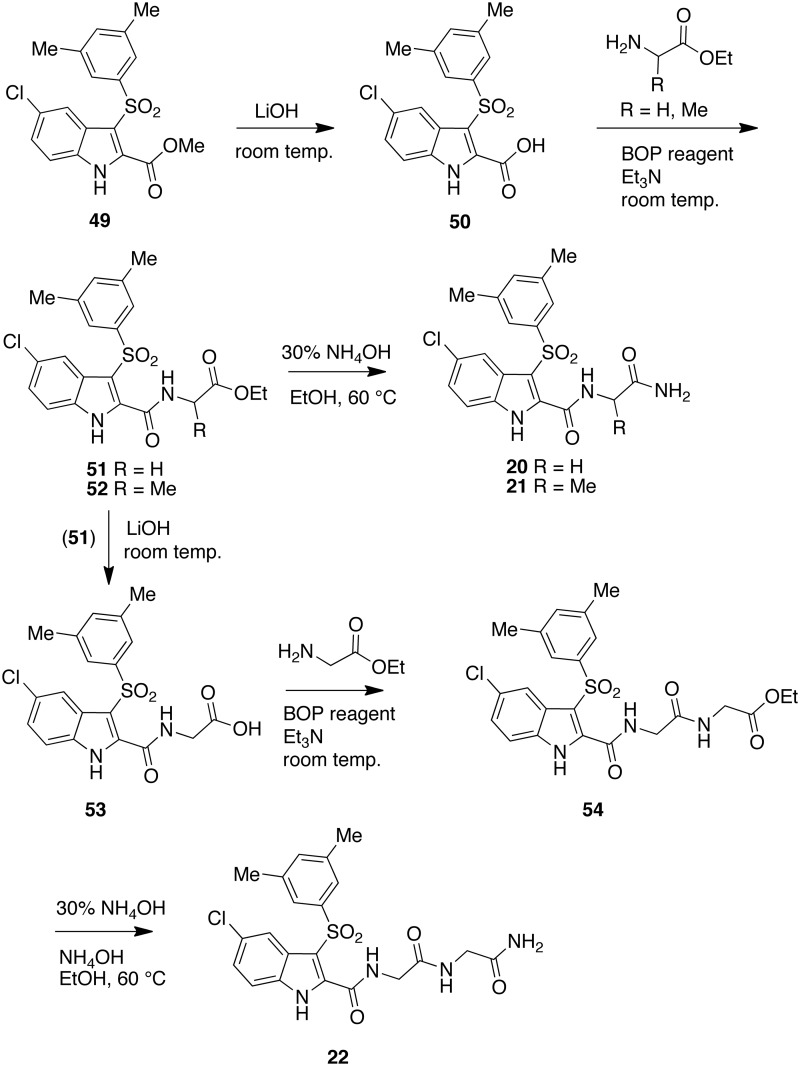
IAS derivatives [20]–[22] were synthesized starting from ester [49]. LiOH hydrolysis of [49] at room temperature yielded the carboxylic acid [50]. The acid was treated with glycine or alanine in the presence of BOP reagent (benzotriazol-l-yl-oxy-tris-(dimethylamino)phosphonium hexafluorophosphate) and triethylamine as coupling reagents to provide esters [51] or [52]. These compounds were transformed into IASs [20] or [21] with concentrated ammonium hydroxide in ethanol at 60 °C. Glycine ester [51] underwent alkaline hydrolysis with LiOH to give acid [53]. Elongation of the chain was performed by coupling [53] with a glycine unit in the presence of BOP reagent and triethylamine. Finally, ester [54] was transformed into IAS [22] by heating at 60 °C with ammonium hydroxide (Scheme 2).
Scheme 2.
Synthesis of IASs [20]–[22].
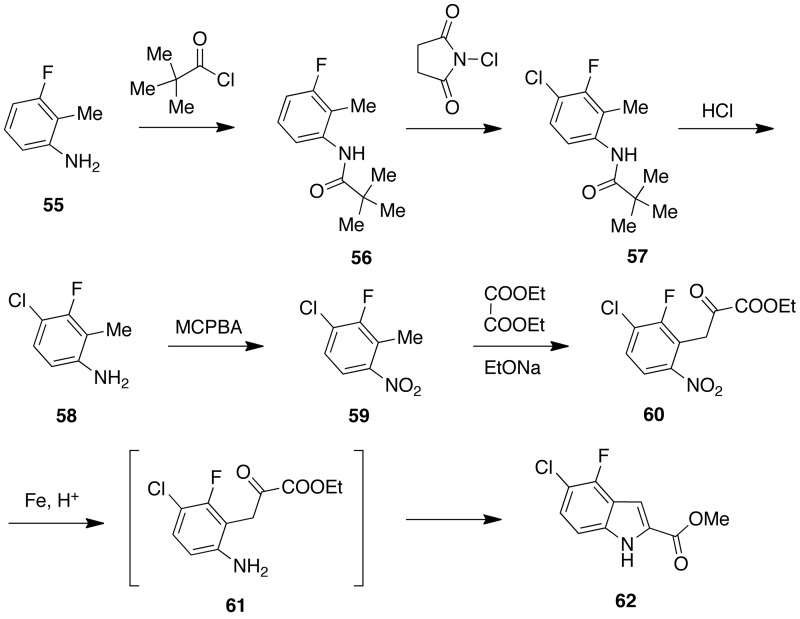
Di-halo-IASs [27] and [28] were synthesized by following the above reported procedure starting from an appropriate ethyl di-halo-1H-indole-2-carboxylate. The required esters were prepared from the corresponding phenylhydrazones80 by Fisher indole synthesis in polyphosphoric acid at 100 °C.81 The isomeric esters were separated with difficulty by column chromatography after repeated passages providing [62] in 5% yield.82 To improve yield, [62] was synthesized starting from 3-fluoro-2-methylaniline [55]. The N-pivaloyl derivative [56] was treated with N-chlorosuccinimide to give the 4-chloro derivative [57]. After hydrolysis of [57] with hydrochloric acid, the aniline [58] was oxidized to nitro [59] with 3-chloroperoxybenzoic acid. Treatment of the latter compound with diethyl oxalate in the presence of sodium ethoxide gave ethyl 3-(3-chloro-2-fluoro-6-nitrophenyl)-2-oxopropanoate [60]. Iron powder reduction of [60] followed by intramolecular cyclization provided [62] in six steps and 37% overall yield83 (Scheme 3).
Scheme 3.
Synthesis of ethyl 5-chloro-4-fluoro-1H-indole-2-carboxylate [62].
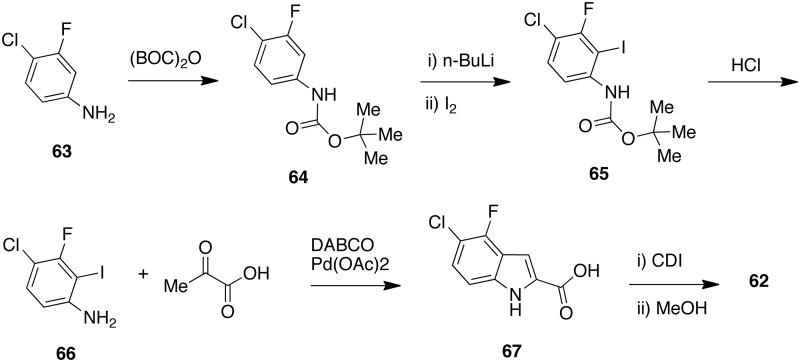
Idenix Pharm. (MA, USA) synthesized [62] starting from 4-chloro-3-fluoroaniline [63].84 C2 iodination of BOC-protected [64] afforded [65]. After deprotection of [65] with hydrochloric acid, the aniline [66] was reacted with pyruvic acid in the presence of palladium(II) acetate and 1,4-diazabicyclo[2.2.2]octane to afford 5-chloro-4-fluoro-indole-2-carboxylic acid [67]. Esterification of [61] to [62] was easily achieved by treatment with 1,1′-carbonyldiimidazolole and then with methanol (Scheme 4).
Scheme 4.
Synthesis of ethyl 5-chloro-4-fluoro-1H-indole-2-carboxylate [56].
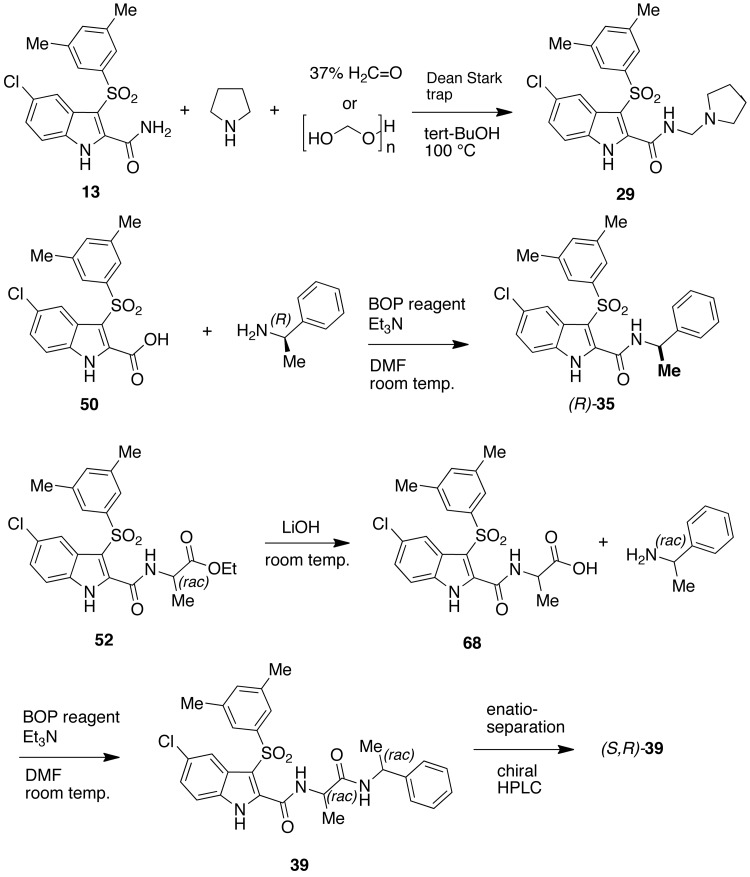
Compound [29] was synthesized according to the Mannich reaction by refluxing [13] in tert-butanol at 100 °C with pyrrolidine in the presence of 37% formaldehyde or paraformaldehyde using a Dean–Stark trap.60 Reaction of (S)-(−)-α-methylbenzylamine with acid [50] in the presence of BOP reagent and triethylamine in DMF at room temperature furnished the chiral IAS (R)-[35]. This compound was used as a reference compound to compare the peaks of enantiomers (R)-[35] and (S)-[35] obtained by enantioseparation of the racemate [35].65 Acid [68] was obtained by LiOH hydrolysis of the racemic ester [52]. Treatment of [52] with the racemic α-methylbenzylamine in the presence of BOP reagent and triethylamine to furnish the diasteromeric compound [39]. HPLC separation of the enantiomers was performed using a polysaccharide-based CSP Chiralpak IC and n-hexane–ethanol–dichloromethane–DEA 40:15:45:0.3 as eluent70 (Scheme 5).
Scheme 5.
Synthesis of IASs [29], (R)-[35] and (S,R)-[39].
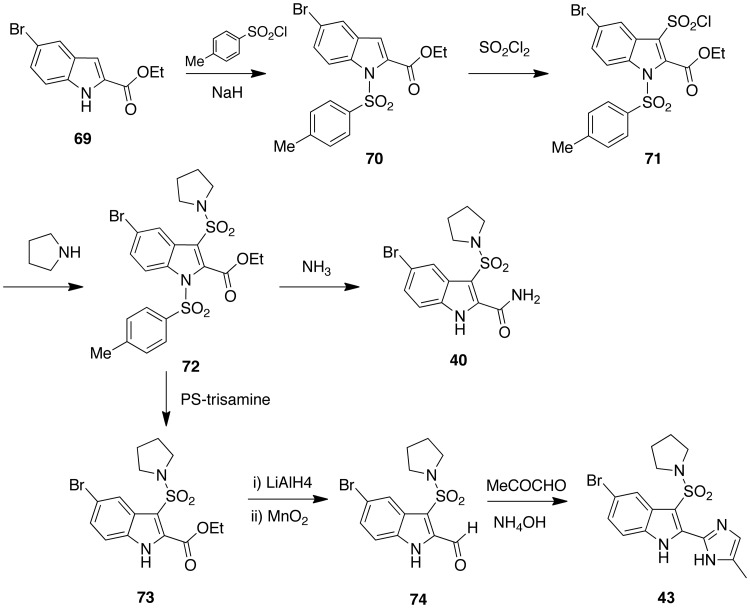
Ethyl 5-bromo-1H-indole-2-caboxylate [69] was protected with 4-toluensulfonyl chloride in the presence of NaH to give [70] and converted to sulfonyl chloride [71] with sulfuryl chloride. The sulfonyl chloride was treated with pyrrolidine to afford [72]. Aminolysis of the 2-ethoxycarbonyl group with ammonia underwent concomitant deprotection of position 1 of the indole to provide [40]. Deprotection of [72] with tris-(2-aminoethyl)aminomethyl polystyrene (PS-trisamine) gave [73]. The ester was transformed to aldehyde [74] by a two-step procedure with lithium aluminium hydride and subsequent oxidation with manganese(IV) oxide. The latter compound was cyclized to imidazole [43] with acetaldehyde and ammonium hydroxide under microwave irradiation (Scheme 6).
Scheme 6.
Synthesis of indolylsulfonamides [40] and [43].
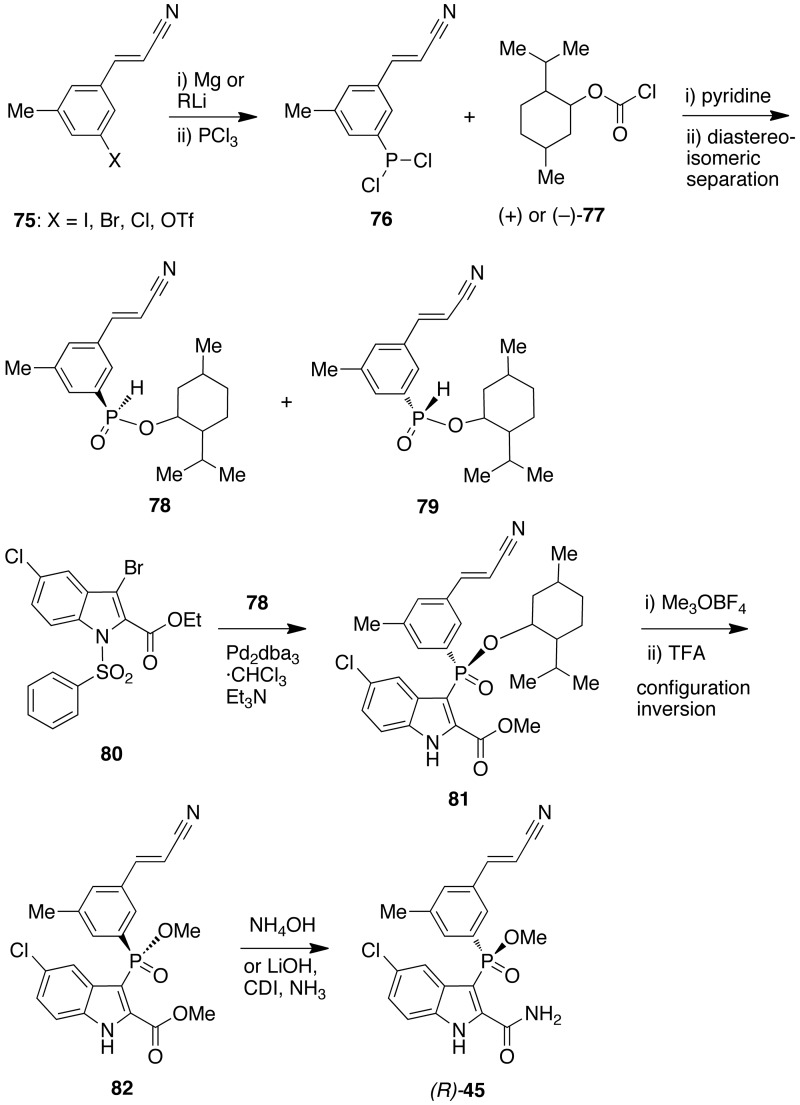
Compound (R)-[45] (IDX899)85 was synthesized starting from an aryl halide or triflate [75] in the presence of Mg or an alkyl Li and PCl3 to provide dichlorophosphite [76]. Compound [76] was treated with (+)- or (−)-menthylchloroformate [77] in pyridine (Hewitt reaction)86 to give the mixture of diastereoisomers [78] and [79] which were separated by crystallization at low temperature in n-hexane. Compound [78] was treated with ethyl 3-bromo-5-chloro-1-phenylsulfonyl-1H-indole-2-carboxylate [80] in the presence of tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct (Pd2(dba)3·CHCl3) and triethylamine to give [81]. Formation of [82] from [80] and trimethyloxonium tetrafluoroborate (Meerwein salt) and then trifluoroacetic acid underwent with inversion of configuration. Finally, (R)-[45] was obtained by reaction of [82] with ammonium hydroxide, or, alternatively, by hydrolysis of the ester with lithium hydroxide and subsequent treatment with ammonia by displacement of the intermediate imidazolide (Scheme 7).
Scheme 7.
Synthesis of API (R)-[45].
Conclusions
IASs are a potent class of HIV-1 NNRTIs. SAR studies led to improve remarkably the profile of L-737,126 discovered by Merck AG. Introduction of the 3′,5′-dimethyl groups at the 3-phenylsulfonyl moiety furnished IAS derivatives with potent and selective activity against HIV-1 mutants carrying NNRTI resistance mutations at positions 103 and 181 of the RT. The presence of a 2-hydroxyethyl tail at the indole-2-carboxamide nitrogen improved the activity against the HIV-1 K103N–Y181C double mutant. Coupling of the carboxamide nitrogen with one or two glycinamide and alaninamide units moieties produced short peptides with potent activity and selectivity against the HIV-1 WT and the mutant strains Y181C, K103N-Y181C and EFVR carrying K103R, V179D and P225H mutations. Introduction of a fluorine atom at position 4 of the indole ring improved the antiviral potency against the HIV-1 WT and the HIV-1 drug-resistant mutants. IASs bearing the third nucleus linked at the carboxamide nitrogen showed potent antiretroviral activity. Chiral derivatives having (R) configuration were significantly more potent than (S) counterparts against the HIV-1 mutant strains. The alanine spacer between the carboxamide nitrogen and the α-methylbenzyl brought attention to a correlation between configuration of the asymmetric centre and linker length: the (R)-enantiomers were superior to the (S)-counterparts when associated to a short linker unit, and the (S)-enantiomers were more potent than the (R)-enantiomers in the presence of a long linker. IAS derivatives are promising drug candidates for the treatment of AIDS/HIV-1 infection in combination with other HIV-1 agents. This review provides some key SARs for the design and synthesis of new potent HIV-1 NNRTIs.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.World Health Organization. HIV/AIDS fact sheet, http://www.who.int/mediacentre/factsheets/fs360/en/ (2016, accessed 9 July 2017).
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medlock J, Pandey A, Parpia AS, et al. Effectiveness of UNAIDS targets and HIV vaccination across 127 countries. Proc Natl Acad Sci USA 2017; 114: 4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. AIDSinfo. FDA-approved HIV medicines, https://aidsinfo.nih.gov/ (accessed 9 July 2017).
- 5.De Clercq E. Antiretroviral drugs. Curr Opin Pharmacol 2010; 10: 507–515. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E. The nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, and protease inhibitors in the treatment of HIV infections (AIDS). Adv Pharmacol 2013; 67: 317–358. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E. Dancing with chemical formulae of antivirals: a personal account. Biochem Pharmacol 2013; 86: 711–725. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E. Dancing with chemical formulae of antivirals: a panoramic view. Biochem. Pharmacol 2013; 86: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. US Food nd Drugs. Approved generic formulations of antiretroviral drugs used in the treatment of HIV infection, https://www.fda.gov/forpatients/ (accessed 9 July 2017).
- 10.Widera M, Dirks M, Bleekmann B, et al. HIV-1 persistent viremia is frequently followed by episodes of low-level viremia. Med Microbiol Immunol 2017; 206: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, https://aidsinfo.nih.gov/guidelines/ (accessed 9 July 2017).
- 12.Menéndez-Arias L. Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res 2013; 98: 93–120. [DOI] [PubMed] [Google Scholar]
- 13.Giacobbi NS andSluis-Cremer N.. In vitro cross-resistance profiles of rilpivirine, dapivirine, and MIV-150, nonnucleoside reverse transcriptase inhibitor microbicides in clinical development for the prevention of HIV-1 infection. Antimicrob Agents Chemother 2017; 61: e00277-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackie N. Resistance to non-nucleoside reverse transcriptase inhibitors. In: Geretti AM (ed) Antiretroviral resistance in clinical practice. Chapter 2. London: Mediscript, 2006. [PubMed]
- 15.Cortez KJ andMaldarelli F.. Clinical management of HIV drug resistance. Viruses 2011; 3: 347–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res 2010; 85: 201–209. [DOI] [PubMed] [Google Scholar]
- 17.Jacobo-Molina A, Clark AD, Jr, Williams RL, et al. Crystals of a ternary complex of human immunodeficiency virus type 1 reverse transcriptase with a monoclonal antibody Fab fragment and double-stranded DNA diffract x-rays to 3.5-Å resolution. Proc Natl Acad Sci USA 1991; 88: 10895–10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlstaedt LA, Wang J, Friedman JM, et al. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 1992; 256: 1783–1790. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YE, Dutschman GE, Bastow KF, et al. Human immunodeficiency virus reverse transcriptase. General properties and its interactions with nucleoside triphosphate analogs. J Biol Chem 1987; 262: 2187–2189. [PubMed] [Google Scholar]
- 20.Jacobo-Molina A, Ding J, Nanni RG, et al. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc Natl Acad Sci USA 1993; 90: 6320–6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers DW, Gamblin SJ, Harris BA, et al. The structure of unliganded reverse transcriptase from the human immunodeficiency virus type 1. Proc Natl Acad Sci USA 1995; 92: 1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen KA, Hopkins J, Ingraham RH, et al. Characterization of the binding site for nevirapine (BI-RG-587), a nonnucleoside inhibitor of human immunodeficiency virus type-1 reverse transcriptase. J Biol Chem 1991; 266: 14670–14674. [PubMed] [Google Scholar]
- 23.Tantillo C, Ding J, Jacobo-Molina A, et al. Locations of anti-AIDS drug binding sites and resistant mutations in the three dimensional structure of HIV-1 reverse transcriptase. J Mol Biol 1994; 243: 369–387. [DOI] [PubMed] [Google Scholar]
- 24.McMahon JB, Gulakowski RJ, Weislow OS, et al. Diarylsulfones, a new chemical class of nonnucleoside antiviral inhibitors of immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother 1993; 37: 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens CE, Felder TM, Sowell JW, Sr, et al. antitumor evaluation of 2-amino- and 2-carboxamido-3-arylsulfonylthiophenes and related compounds as a new class of dirarylsulfones. Bioorg Med Chem 2001; 9: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 26.Buckheit RW, Jr, Kliakas-Boltz V, Russel DJ, et al. Inhibitor with a unique sensitivity profile to drug resistance virus isolates. Antivir Chem Chemother 1996; 7: 243–252. [Google Scholar]
- 27.Chan JH, Hong SJ, Hunter RN III, et al. 2-Amino-6-arylsulfonylbenzonitriles as non-nucleoside reverse transcriptase inhibitors of HIV-1. J Med Chem 2001; 44: 1866–1882. [DOI] [PubMed] [Google Scholar]
- 28.Artico M, Silvestri R, Stefancich G, et al. Synthesis of pyrryl aryl sulfones targeted at the HIV-1 reverse transcriptase. Arch Pharm 1995; 328: 223–229. [DOI] [PubMed] [Google Scholar]
- 29.Artico M, Silvestri R, Massa S, et al. 2-Sulfonyl-4-chloroanilino moiety: a potent pharmacophore for the anti-human immunodeficiency virus type 1 activity of pyrrolyl aryl sulfones. J Med Chem 1996; 39: 522–530. [DOI] [PubMed] [Google Scholar]
- 30.Artico M, Silvestri R, Pagnozzi E, et al. Structure-based design, synthesis and biological evaluation of novel pyrrolyl aryl sulfones (PASs), HIV-1 non-nucleoside reverse transcriptase inhibitors active at nanomolar concentrations. J Med Chem 2000; 43: 1886–1891. [DOI] [PubMed] [Google Scholar]
- 31.Artico M, Silvestri R, Massa S, et al. Pirril-(indolil-)aril-solfoni e relativo processo di produzione ed impiego nella terapia delle infezioni da virus. Patentapplication MI95A000812, 1995.
- 32.Artico M, Massa S, Silvestri R, et al. 1H-pyrrol-1-yl and 1H-indol-1-yl sulphones, processes for their preparation and use for therapy of HIV-1 infections. Patentapplication WO 96/33171, 1996.
- 33.Artico M, Silvestri R, Pagnozzi E, et al. 5H-pyrrolo[1,2-b][1,2,5]benzothiadiazepines (PBTDs): a novel class of HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem 1996; 4: 837–850. [DOI] [PubMed] [Google Scholar]
- 34.Silvestri R, Artico M, Pagnozzi E, et al. Synthesis and anti-HIV activity of 10,11-dihydropyrrolo[1,2-b][1,2,5]benzothiadiazepine-11-acetic acid 5,5-dioxide derivatives and related compounds. Farmaco 1996; 51: 425–430. [PubMed] [Google Scholar]
- 35.Williams TM, Ciccarone TM, MacTough SC, et al. 5-Chloro-3-(phenylsulfonyl)indole-2-carboxamide: a novel, non-nucleoside inhibitor of HIV-1 reverse transcriptase. J Med Chem 1993; 36: 1291–1294. [DOI] [PubMed] [Google Scholar]
- 36.Williams TA, Ciccarone TM, Saari WS, et al. Indoles as inhibitors of HIV reverse transcriptase. Patentapplication EP 0 530 907 A1, 1992.
- 37.Williams TM, Ciccarone TM, Saari WS, et al. Theoharides AD. Inhibitors of HIV reverse transcriptase. Patent WO 94/19321.
- 38.Silvestri R, De Martino G, La Regina G, et al. Novel indolyl aryl sulfones active against HIV-1 carrying NNRTI resistance mutations: synthesis and SAR studies. J Med Chem 2003; 46: 2418–2493. [DOI] [PubMed] [Google Scholar]
- 39.Young SD, Amblard M, Brichter SF, et al. 2-Heterocyclic indole-3-sulfones as inhibitors of HIV-1 reverse transcriptase. Bioorg Med Chem Lett 1995; 5: 491–496. [Google Scholar]
- 40.Silvestri R, De Martino G, La Regina G, et al. Novel indolyl aryl sulfones active against HIV-1 carrying NNRTI resistance mutations: synthesis and SAR studies. J Med Chem 2003; 46: 2482–2493. [DOI] [PubMed] [Google Scholar]
- 41.Ragno R, Artico M, de Martino, et al. QSAR studies on indolyl aryl sulfones (IASs). Binding mode exploration at the HIV-1 reverse transcriptase non-nucleoside binding site and design of highly active N-(2-hydroxyethyl)carboxyamide and N-(2-hydroxyethyl)carboxyhydrazide derivatives. J Med Chem 2005; 48: 213–223. [DOI] [PubMed] [Google Scholar]
- 42.De Martino G, La Regina G, Ragno R, et al. Indolyl aryl sulphones as HIV-1 non-nucleoside reverse transcriptase inhibitors: synthesis, biological evaluation and binding mode studies of new derivatives at indole-2-carboxamide. Antivir Chem Chemother 2006; 17: 59–77. [DOI] [PubMed] [Google Scholar]
- 43.Ragno R, Coluccia A, La Regina G, et al. Design, molecular modeling, synthesis, and anti-HIV-1 activity of new indolyl aryl sulfones. Novel derivatives of the indole-2-carboxamide. J Med Chem 2006; 49: 3172–3184. [DOI] [PubMed] [Google Scholar]
- 44.Silvestri R, Artico M, De Martino G, et al. Simple, short peptide derivatives of a sulfonylindolecarboxamide (L-737,126) active in vitro against HIV-1 wild type and variants carrying non-nucleoside reverse transcriptase inhibitor resistance mutations. J Med Chem 2004; 47: 3892–3896. [DOI] [PubMed] [Google Scholar]
- 45.Piscitelli F, Coluccia A, Brancale A, et al. Indolylarylsulfones bearing natural and unnatural amino acids. Discovery of potent inhibitors of HIV-1 non-nucleoside wild type and resistant mutant strains reverse transcriptase and coxsackie B4 virus. J Med Chem 2009; 52: 1922–1934. [DOI] [PubMed] [Google Scholar]
- 46.Corbett JW andRodgers JD.. Discovery of second generation quinazolinone non-nucleoside reverse transcriptase inhibitors of HIV-1. Prog Med Chem 2002; 40: 63–105. [DOI] [PubMed] [Google Scholar]
- 47.Hoetelmans RMV. Pharmacology of antiretroviral drugs. Antivir Ther 1999; 4: 29–41. [PubMed] [Google Scholar]
- 48.Dong BJ. Efavirenz DuPont Pharmaceuticals Co. IDrugs 1998; 6: 700–711. [PubMed] [Google Scholar]
- 49.Corbett JW, Ko SS, Rodgers JD, et al. Expanded-spectrum nonnucleoside reverse transcriptase inhibitors inhibit clinically relevant mutant variants of human immunodeficiency virus type 1. Antimicrob Agents Chemother 1999; 43: 2893–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel M, McHugh RJ, Jr, Cordova BC, et al. Synthesis and evaluation of benzoxazinones as HIV-1 reverse transcriptase inhibitors. Analogs of Efavirenz (SUSTIVA). Bioorg Med Chem Lett 1999; 9: 3221–3224. [DOI] [PubMed] [Google Scholar]
- 51.Corbett JW, Ko SS, Rodgers JD, et al. Inhibition of clinically relevant mutant variants of HIV-1 by quinazolinone non-nucleoside reverse transcriptase inhibitors. J Med Chem 2000; 43: 2019–2030. [DOI] [PubMed] [Google Scholar]
- 52.Regina GL, Coluccia A, Piscitelli F, et al. Indolyl aryl sulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: role of two halogen atoms at the indole ring in developing new analogues with improved antiviral activity. J Med Chem 2007; 50: 5034–5038. [DOI] [PubMed] [Google Scholar]
- 53.Samuele A, Kataropoulou A, Viola M, et al. Non-nucleoside HIV-1 reverse transcriptase inhibitors di-halo-indolyl aryl sulfones achieve tight binding to drug-resistant mutants by targeting the enzyme-substrate complex. Antiviral Res 2009; 81: 47–55. [DOI] [PubMed] [Google Scholar]
- 54.Cancio R, Silvestri R, Ragno R, et al. High potency of indolyl aryl sulfone nonnucleoside inhibitors towards drug-resistant human immunodeficiency virus type 1 reverse transcriptase mutants is due to selective targeting of different mechanistic forms of the enzyme. Antimicrob Agents Chemother 2005; 49: 4546–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.La Regina G Coluccia A andSilvestri R.. Looking for an active conformation of the future HIV type-1 non-nucleoside reverse transcriptase inhibitors. Antivir Chem Chemother 2010; 20: 231–237. [DOI] [PubMed] [Google Scholar]
- 56.Ludovici DW, Kavash RW, Kukla MJ, et al. Evolution of anti-HIV drug candidates. Part 2: diaryltriazine (DATA) analogues. Bioorg Med Chem Lett 2001; 11: 2229–2234. [DOI] [PubMed] [Google Scholar]
- 57.Klunder JM, Hoermann M, Cywin CL, et al. Novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 7. 8-Arylethyldipyridodiazepinones as potent broad-spectrum inhibitors of wild-type and mutant enzymes. J Med Chem 1998; 41: 2960–2971. [DOI] [PubMed] [Google Scholar]
- 58.Cywin CL, Klunder JM, Hoermann M, et al. Novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 8. 8-Aryloxymethyl- and 8-arylthiomethyldipyridodiazepinones. J Med Chem 1998; 41: 2972–2984. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Z, Wolkenberg SE, Sanderson PEJ, et al. Novel indole-3-sulfonamides as potent HIV non-nucleoside reverse transcriptase inhibitors (NNRTIs). Bioorg Med Chem Lett 2008; 18: 554–559. [DOI] [PubMed] [Google Scholar]
- 60.La Regina G, Coluccia A, Brancale A, et al. Indolylarylsulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: new cyclic substituents at indole-2-carboxamide. J Med Chem 2011; 54: 1587–1598. [DOI] [PubMed] [Google Scholar]
- 61.Samuele A, Bisi S, Kataropoulou A, et al. Mechanism of interaction of novel indolylarylsulfone derivatives with K103N and Y181I mutant HIV-1 reverse transcriptase in complex with its substrates. Antivir Chem Chemother 2011; 22: 107–118. [DOI] [PubMed] [Google Scholar]
- 62.La Regina G, Coluccia A, Brancale A, et al. New nitrogen containing substituents at the indole-2-carboxamide yield high potent and broad spectrum indolylarylsulfone HIV-1 non-nucleoside reverse transcriptase inhibitors. J Med Chem 2012; 55: 6634–6638. [DOI] [PubMed] [Google Scholar]
- 63.Manetti F, Esté JA, Clotet-Codina I, et al. Parallel solution-phase and microwave-assisted synthesis of new S-DABO derivatives endowed with subnanomolar anti-HIV-1 activity. J Med Chem 2005; 48: 8000–8008. [DOI] [PubMed] [Google Scholar]
- 64.Famiglini V and, Silvestri R. Focus on chirality of HIV-1 non-nucleoside reverse transcriptase inhibitors. Molecules 2016; 21: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Famiglini V, La Regina G, Coluccia A, et al. New indolylarylsulfones as highly potent and broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitors. Eur J Med Chem 2014; 80: 101–111. [DOI] [PubMed] [Google Scholar]
- 66.Geitmann M Unge T andDanielson UH.. Interaction kinetic characterization of HIV-1 reverse transcriptase non-nucleoside inhibitor resistance. J Med Chem 2006; 49: 2375–2387. [DOI] [PubMed] [Google Scholar]
- 67.Famiglini V, La Regina G, Coluccia A, et al. Indolylarylsulfones carrying a heterocyclic tail as very potent and broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitors. J Med Chem 2014; 57: 9945–9957. [DOI] [PubMed] [Google Scholar]
- 68.Hsiou Y, Ding J, Das K, et al. The Lys103Asn mutation of HIV-1 RT: a novel mechanism of drug resistance. J Mol Biol 2001; 309: 437–445. [DOI] [PubMed] [Google Scholar]
- 69.Wodak SJ andJanin J.. Analytical approximation to the accessible surface area of proteins. Proc Natl Acad Sci USA 1980; 77: 1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Famiglini V, La Regina G, Coluccia A, et al. Chiral indolylarylsulfone non-nucleoside reverse transcriptase inhibitors as new potent and broad spectrum anti-HIV-1 agents. J Med Chem 2017; 60: 6528–6547. [DOI] [PubMed]
- 71.May MT andIngle SM.. Life expectancy of HIV-positive adults: a review. Sex Health 2011; 8: 526–533. [DOI] [PubMed] [Google Scholar]
- 72.Clifford DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med 2008; 16: 94–98. [PubMed] [Google Scholar]
- 73.Grovit-Ferbas K andHarris-White ME.. Thinking about HIV: the intersection of virus, neuroinflammation and cognitive dysfunction. Immunol Res 2010; 48: 40–58. [DOI] [PubMed] [Google Scholar]
- 74.Joska JA, Gouse H, Paul RH, et al. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol 2010; 16: 101–114. [DOI] [PubMed] [Google Scholar]
- 75.Alexandre FR, Amador A, Bot S, et al. Synthesis and biological evaluation of aryl-phospho-indole as novel HIV-1 non-nucleoside reverse transcriptase inhibitors. J Med Chem 2011; 54: 392–395. [DOI] [PubMed] [Google Scholar]
- 76.Dousson C, Alexandre FR, Amador A, et al. Discovery of the aryl-phospho-indole IDX899, a highly potent anti-HIV non-nucleoside reverse transcriptase inhibitor. J Med Chem 2016; 59: 1891–1898. [DOI] [PubMed] [Google Scholar]
- 77.Klibano OM andKaczor RL.. IDX-899, an aryl phosphinate-indole non-nucleoside reverse transcriptase inhibitor for the potential treatment of HIV infection. Curr Opin Investig Drugs 2010; 11: 237–245. [PubMed] [Google Scholar]
- 78.Zala C, St Clair M, Dudas K, et al. Safety and efficacy of GSK2248761, a next-generation nonnucleoside reverse transcriptase inhibitor, in treatment-naive HIV-1-infected subjects. Antimicrob Agents Chemother 2012; 56: 2570–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atkinson JG Hamel P andGirard Y.. A new synthesis of 3-arylthioindoles. Synthesis 1988; 480–481. [Google Scholar]
- 80.Heath-Brown B andPhilpott PG.. The indole series. III. The Japp-Klingemann reaction. J Chem Soc 1965; 7185–7193. [Google Scholar]
- 81.Robinson B. The Fischer indole synthesis. Chichester: John Wiley and Sons, 1982. [Google Scholar]
- 82.Silvestri R, Artico M, la Regina G, et al. Synthetic approaches to difluoroindole carboxylic acid ethyl esters. Arkivoc 2004; 5: 26–31. [Google Scholar]
- 83.Silvestri R de Martino G andSbardella G.. A simplified synthesis of ethyl 5-chloro-6-fluoro-1H-indole-2-carboxylate and ethyl 5-chloro-4-fluoro-1H-indole-2-carboxylate. Organic Prep Proc Int 2002; 34: 507–510. [Google Scholar]
- 84.Mayes BA, Chaudhuri NC, Hencken CP, et al. Robust synthesis of methyl 5-chloro-4-fluoro-1H-indole-2-carboxylate: a key intermediate in the preparation of an HIV NNRTI candidate. Organic Process Res Dev 2010; 14: 1248–1253. [Google Scholar]
- 85.Storer R, Alexande FR, Dousson C, et al. Enantiomerically pure phosphoindoles as HIV inhibitors. PatentPCT WO/2008/042240, 2008.
- 86.Afarinkia K andYu HW.. Hewitt reaction revisited. Tetrahedron Lett 2003; 44: 781–783. [Google Scholar]