Abstract
Background & objective:
It has been shown that the combined use of alcohol before radiofrequency ablation (RFA) helps to augment the therapeutic advantage of RFA. The present study was conducted to compare the outcome of treatment with RFA alone and RFA with alcohol as ablative technique in patients with small hepatocellular carcinomas (HCCs), who were not candidates for surgery.
Methods:
Fifty patients with chronic liver disease and concurrent HCC were enrolled in this prospective study. The patients were treated with either RFA alone (n=25) or RFA combined with alcohol (n=25). Patient outcome was evaluated, and the tumour recurrence and survival of the patients were assessed in the two groups.
Results:
The survival rates at six months in patients who completed at least six months of follow up were 84 and 80 per cent in patients treated with RFA alone and combination therapy, respectively. During the follow up period, 11 and four patients treated with RFA alone showed local and distant intrahepatic tumour recurrence, respectively. All local recurrences were at one to 18 months of the follow up period. The distant recurrences occurred at 6-36 months of the follow up period. During the follow up period, eight and six patients treated with combination therapy showed local and distant intrahepatic tumour recurrence, respectively. All local recurrences were at 1.5-15 months during the follow up period. The distant intrahepatic recurrences occurred at 6-72 months during the follow up period.
Interpretation & conclusions:
No significant difference was seen between the survival time of the patients treated with RFA alone and RFA with alcohol as well as in the local recurrences and distant intrahepatic recurrences in RFA compared to RFA and alcohol group patients. Combined use of RFA and alcohol did not improve the local tumour control and survival in patients with HCC compared to RFA alone.
Keywords: Alcohol, hepatocellular carcinomas, outcome, radiofrequency ablation, survival
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, especially in people belonging to Asian countries due to a high incidence of viral hepatitis. There have been exhaustive efforts made to develop different treatment options for HCC; however, the prognosis continues to be poor1.
The various treatment options for HCC include locoregional minimally invasive therapies, surgical resection and orthotopic liver transplantation (OLT)1,2. Various endovascular and percutaneous interventions constitute locoregional therapies for the treatment. Endovascular interventions include transarterial chemoembolization (TACE), which includes the use of drug-eluting beads, transarterial embolization (TAE) and transarterial radionuclide therapy (TART) using yttrium microspheres. The percutaneous interventions are based on thermal, chemical or non-thermal non-chemical ablation techniques. The thermal ablation therapies include radiofrequency ablation (RFA), interstitial laser therapy, cryotherapy and microwave coagulation. Ethanol and acetic acid are used during the chemical ablation techniques1,2. Irreversible electroporation is the recent non-thermal non-chemical technique that is available for the treatment of unresectable HCC1.
Percutaneous ethanol and acetic acid injections are commonly used for the treatment; however, these have been found to be effective in small and encapsulated HCC only. As against chemical ablation techniques, thermal ablation techniques have been found effective in treating primary HCC3,4,5,6,7,8 as well as liver metastasis9,10. Larger areas of coagulated necrosis can be obtained using novel RFA techniques. RFA plays a major role in the local treatment of HCC due to its capability of controlled area of coagulative necrosis in less number of sessions without major procedure-related complications as against the use of ethanol and acetic acid alone. Although RFA has been shown to be effective, it carries a few disadvantages, such as the limited area of necrosis induced by RFA and more frequent local site tumour recurrences during the follow up11,12.
Studies have shown that the use of alcohol before RFA helps to augment the therapeutic advantage of RFA due to the requirement of less energy in the combination therapy compared with RFA alone13,14,15,16. However, only a few studies have compared the efficacy and survival in patients treated with RFA alone and RFA combined with alcohol17,18,19,20,21. The present study was conducted to compare the outcome of treatment with RFA alone and RFA with alcohol as an ablative technique in patients with small HCCs, who were not candidates for surgery.
Material & Methods
This prospective study was conducted in the department of Radiodiagnosis and Imaging of the Postgraduate Institute of Medical Education and Research, Chandigarh, India, over a period of six years (January 2009 to December 2014). Fifty patients with chronic liver disease with cirrhosis and concurrent HCC were enrolled in this study which was approved by the Institute Ethics Committee. The aetiology of the cirrhosis cases included hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol, non-alcoholic steatohepatitis (NASH) related and also cryptogenic cases of chronic liver disease were enrolled. Practice guidelines of American Association for the Study of Liver Diseases were used for establishing the diagnosis of HCC22. On the basis of Barcelona Clinic Liver Cancer (BCLC) Staging System guidelines, patients were selected for RFA treatment23.
Inclusion criteria included HCCs which were considered unresectable or patients were unfit for surgery (presence of varices as seen on helical CT/magnetic resonance angiography, platelet count <100,000/μl), absence of vascular invasion and extrahepatic metastases, Child-Pugh class A and B, prothrombin time index (PTI) >75 per cent, platelet count higher than 50,000 per μl and no previous treatment for HCC. Exclusion criteria included extrahepatic spread of HCC (metastasis, lymph nodal metastasis, peritoneal deposits), extensive intrahepatic burden (involving more than 50% volume of liver), poor coagulation profile (platelet count <50,000/μl, PTI <75%), severe hepatic dysfunction with Child-Pugh class C and pre-existing cardiac disease and severe cardiac and renal failure.
All patients were randomized into treatment with either RFA alone (n=25) or RFA combined with alcohol (n=25) groups using computer-generated random table. A sample size of at least 22 patients was required in each of the two groups to detect a difference in tumour necrosis and local recurrence at effect size of 30 per cent, power of 80 per cent and a level of confidence of 95 per cent. Written informed consent was obtained from all the patients.
Protocol and technique of the percutaneous interventions: All the procedures were performed under conscious sedation on an inpatient basis in the interventional radiology suite of the department using a commercially available system (Covidien, Mansfield, MA, USA). Single or clustered needle electrodes were used with length of the burning tip of the radiofrequency (RF) probe ranging from 1 to 3 cm depending on the size of tumour. In the patients with combined treatment group, initially, a RFA needle was placed inside the tumour. This was followed by placement of Chiba or lumbar puncture needle in the tumour adjacent to the RFA needle, through which absolute alcohol was injected slowly. The alcohol was first injected into tumour part which was farthest from the puncture site on the skin and then injected into the remaining portion of the tumour. A volume of 4-14 ml of alcohol was injected depending on the size of tumour. Twelve minute RF cycle was given in automode, and one minute cycle was given for tract ablation.
Post-treatment follow up: The patient outcome and recurrence of tumour and patient survival were evaluated in the two groups. The tumours were considered as ablated completely if no viability was found on dynamic contrast-enhanced multiphasic computed tomography (CT) scan done at one month after the intervention.
During the follow up imaging, two types of tumour recurrence were looked for: local tumour progression (LTP) and intrahepatic distant recurrence (IDR). LTP is defined as tumour recurrence along the periphery of the ablated lesion4. IDR is defined as a new HCC seen distant from the margin of the ablated lesion within the liver parenchyma4.
The follow up period ranged from six months to six years. The follow up imaging included a multiphasic CT scan at 1, 3 and 6 months after the first ablation and every six months thereafter. The complications during the treatment were classified into four grades according to the classification of Accordion Severity Grading System of Surgical Complications24.
Statistical analysis: All analyses were conducted using SPSS for Windows (version 17.0; SPSS Inc., Chicago, IL, USA). Discrete categorical data are presented as n (%); continuous data are given as mean±standard deviation (SD). Categorical data between the two groups were analyzed using Chi-square/Fischer's exact test, whichever was appropriate. Age is presented as mean±SD, and it was compared between the two groups by t test. Cycles and sittings are presented as median and interquartile range, which were compared between the two groups using Mann-Whitney test. Kaplan-Meier survival curve and log rank test were applied to test the significance of difference between the survival times of the different groups.
Results
The Table shows the characteristics of the study population, HCC characteristics and treatment cycles during the follow up in the two different groups. No significant difference was there in the gender and age distribution, size and number of lesions, child score distribution, aetiology of cirrhosis, recurrence and number of sittings in the patients in the two groups.
Table.
Characteristics of the study population, hepatocellular carcinoma characteristics and treatment sittings during the follow up in the two different groups
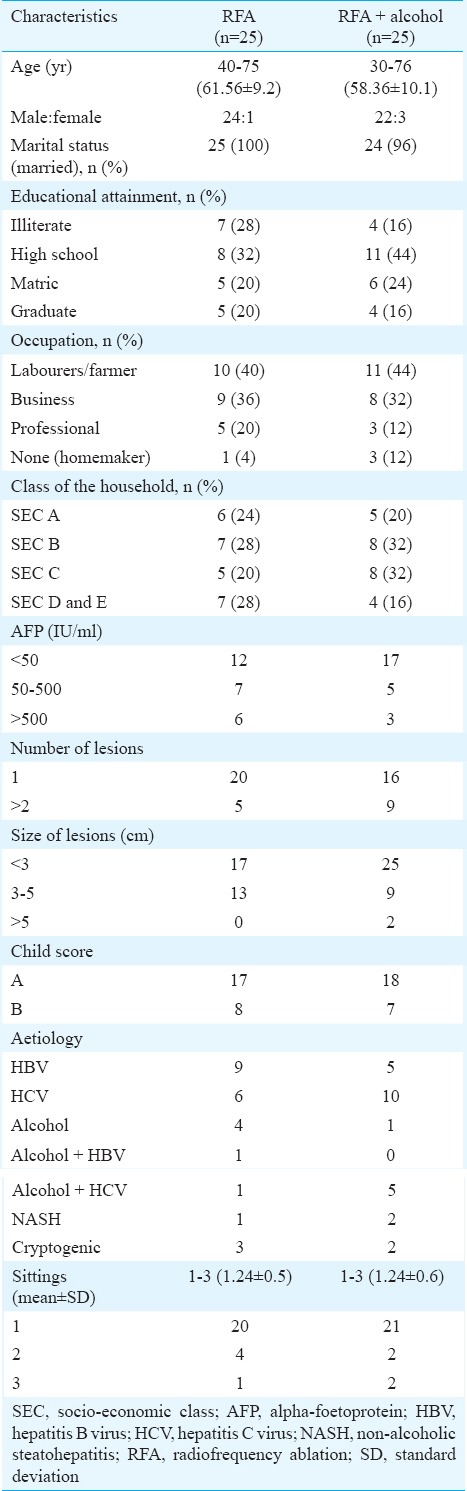
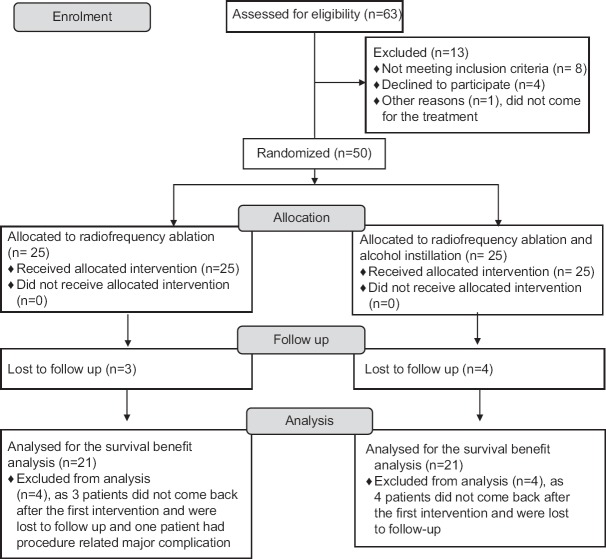
Fig. 1 summarizes the inclusion of the study population in our study. The survival rate at six months in patients who completed at least six months of follow up was 84 per cent in patients treated with RFA alone, while it was 80 per cent in patients treated with RFA and alcohol (Figs 2 and 3). This difference was not found to be significant.
Fig. 1.
Flow diagram summarizing the study population. RFA, radiofrequency ablation.
Fig. 2.
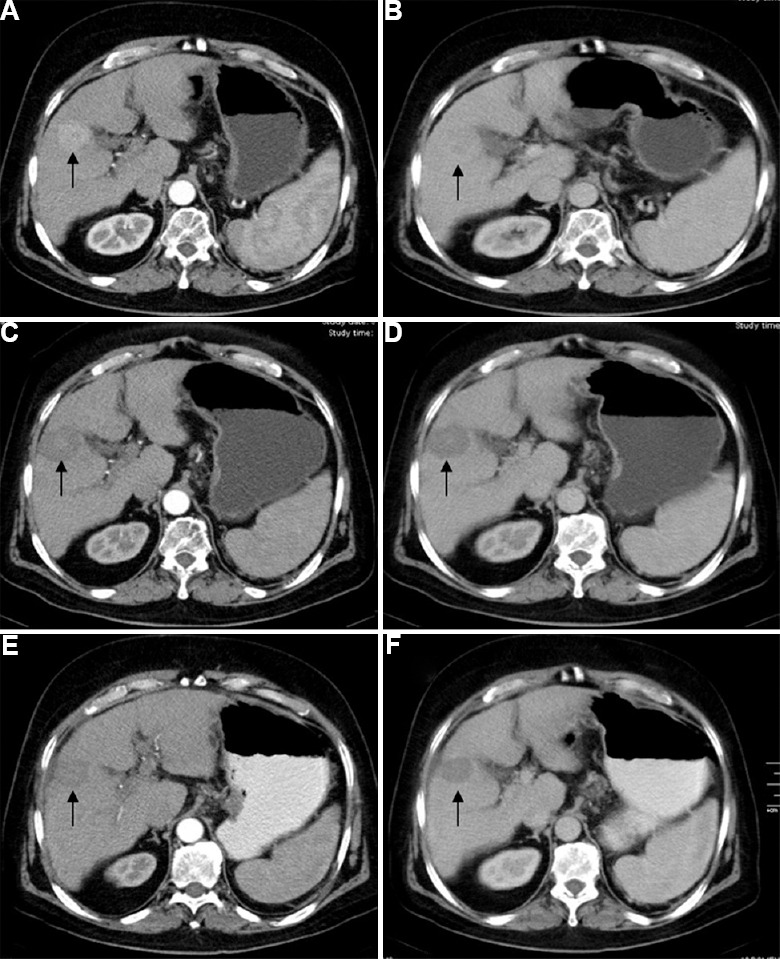
A 65 yr old male with hepatocellular carcinoma in segment 5 of liver treated with radiofrequency ablation alone. Pre-treatment arterial (A) and venous (B) phase images showing hypervascular lesion in segment 5 of liver (arrows). Post-radiofrequency ablation follow up imaging at three months (C & D) and six months (E & F) shows no residual enhancement in the lesion (arrows).
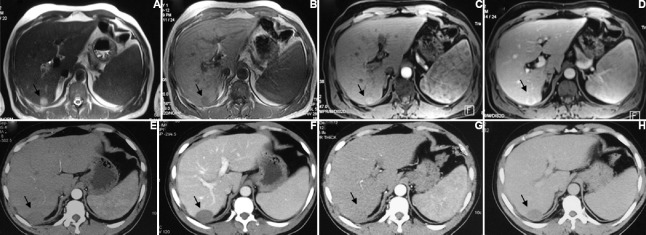
Fig. 3.
A 50 yr old male with hepatocellular carcinoma in segment 7/6 of liver treated with radiofrequency ablation and alcohol. Pre-treatment MRI shows a hyperintense lesion on T2-weighted images (A) which is hypointense on T1-weighted images (B) and is showing arterial hypervascularity (C) and wash out on portal venous phase (D) (arrows). Post-treatment follow up imaging at three months (E & F) and six months (G & H) shows no residual enhancement in the lesion (arrows).
No significant difference was seen between the survival time of the patients in RFA and RFA+alcohol groups using log rank test (Fig. 4). Since no difference was there in the survival time of the patients in the two groups, further analysis was done by combining the survival of the patients in two groups. The analysis showed no significant correlation in the survival with the age group, child score, alpha-foetoprotein levels and number of lesions. There was significant association between the survival of the males (mean of 22.47 months) and females (mean of 4.67 months), with P=0.002.
Fig. 4.
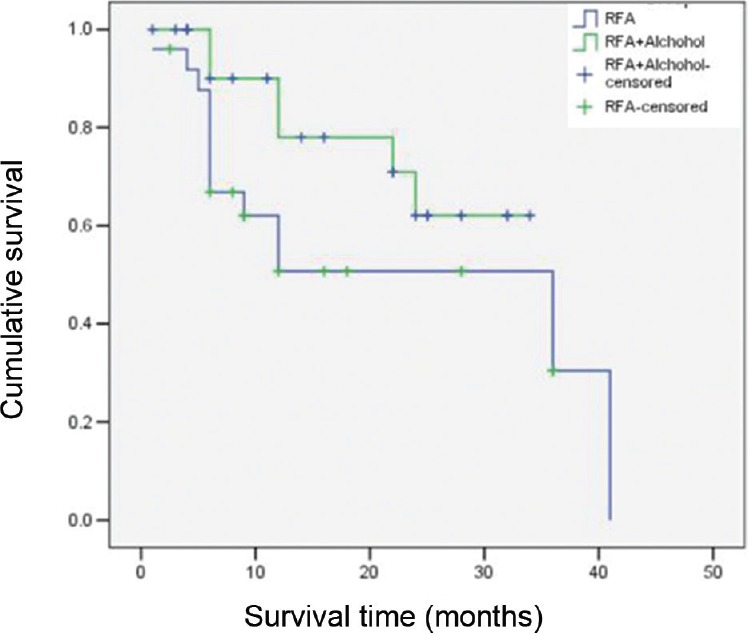
Kaplan-Meier curve showing no significant difference in the survival in the patients in the two groups (P=0.131).
During the follow up period, 11 and four patients treated with RFA alone showed local and distant intrahepatic tumour recurrence, respectively. All local recurrences were at 1-18 months of the follow up period. The distant recurrences occurred at 6-36 months of the follow up period.
During the follow up period, eight and six patients treated with RFA and alcohol showed local and distant intrahepatic tumour recurrence, respectively. All local recurrences were at 1.5-15 months during the follow up period. The distant intrahepatic recurrences occurred at 6-72 months during follow up period.
No significant difference was observed in the local recurrences in RFA alone and RFA and alcohol group patients. Similarly, no significant difference was noted in the distant intrahepatic recurrences in RFA alone and RFA and alcohol group patients.
Extrahepatic dissemination was seen in none of the patients during the follow up period. In our study, only two HCCs were >5 cm and were treated with the combination therapy. Both of these lesions were seen in a single patient only. The patient, however, showed persistent residual lesions with multifocal disease progression at six month follow up and died eight months after the initial treatment.
According to the classification of Accordion Severity Grading System of Surgical Complications, Grade I complication was seen in all the patients in the form of requirement of analgesics for alleviation of pain (100%). Grade II and III complications were not encountered in our study. Grade IV complication was seen in one patient in the form of hemoperitoneum during the procedure.
At the time of study censoring (March 2015), 14 patients had died within one month to 41 months of treatment with RFA during the follow up period. Six patients had died within six months to 24 months of treatment with RFA and alcohol during the follow up period.
Discussion
Percutaneous RFA is being used widely for treating the patients with HCC, especially in those patients who are not suitable for surgery1,2. Increase in tumour size is associated with a decrease in the therapeutic effect of RFA18. The region of complete coagulative necrosis which is induced by RFA is limited and decreases with the size of the tumour (especially the tumours with size >3 cm). In our study RFA was offered as the treatment option to patients with HCC up to 5 cm to improve the survival. Tumour recurrences in small size HCC treated with RFA have been shown to be comparable with larger lesions9.
Furthermore, it has also been shown that local tumour recurrences occur more frequently after RFA treatment. Increasing the ablation zone to decrease tumour recurrence is required so as to improve upon the efficacy of RFA in treating the tumours of size greater than 3.0 cm18. Therefore, the need arises to further develop the RFA technique to improve the remedial effects of this treatment. Among the various optional treatment combinations available, we used a combination of RFA and alcohol to compare the results with the patients treated with RFA alone. Livraghi et al7 reported complete necrosis in 90 per cent tumours with RFA versus 80 per cent with percutaneous ethanol injection (PEI) with an average of 1.2 sessions per tumour with RFA versus 4.8 sessions per tumour with PEI. The cumulative five-year survival after RFA has been reported ranging from 54.3 to 80 per cent25,26. Indications for RFA in these studies included single tumour up to 5 cm in maximum diameter, up to three tumours (no one exceeding 3 cm in maximum diameter) and no extrahepatic spread of tumour. Contraindications included extensive tumour burden [>5 cm (single tumour), >3 tumours (up to 3 cm in size)], extrahepatic spread and abnormal coagulation profile. Cho et al26 have shown three-year survival of 80 per cent with RFA and 77.4 per cent with surgical resection in 160 cases.
Comparison has also been carried out between RFA alone or in combination with PEI. Shankar et al27 have reported achieving a significantly larger ablation volume with the combined therapy (84.6 cm2) than with RFA alone (32.3 cm2) with no increase in the complication rate. Kurokohchi et al17 have also reported an increase in the volume of coagulative necrosis produced with a combination of RFA and PEI with administration of similar amounts of energy.
Studies have shown that RFA combined with PEI facilitates better long-term survival and local tumour recurrence, compared with RFA alone18,20,28. Zhang et al18 showed that the overall survival rates with RFA only was significantly less than RFA-PEI combined therapy. However, in their study, use of combination therapy improved the overall survival of those patients only who had tumours of size between 3.1 and 5.0 cm and not those of patients with tumour size of 3.0 cm or smaller or those of patients with size between 5.1 and 7.0 cm. Kurokohchi et al13 and Watanabe et al29 reported that the ablative zone in HCC can be increased with the combination of RFA and PEI treatment both in in vivo and ex vivo studies. In our study, only two HCCs were >5 cm and were treated with the combination therapy. Both of these lesions were seen in a single patient only. The patient however, showed persistent residual lesions with multifocal disease progression at six month follow up and died around eight months after the initial treatment. Majority of the tumours in our study group were <3 cm in both the groups; however, no significant difference was found in the six-month survival rate in the patients in the two groups. Zhang et al18 also had shown that the use of combination therapy did not improve the overall survival of those patients with tumour size of 3.0 cm or smaller. No significant difference was there between the survival time of the patients treated with RFA only and patients treated with combined therapy. Similarly, Kai et al21 in their retrospective study found no significant difference in overall survival of the patients treated with RFA alone and combined therapy with RFA and alcohol.
Tumour recurrence has been the main reason for the futile RFA. It is said that an obvious safety margin of 1.0 cm is crucial for the surgical resection as well as for percutaneous ablative therapies. Inability to achieve adequate safety margins results in local recurrence18. Results have shown that RFA combined with alcohol helps to achieve a larger ablative zone compared to RFA alone, and therefore, the use of combination treatment in small HCCs can result in a decrease in local tumour recurrence13,18,26. In our study, although less local recurrence was seen in the patients treated with combined therapy (n=8) than the patients treated with RFA only (n=11), the difference was not significant. Zhang et al18 showed that that use of combination treatment significantly decreased the local recurrence but did not show benefit in reducing intrahepatic recurrence or extrahepatic metastasis. Similarly, in our study, six patients treated with combination treatment showed distant intrahepatic recurrence, while four patients treated with RFA alone showed distant intrahepatic recurrence during the follow up period. The difference however, was not significant. We did not encounter extrahepatic metastasis in any of the patients belonging to both the groups in our study.
Complication rates vary widely in patients undergoing percutaneous treatment with RFA, with a low mortality reported to be <1 per cent. Multiple factors have been attributed for the complications including tumour size, location, presence or absence of underlying liver disease, number of lesions and experience of the interventionist30. Our patients in both the groups tolerated the procedures well. Grade I complication was seen in all the patients in the form of requirement of analgesics for alleviation of pain (100%). Grade II and III complications were not encountered in our study. Grade IV complication was seen in one patient in the form of hemoperitoneum during the procedure where the HCC was located in the subcapsular location in segment VIII.
There were certain limitations in our study. The small sample size was one of the major limitations of our study. A small number of patients with nodules more than 3 cm in the two groups may have confounded our results. In addition, only a few of the patients had a very long follow up period after their enrolment in both the groups. This could probably be the cause for the contradictory results of our study when compared to some of the previous studies.
In conclusion, our results showed that the combination of RFA and alcohol did not have a significant difference than the use of RFA alone in local tumour recurrence as well as increasing the survival in patients with HCC.
Acknowledgment
The study was financially supported by the Indian Council of Medical Research, New Delhi.
Footnotes
Conflicts of Interest: None.
References
- 1.Kalra N, Gupta P, Chawla Y, Khandelwal N. Locoregional treatment for hepatocellular carcinoma: The best is yet to come. World J Radiol. 2015;7:306–18. doi: 10.4329/wjr.v7.i10.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalra N, Kang M, Bhatia A, Duseja AK, Dhiman RK, Arya VK, et al. Role of radiofrequency ablation in unresectable hepatocellular carcinoma: An Indian experience. Indian J Radiol Imaging. 2013;23:139–44. doi: 10.4103/0971-3026.116569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allgaier HP, Deibert P, Zuber I, Olschewski M, Blum HE. Percutaneous radiofrequency interstitial thermal ablation of small hepatocellular carcinoma. Lancet. 1999;353:1676–7. doi: 10.1016/S0140-6736(99)00368-2. [DOI] [PubMed] [Google Scholar]
- 4.Nagata Y, Hiraoka M, Akuta K, Abe M, Takahashi M, Jo S, et al. Radiofrequency thermotherapy for malignant liver tumors. Cancer. 1990;65:1730–6. doi: 10.1002/1097-0142(19900415)65:8<1730::aid-cncr2820650812>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–8. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Gazelle GS, Solbiati L, Livraghi T, Tanabe KK, Hahn PF, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023–8. doi: 10.2214/ajr.170.4.9530053. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: Treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–61. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–91. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: Treatment and follow-up in 16 patients. Radiology. 1997;202:195–203. doi: 10.1148/radiology.202.1.8988211. [DOI] [PubMed] [Google Scholar]
- 10.Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, et al. Hepatic metastases: Percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–73. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 11.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–8. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, et al. Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocelluar carcinoma. Int J Oncol. 2002;21:841–6. doi: 10.3892/ijo.21.4.841. [DOI] [PubMed] [Google Scholar]
- 14.Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Funaki T, Arima K, et al. Combination therapy of percutaneous ethanol injection and radiofrequency ablation against hepatocellular carcinomas difficult to treat. Int J Oncol. 2002;21:611–5. [PubMed] [Google Scholar]
- 15.Kurokohchi K, Masaki T, Miyauchi Y, Hosomi N, Yoneyama H, Yoshida S, et al. Efficacy of combination therapies of percutaneous or laparoscopic ethanol-lipiodol injection and radiofrequency ablation. Int J Oncol. 2004;25:1737–43. doi: 10.3892/ijo.25.6.1737. [DOI] [PubMed] [Google Scholar]
- 16.Kurokohchi K, Masaki T, Watanabe S, Nakai S, Deguchi A, Morishita A, et al. Time-lag performance of radiofrequency ablation after percutaneous ethanol injection for the treatment of hepatocellular carcinoma. Int J Oncol. 2006;28:971–6. [PubMed] [Google Scholar]
- 17.Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, et al. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11:1426–32. doi: 10.3748/wjg.v11.i10.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: A prospective randomized trial. Radiology. 2007;244:599–607. doi: 10.1148/radiol.2442060826. [DOI] [PubMed] [Google Scholar]
- 19.Lin JW, Lin CC, Chen WT, Lin SM. Combining radiofrequency ablation and ethanol injection may achieve comparable long-term outcomes in larger hepatocellular carcinoma (3.1-4 cm) and in high-risk locations. Kaohsiung J Med Sci. 2014;30:396–401. doi: 10.1016/j.kjms.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azab M, Zaki S, El-Shetey AG, Abdel-Moty MF, Alnoomani NM, Gomaa AA, et al. Radiofrequency ablation combined with percutaneous ethanol injection in patients with hepatocellular carcinoma. Arab J Gastroenterol. 2011;12:113–8. doi: 10.1016/j.ajg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Kai L, Jia L, Zhi-Gang W, Lei Y. Ultrasonic guided percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas. Indian J Cancer. 2015;52(Suppl 2):e102–4. doi: 10.4103/0019-509X.172503. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 24.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–86. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–9. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 26.Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Hwang YJ, et al. The comparative results of radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma. Korean J Hepatol. 2005;11:59–71. [PubMed] [Google Scholar]
- 27.Shankar S, vanSonnenberg E, Morrison PR, Tuncali K, Silverman SG. Combined radiofrequency and alcohol injection for percutaneous hepatic tumor ablation. AJR Am J Roentgenol. 2004;183:1425–9. doi: 10.2214/ajr.183.5.1831425. [DOI] [PubMed] [Google Scholar]
- 28.Shi F, Tan Z, An H, Wang X, Xu Y, Wang S. Hepatocellular carcinoma ≤4 cm treated with radiofrequency ablation with or without percutaneous ethanol injection. Ann Hepatol. 2016;15:61–70. doi: 10.5604/16652681.1184219. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe S, Kurokohchi K, Masaki T, Miyauchi Y, Funaki T, Inoue H, et al. Enlargement of thermal ablation zone by the combination of ethanol injection and radiofrequency ablation in excised bovine liver. Int J Oncol. 2004;24:279–84. [PubMed] [Google Scholar]
- 30.Fonseca AZ, Saad WA, Ribeiro MA., Jr Complications after radiofrequency ablation of 233 hepatic tumors. Oncology. 2015;89:332–6. doi: 10.1159/000439089. [DOI] [PubMed] [Google Scholar]