Abstract
Background & objectives:
Hyperosmolar hyperglycaemic state (HHS) is a medical emergency, but there is a paucity of studies reporting the spectrum of neurological manifestations of HHS. We, therefore, report the neurological spectrum, triggering factors and outcome of HHS in general neurology practice.
Methods:
The records of the patients with HHS were extracted from computerized hospital information system and those managed currently were prospectively included. The demographic, clinical manifestations, duration of diabetes and precipitating events such as infection, stress and stroke were noted. Patients with HHS were categorized into seizure, movement disorder and encephalopathy groups. Their electroencephalography, magnetic resonance imaging (MRI) findings and outcome were noted.
Results:
There were 17 patients with HHS (age range 40 and 75 yr) and seven were females. Seven patients were diabetic for five years, one for four years, one for one year and four were diagnosed after the occurrence of HHS. Four patients had epilepsia partialis continua persisting for 72-360 h, one patient had focal seizures and his MRI revealed T2 hyperintensity in frontal region in one patient and cerebellar vermian hyperintensity in another. All the five patients improved, but two had neurological deficits on discharge. Nine patients had encephalopathy which was precipitated by stroke in six patients, urinary infections in two and meningitis in one. Three females had hemichorea-hemiballismus syndrome, which was triggered by infections. Abnormal movements lasted 5-10 days and responded to correction of hyperosmolarity. Nine out of 17 patients improved completely whereas the remaining eight had partial recovery, these patients had stroke, ventilator-related complications or meningoencephalitis.
Interpretation & conclusions:
The most common presentation of HHS was encephalopathy (9) followed by seizure (5) and hemichorea-hemiballismus syndrome (3) which responded to the correction of hyperosmolar state.
Keywords: Encephalopathy, epilepsia partialis continua, hemiballismus, hemichorea, hyperosmolar hyperglycaemic state, movement disorder, magnetic resonance imaging
Hyperosmolar hyperglycaemic state (HHS) was first described more than 100 years ago by Frerichs1 although this condition received little attention until the report of Sament and Schwartz in 19572. HHS is characterized by hyperglycaemia, hyperosmolarity and dehydration in the absence of ketoacidosis. HHS is the most serious complication of hyperglycaemia and may account for 10 to 47 per cent cases of hyperglycaemia and has a mortality of 5- 20 per cent which is about 10 times higher than diabetic ketoacidosis (DKA)3. HHS is attributed to deficient insulin function along with increase in counter regulatory hormones. The neurological manifestations in HHS are diverse and include encephalopathy, focal seizure and hyperkinetic movement disorders.
In a retrospective analysis of 51 patients with HHS, 16 per cent died4. Low Glasgow Coma Scale (GCS) score, high plasma glucose and mild acidosis were predictors of mortality4. The neurological complications of HHS such as seizures, particularly partial seizures5,6, hyperkinetic movement disorders7 and coma3, have been reported in isolated case reports or small case series. There is a paucity of studies reporting the spectrum of neurological manifestations of HHS and their outcome. Here we report the neurological manifestations of HHS, their triggering factors, magnetic resonance imaging (MRI) findings and outcome.
Material & Methods
This was both a retrospective and prospective analysis of patients with HHS admitted in Neurology Service of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, from 2004 to 2015. The data of 10 retrospective patients with HHS were retrieved from the computerized hospital information system and seven patients admitted during 2014-2015 were prospectively evaluated. The diagnosis of HHS was based on hyperglycaemia (blood glucose >600 mg/dl) or hyperosmolarity (>300 mOsmol/l) and absence of urinary ketone.
Parameters: Patients’ demographic information (age, gender and residence) and associated comorbidities such as hypertension, dyslipidaemia, cardiovascular disorder, renal failure, chronic liver disease and chronic obstructive lung disease were noted. Precipitating factors of HHS such as pneumonia, urinary tract infection, fungal diseases and otitis media were noted. A history of focal or generalized seizure and its duration were recorded. Status epilepticus was defined as continuous seizures for >five minutes or discrete seizures without regaining full consciousness in between. Any movement disorders such as chorea, athetosis, ballism and tremor were noted. The distribution of movement disorder, severity and duration were recorded. Any impairment in consciousness was also noted, and the depth of coma was assessed by GCS. Presence of cranial nerve palsy including papilloedema was noted. Focal weakness was categorized into monoplegia, hemiplegia or quadriplegia. Cranial computed tomography (CT) scan and/or MRI with contrast were done. The location and type of abnormalities on imaging were noted. The MRI scans were reviewed, and the signal changes of the lesions in different sequences were noted. Blood counts, haemoglobin, blood urea nitrogen, serum creatinine, protein, albumin, transaminase, calcium, phosphorus, sodium and potassium were noted. Blood sugar levels were noted, and the time taken to normalize was also recorded. Arterial blood gas analysis was done on admission and the osmolarity was noted. In patients who had already received insulin before osmolarity study, their serum osmolarity was calculated by the initial blood sugar, serum sodium, serum potassium and blood urea nitrogen values [2×(Na+K)+BUN/2.8)+glucose/18]. Chest radiograph and ECG findings were also recorded. The patients were categorized into movement disorder, seizure and altered sensorium groups.
Management: The patients were managed with intravenous saline 8-10 l/day. Intravenous insulin was prescribed in a dose of 0.1 units/kg/h, followed by subcutaneous maintenance dose once the blood sugar was below 250 mg/dl. Patients with hyperkinetic movement disorders received tetrabenazine and/or clonazepam; those with seizure received intravenous antiepileptic drugs which were then gradually withdrawn after confirmation of diagnosis. The outcome was defined as death during hospital stay or disability at the time of discharge.
The study was approved by the ethics committee of the institute and written informed consents were obtained from patients included in the prospective analysis.
Statistical analysis: The biochemical, MRI and outcome of HHS patients with movement disorder, seizure and encephalopathy were compared by Fisher's exact test for categorical and ANOVA for continuous variables. The clinical, biochemical and MRI findings of the patients who recovered completely were also compared with those who developed disability using Fisher's exact test for categorical and Mann–Whitney U-test for continuous variables. The variable having a two-tailed P<0.05 was considered significant. The statistical analysis was done by SPSS version 16 software (SPSS Inc., Chicago, IL, USA).
Results
There were 17 patients with HHS (mean age 58.5±12.6 yr, range: 40-75 yr) and seven were females. They had a long duration of diabetes mellitus (median: 20 yr); however, in four patients, HHS led to the diagnosis of diabetes mellitus. The diagnosis of diabetes mellitus in these patients was based on HbA1c. The patients reported to our institute after a mean duration of 4.8 days (range: 1-15 days) from the onset of neurological manifestations. The precipitating factors of HHS could be identified in 13 patients; infections in seven and stroke in six patients. The comorbidities included ischaemic heart disease in one patient, rheumatic heart disease in one, tubercular meningitis in two and renal failure in six patients.
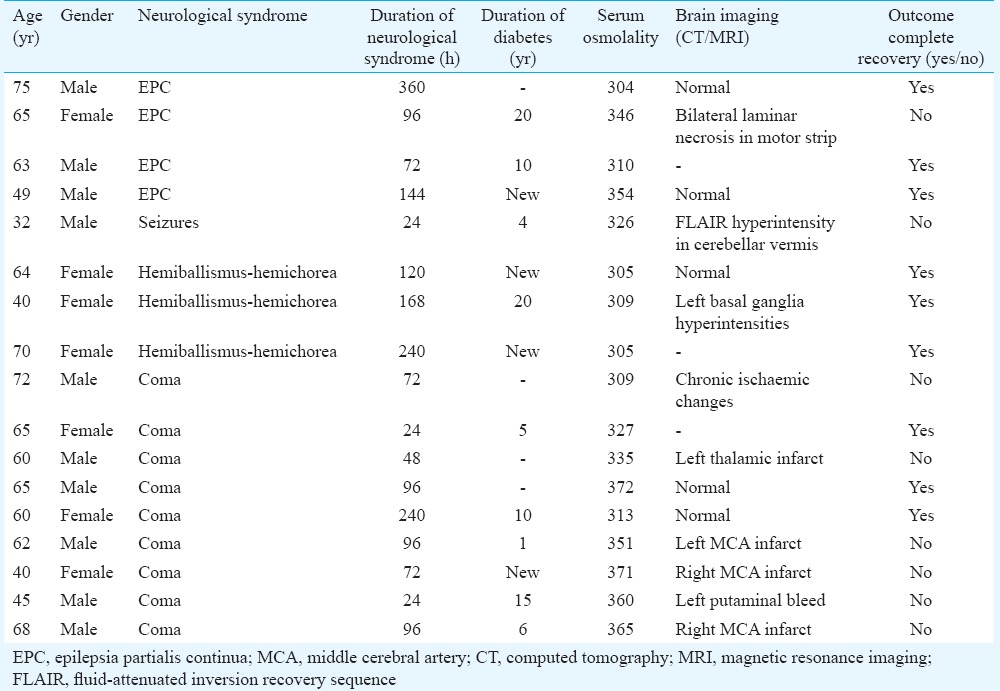
The neurological manifestations of HHS were seizure in five, hemichorea-hemiballismus in three and coma in nine patients. The details of these patients are summarized in the Table.
Table.
Clinical imaging and outcome of patients with hyperosmolar hyperglycaemic state
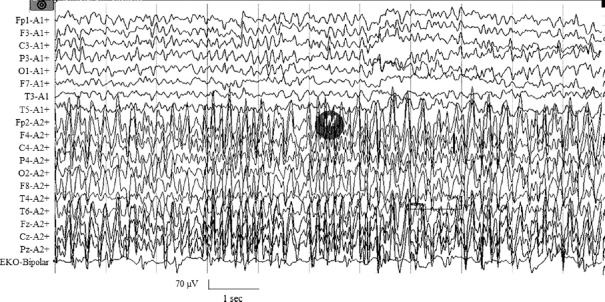
Seizures: Four patients presented with epilepsia partialis continua (EPC) and one had discrete focal seizures. The duration of EPC ranged between 72 and 360 h. Their mean age was 56.8±16.7 yr (range 32-75 yr) and one was female. MRI could be reviewed in four patients and one revealed T2 hyperintensity in the frontal cortex. The retrospective analysis of electroencephalography (EEG) in the first three patients was normal, but the exact time from the EPC to EEG in them was not available. EEG of one out of two prospectively analyzed patients showed focal spike and wave discharges with background delta slowing (Fig. 1) and the other patient had delta slowing which was done after termination of seizure.
Fig. 1.
Electroencephalography recording of a 51 yr old male who was a newly diagnosed diabetic with chronic renal disease having epilepsia partialis continua. At the time of recording, the serum osmolarity was 354 mOsmol/l which was controlled to 304 mOsmol/l after 24 h when his seizures subsided.
In two patients, seizures subsided following control of osmolarity and blood sugar level. Three patients received multiple antiepileptic drugs (lorazepam, divalproate, carbamazepine and levetiracetam) and their EPC continued till 4-15 days. The blood sugar in this group ranged between 329 and 593 mg/dl and osmolarity ranged between 304 and 354 mOsmol/l. Three patients recovered completely and two had disability on discharge due to ventilator-associated complication in one and coexistent tubercular meningitis in the other.
Chorea-hemiballismus syndrome: Three patients had chorea-hemiballismus and all were females between 40 and 70 yr of age. The duration of hemichorea was 5-10 days. Two of them were diagnosed as diabetic after the neurological manifestations and the other was poorly controlled on oral hypoglycaemic drug. In all the three patients, the hemichorea was triggered by urinary tract infection and two of them had fever. MRI revealed T2- and fluid-attenuated inversion recovery hyperintensities in basal ganglia which were non-enhancing in one and enhancing in another patient (Fig. 2). In the other patient, the MRI was normal. Serum osmolarity of these patients ranged between 305 and 309 mOsmol/l. All the three patients responded to control of hyperglycaemia and hyperosmolar state in 3-4 days and were discharged without any sequelae.
Fig. 2.
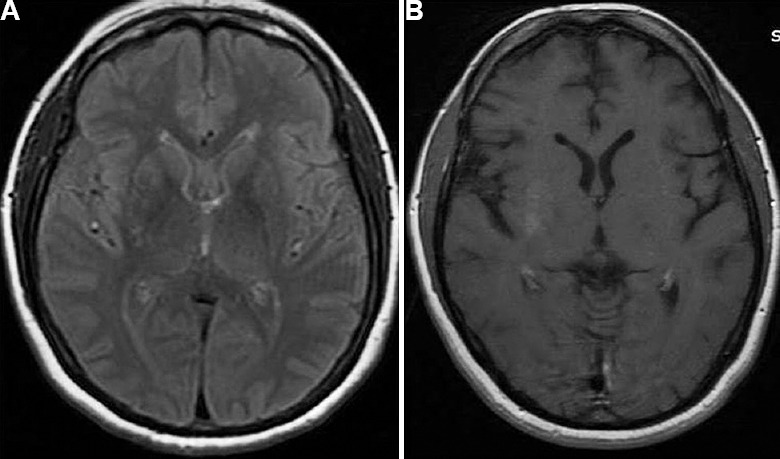
Cranial magnetic resonance imaging axial section (A) fluid-attenuated inversion recovery and (B) T1 contrast of a 40 yr old female presenting with hemiballismus-hemichorea syndrome with admission serum osmolarity of 309 mOsmol/l (patient no. 7).
Coma: Altered sensorium was the presenting symptom in nine patients and their age ranged between 40 and 72 years. Seven had poorly controlled diabetes for 5-15 yr, one was diabetic for one year and the other was diagnosed as diabetic after the neurological manifestation. The patients were in altered sensorium for 1-10 days prior to admission. Precipitating causes were noted in six patients and included stroke in four and urinary tract infection and lymphocytic meningitis in one patient each. Their serum osmolarity ranged between 309 and 372 mOsmol/l. They were treated with antibiotics, fluids and insulin and recovered completely within 4-6 days except for six patients who had associated stroke.
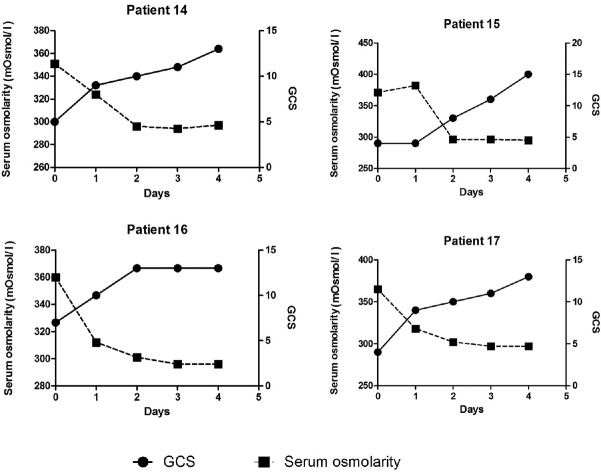
Correlation of serum osmolarity with consciousness: In four patients, stroke triggered HHS who were prospectively evaluated for relationship of serum osmolarity and consciousness level, the initial median GCS score was 4.5 (range: 4-7) and serum osmolarity 361.75 mOsmol/l (range: 351-371 mOsmol/l) which improved as the serum osmolarity normalized (Fig. 3).
Fig. 3.
Relationship of serum osmolarity and Glasgow Coma Scale in four patients who presented with coma as a manifestation of hyperosmolar hyperglycaemic state (patient nos. 14, 15, 16 and 17).
Comparison: The presenting syndrome of encephalopathy, seizure and movement disorders were not related to age, gender, serum osmolarity on admission, associated comorbidities, brain imaging findings and duration of illness. The sequelae, however, were more common in the patients with abnormal brain imaging (8 vs 1, P<0.001) and insignificantly higher serum osmolarity (345.36± 21.0 vs 322.11± 24.61 mOsmol/l, respectively, P<0.06).
Discussion
In the present study, the most common neurological presentation of HHS was coma followed by focal seizures and hemichorea-hemiballismus. None of the patients died and nine had complete recovery. Hemichorea-hemiballismus was noted only in females and they responded well to treatment of hyperosmolar state.
Hemichorea-hemiballismus is attributed to the abnormality in contralateral striatum by ischaemia, inflammation, degenerative disorders or systemic disease such as systemic lupus erythematosus. Hemichorea-hemiballismus has also been reported in patients with HHS having long-standing poorly controlled diabetes mellitus and is attributed to the mild ischaemic changes in the distribution of lateral striate arteries which are branch of middle cerebral artery. The abnormal movement subsides after control of blood sugar and hyperosmolarity7. These lesions are non-enhancing and T1 hyperintense on MRI and hyperdense on CT scan8. On single-photon emission CT study, there was reduced blood flow in subthalamic nuclei, caudate, putamen, pallidum and thalamus9. The inhibition of caudate and putamen by indirect pathways is reduced following ischaemia, and the direct pathways become burst generating and are responsible for hyperkinetic movement disorder. The overactivity of the direct pathway results in overactivity of neurons and glia which has been attributed to shortening of T1. It has been suggested that the metabolic changes in HHS result in the production of acanthocytes which may in turn result in cross-activation of other antigens in striatum10. All the patients in our study who had hemichorea-hemiballismus were postmenopausal women. In a study on hemichorea-hemiballismus in HHS, the female-to-male ratio was 1.8:1 and Asian postmenopausal women were more commonly affected11. Common occurrence of this syndrome in Asian women may be due to a genetic susceptibility or higher dopamine sensitivity of striatum in postmenopausal women12.
Five patients in our study had seizure and two patients with EPC were refractory to multiple antiepileptic drugs. Generalized seizure is the rule in metabolic abnormality whereas focal seizure is common in HHS. In HHS, focal seizure occurs in 10-25 per cent of patients5,13. In a report of 158 cases of HHS, 19 per cent of patients had focal seizures which were refractory to antiepileptic drugs and responded to insulin therapy and hydration5. In a review of 162 patients with EPC, hyperosmolar non-ketotic coma was the underlying cause in 58.8 per cent of patients6. Rarely, occipital lobe seizures14 and reflex- or posture-induced focal seizure have been reported in HHS15,16,17. The aetiology of focal seizure is not well understood in hyperglycaemia but seizure is rare in DKA. The lower incidence of seizure in ketoacidosis may be due to acidic pH which reduces the neuronal excitability. The anticonvulsant effect of ketones may result in intracellular acidosis and increased gamma-aminobutyric acid (GABA) level due to increased activity of glutamic acid decarboxylase15. In HHS, Krebs cycle is inhibited and GABA metabolism is increased which may lower the seizure threshold18,19. The additional factors such as hyperosmolarity, dehydration and hyponatremia may also be responsible for focal seizures5. It has also been suggested that hyperglycaemia-induced neuronal ischaemia or pre-existing cortical ischaemic lesions may also contribute to HHS-associated seizures after acquiring epileptogenic character under altered metabolic conditions20.
It is interesting to note that focal syndromes (focal seizure and hemichorea-hemiballismus) in HHS occur in spite of diffuse metabolic abnormality (unpublished observations). Hyperglycaemia results in reduction of blood flow to striatum and cerebral cortex. Elevation of plasma glucose from 162 mg/dl to 702 mg/dl reduced the cerebral blood flow by 24 per cent compared to control20. Hyperglycaemia results in alteration of blood-brain barrier with altered transport of glucose, insulin, choline, amino acids and oxidative stress in central nervous system (CNS) micro-capillaries. Hyperglycaemia also results in the progression of cerebral ischaemia and consequent injury to CNS21. The most common manifestation of HHS was altered sensorium. A dose-response relationship with serum osmolarity and altered sensorium has been reported, serum osmolarity of 324 mOsmol/l or more results in mental obtundation and that above 349 mOsmol/l results in coma22. In our study, blood osmolarity, however, was similar in all the three groups of neurological manifestations; eight patients had sequelae due to the underlying disease and none due to HHS. HHS may be precipitated by concomitant and acute medical conditions in long-standing diabetics. Infection was the most common trigger of HHS in our study. Acute infection may contribute to encephalopathy, especially in elderly patients. In our study, there was no death and only eight patients had sequelae at the time of discharge and the remaining improved completely. The mortality in HHS has been reduced from 60 to 11 per cent which may be due to early detection and improved treatment strategies23. The elderly patients have higher mortality (~20 %) with HHS than with DKA (2 %)24.
Our study was limited by a retrospective design, a small sample size and calculation of osmolarity with retrospective parameters as many of them did not fulfil HHS at the time of admission because of prior fluid and insulin treatment. This limitation has also been highlighted in a study on 21 patients in whom the mean serum osmolarity was 302 mOsmol/l and mean blood glucose was 587.56 mg/dl25. Our study also had a referral bias of a tertiary care centre where severe or refractory cases are referred; therefore, the results of the present study may not be applicable to HHS as a whole.
In conclusion, our study suggests that prompt diagnosis, aggressive management of osmolarity, hyperglycaemia and comorbidities may improve the outcome of HHS patients. The therapy has to be targeted to the underlying pathophysiology of HHS rather than antiepileptic drugs in seizure and antidystonia drugs in hemichorea-hemiballismus.
Acknowledgment
Authors thank Sarvshri Shakti and Deepak Kumar Anand for secretarial help.
Footnotes
Conflicts of Interest: None.
References
- 1.Frerichs FT. About sudden death and coma in diabetes. Z Klin Med (Berlin) 1883;6:1. [Google Scholar]
- 2.Sament S, Schwartz MB. Severe diabetic stupor without ketosis. S Afr Med J. 1957;31:893–4. [PubMed] [Google Scholar]
- 3.Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Diabetes Spectr. 2002;15:28–36. [Google Scholar]
- 4.Fadini GP, de Kreutzenberg SV, Rigato M, Brocco S, Marchesan M, Tiengo A, et al. Characteristics and outcomes of the hyperglycemic hyperosmolar non-ketotic syndrome in a cohort of 51 consecutive cases at a single center. Diabetes Res Clin Pract. 2011;94:172–9. doi: 10.1016/j.diabres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Singh BM, Strobos RJ. Epilepsia partialis continua associated with nonketotic hyperglycemia: Clinical and biochemical profile of 21 patients. Ann Neurol. 1980;8:155–60. doi: 10.1002/ana.410080205. [DOI] [PubMed] [Google Scholar]
- 6.Löhler J, Peters UH. Continuous partial epilepsy (Kozhevnikov epilepsy) Fortschr Neurol Psychiatr Grenzgeb. 1974;42:165–212. [PubMed] [Google Scholar]
- 7.Bhagwat NM, Joshi AS, Rao G, Varthakavi PK. Uncontrolled hyperglycaemia: A reversible cause of hemichorea-hemiballismus. BMJ Case Rep 2013. 2013 doi: 10.1136/bcr-2013-010229. pii : bcr2013010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004;25:975–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26:905–11. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Pisani A, Diomedi M, Rum A, Cianciulli P, Floris R, Orlacchio A, et al. Acanthocytosis as a predisposing factor for non-ketotic hyperglycaemia induced chorea-ballism. J Neurol Neurosurg Psychiatry. 2005;76:1717–9. doi: 10.1136/jnnp.2005.067033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedwell SF. Some observations on hemiballismus. Neurology. 1960;10:619–22. doi: 10.1212/wnl.10.6.619. [DOI] [PubMed] [Google Scholar]
- 12.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: A meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200:57–62. doi: 10.1016/s0022-510x(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 13.Cochin JP, Hannequin D, Delangre T, Guegan-Massardier E, Augustin P. Continuous partial epilepsy disclosing diabetes mellitus. Rev Neurol (Paris) 1994;150:239–41. [PubMed] [Google Scholar]
- 14.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739–48. doi: 10.2337/dc06-9916. [DOI] [PubMed] [Google Scholar]
- 15.Hennis A, Corbin D, Fraser H. Focal seizures and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry. 1992;55:195–7. doi: 10.1136/jnnp.55.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tedrus GM, Albertin MC, Odashima NS, Fonseca LC. Partial motor seizures induced by movement in diabetic patients. Arq Neuropsiquiatr. 1991;49:442–6. doi: 10.1590/s0004-282x1991000400013. [DOI] [PubMed] [Google Scholar]
- 17.Suárez-Moro R, Salas-Puig J, Amorín M, Róiz C, Lahoz C. SPECT findings in reflex seizures induced by movement in non-ketotic hyperglycemia. Epileptic Disord. 1999;1:199–201. [PubMed] [Google Scholar]
- 18.Roberts E, Rothstein M, Baxter CF. Some metabolic studies of gamma-aminobutyric acid. Proc Soc Exp Biol Med. 1958;97:796–802. doi: 10.3181/00379727-97-23883. [DOI] [PubMed] [Google Scholar]
- 19.Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: Correlation with biochemical alterations in the brain. Metabolism. 1975;24:665–79. doi: 10.1016/0026-0495(75)90146-8. [DOI] [PubMed] [Google Scholar]
- 20.Duckrow RB. Decreased cerebral blood flow during acute hyperglycemia. Brain Res. 1995;703:145–50. doi: 10.1016/0006-8993(95)01077-7. [DOI] [PubMed] [Google Scholar]
- 21.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: An overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pishdad G, Ghavanini AA. Factors contributing to alteration in the level of consciousness in the patients with diabetic ketoacidosis: Analysis of 189 cases. Med J Islam Repub Iran. 1999;13:83–7. [Google Scholar]
- 23.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(Suppl 1):S94–102. doi: 10.2337/diacare.27.2007.s94. [DOI] [PubMed] [Google Scholar]
- 24.Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93:1541–52. doi: 10.1210/jc.2007-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiamkao S, Pratipanawatr T, Tiamkao S, Nitinavakarn B, Chotmongkol V, Jitpimolmard S. Seizures in nonketotic hyperglycaemia. Seizure. 2003;12:409–10. doi: 10.1016/s1059-1311(02)00353-9. [DOI] [PubMed] [Google Scholar]