Abstract
Background & objectives:
Earlier reports have shown hypocholesterolaemia in cancer patients and high number of lipid rafts in cancer cells. The primary objective of this study was to compare the intracellular cholesterol turnover in non-cancerous (benign) prostatic hyperplasia (BPH) and carcinoma prostate (CAP) with normal prostate cells obtained from patients undergoing radical cystectomy for carcinoma bladder (sham control).
Methods:
ELISA-based estimation of prostate-specific antigen (PSA), evaluation of expression of low-density lipoprotein receptor (LDLR), peripheral-type benzodiazepine receptor (PBR) and cyclin E, immunohistochemistry and confocal microscopy, measurement of integrated optical density of the diaminobenzidine (DAB)-stained immunohistograms, isolation of nucleus and cell cytoplasm from prostate tissue by ultracentrifugation followed by estimation of cholesterol spectrophotometrically in isolated nuclear and cytoplasmic fractions were performed.
Results:
Seventy five individuals, 25 for each group (BPH n=25; CAP n=25 and sham control n=25), were included in the study. Cholesterol was increased in the cytoplasm and nucleus of the prostate cancer cells along with elevated expression of LDLR. Increased cholesterol concentration in the cell nucleus was found comparable with the increased expression of cholesterol transporter viz. PBR in the prostate tumour tissues as compared to its expression in normal prostate cells obtained from individuals undergoing radical cystectomy for carcinoma bladder. Cell cycle protein cyclin E was also highly expressed in cancer tissues.
Interpretation & conclusions:
The present findings along with increased expression of cell cycle protein cyclin E in the cell nucleus of the tumour tissue suggested the possibility of an intriguing role of cholesterol in the mechanism of cell cycle process of prostate cell proliferation.
Keywords: Cholesterol, cyclin E, low-density lipoprotein receptor, peripheral-type benzodiazepine receptor, prostate cancer
Although cholesterol is primarily involved in steroid hormone synthesis, bile production and cell membrane formation, it has also been found to be implicated in a number of human diseases. While hypercholesteraemia is a known cause of atherosclerosis1 for a long time, hypocholesteraemia has been found to be associated with cancers2. In an animal study on bone marrow hyperplasia exponential fall of plasma lipoproteins was observed, whereas triglycerides were significantly elevated without any know reason3. A decrease of total cholesterol content in the blood of patients with metastatic prostatic carcinoma has been reported4,5. It is still unclear whether this phenomenon is due to an increased cholesterol uptake by the cells, even though the overexpression of low-density lipoprotein receptor (LDLR) in prostate cancer cell has been documented6. The breast cancer and prostate cancer cell lines contained more lipid rafts and were more sensitive to cholesterol depletion-induced cell death than their normal counterparts7. These results indicated that cancer cells contained increased levels of rafts and suggested a potential use of raft-modulating agents as anti-cancer drugs. Lipid rafts have been shown as master regulators of cancer cell function, cell adhesion, migration, cell growth and signalling pathways7,8.
There are ample data supporting a functional relationship between cholesterol metabolism and prostate cancer, ranging from an epidemiologic association to cholesterol abnormality and to prostate cancer illustrating dysregulation of cholesterol metabolism at the cellular level in prostate cancer9,10. It has been postulated that cholesteryl ester accumulation in lipid droplets within prostate cancer cells is a causative factor underlying prostate cancer aggressiveness11 and that their occurrence increases with prostate cancer grade.
The findings on nuclear localization and chromatin-associated cholesterol12 have indicated that cholesterol might also be a participant in the mechanism of cell cycle processes. It has been shown that the level of chromatin-associated cholesterol increases just before initiation of S phase12. The translocation of cytoplasmic cholesterol into nucleus is probably facilitated by peripheral-type benzodiazepine receptor (PBR), which has previously been reported to be a regulator of cholesterol transport to the inner mitochondrial membrane, as well as the rate determining step in steroid biosynthesis13. Although PBR is known to be involved in the regulation of steroidogenesis, Hardwick et al14 have suggested its presence on nuclear membrane and its involvement to cholesterol transport into the nucleus in human breast carcinoma. Exorbitant phenotypic expression of PBR and aggressive proliferation of cancer cells might be correlated phenomena in the advancement of the disease process. Whether PBR has the same role in other tumours or is an isolated phenomenon for breast carcinoma is not known. Besides, the mechanism through which cholesterol may be associated with cellular proliferation remains to be unexplored. Therefore, the aim of the present study was to find the impact of cholesterol in the cell nucleus, particularly on cell cycle process in prostate carcinoma cells.
Material & Methods
Histopathologically examined human prostate tissues representing non-cancerous (benign) prostatic hyperplasia (n=25) and carcinoma prostate (CAP) (n=25) were obtained from consecutive patients visiting the surgical Outpatient department of Urology, All India Institute of Medical Sciences (AIIMS), New Delhi, India, during April 2010 to March 2012. Normal prostate tissues for representing control samples were obtained from patients undergoing radical cystectomy (n=25) for carcinoma bladder (sham controls). Normal tissues were examined histopathologically as well. Prostate-specific antigen (PSA) estimation kit was procured from Calbiotech Inc. CA, USA. Antibodies for immunohistochemistry (IHC) were purchased from Santa Cruz Biotechnology with the following specifications e.g. LDLR (N-17: SC-11822), PBR (W-12: SC-23418), cyclin E (C-19: SC-198) and goat anti-rabbit IgG-fluorescein isothiocyanate (FITC) (SC-2012). Diaminobenzidine (DAB) substrate, secondary antibodies and streptavidin peroxidase kit for IHC were procured from Vector Laboratories, Inc., Burlingame, USA.
All the specimens were collected following approval of the study protocol by the Institute Ethics Committee (AIIMS, New Delhi, India). Each patient was given an information sheet having description of the study and its importance. Written informed consents were obtained from the patients or their bystanders.
Selection criteria: Only histopathological examination-proven cases of BPH and CAP were included in the study. Prostate tissues obtained from patients undergoing radical cystectomy were considered as sham control. PSA estimation in the blood plasma from all patients and normal healthy control was the distinguishing parameter to define CAP and control individuals. Those suffering from any other acute or chronic disease and those not willing to give consent were excluded from the study.
ELISA sandwich assay: Routine PSA (tumour marker) estimation was carried out by microplate immunoenzymometric (ELISA sandwich) assay as reported earlier2.
Immunohistochemistry (IHC)
Low-density lipoprotein receptor (LDLR): Surgically removed prostate tissues were washed thoroughly in normal saline and small 1 cm2 cubes were dissected, snap-frozen in liquid nitrogen and blocks were prepared using the cryogel at −25°C. Sections (5-10 μm) were taken on glass slides, air-dried and fixed with absolute acetone at 4°C for 10 min. After fixation, the slides were rinsed thrice, five minutes each, with phosphate-buffered saline containing 0.01 per cent Triton X-100 (PBST). Processing of sections for IHC was carried out using DAB staining and images acquired using a Nikon Microphot FXA microscope with Nikon DXM 1200 Digital Camera (Nikon, Japan) as per the procedure described previously15.
Peripheral-type benzodiazepine receptor (PBR) and cyclin E: The procedure was same as LDLR described above except the Triton concentration used in PBST wash. The three repeated five-minute PBST washes in the above protocol were substituted with serially increased concentration of Triton X-100 viz. 0.01, 0.025 and 0.05 per cent, respectively, instead of only 0.01 per cent Triton X-100.
Estimation of protein expression from immunohistogram: Expressions of LDLR, PBR and cyclin E on their respective immunohistograms were estimated by measuring integrated optical density (IOD) of the DAB stain. Multiple fields were chosen randomly to determine their expression densities per 25 cells as reported earlier15. Mean±standard deviation (SD) was determined to show the respective IOD of the expression.
Confocal microscopy: Prostatic tissues on slides were fixed in absolute acetone at 4°C. Blocking was done in 1 per cent bovine serum albumin at room temperature for one hour to protect non-specific expression followed by addition of primary antibody (anti-cyclin E rabbit polyclonal) at 1:50 dilution in PBS. This was incubated at room temperature for two hours in a humid chamber. The cells were then labelled with the secondary antibody (anti-rabbit FITC conjugate) at 1:50 dilution in the dark. Incubation and washing were carried out as routine protocol. Slides were incubated with propidium iodide (1 mg/ml) at 1:3000 dilution in PBS for one minute and then mounted in glycerol - PBS (1:1) with cover glasses and stored in the dark at −20°C. The images were then captured on a Leica SP-2 confocal microscope (Germany) under the ×20 and ×60 oil-immersion objectives.
Isolation of nucleus and cell cytoplasm from prostate tissue: Prostate tissue was made blood free by washing with ice-cold sucrose and weight was measured. The tissue was minced into small fragments and homogenized in isotonic sucrose solution (0.25 M sucrose). The homogenate of prostate tissue (20% in ice cold 0.25 M sucrose) was centrifuged at 800 g for 10 min at 4°C. The pellet was used as nuclear pellet. The supernatant was spun at 100,000 g for 60 min to obtain cytoplasmic fraction. The nuclear pellet was resuspended in lysis buffer [50 mM Tris–HCl (p H 7.4), 300 mM NaCl, 0.5% (v/v) Triton X-100, 5 mM EDTA with 2 mM PMSF, 10 mg/ml leupeptin and 10 U/ml aprotinin] and vortexed. The purity of the isolates was verified by relative activity/expressions of site-specific bio-markers16 e.g. DNA estimation and lactate dehydrogenase (LDH) activity.
Total cholesterol estimation in nuclear and cytoplasmic fractions: Total cholesterol from isolated nuclear and cytoplasmic fractions were extracted in 1ml of hexane: isopropanol (3:2, v:v), and the extract was transferred to microcentrifuge tubes and air dried. The cholesterol standard, samples and blank were dissolved in 1 ml of isopropanol:nonidet P-40 (9:1, v:v). Cholesterol content in cell nucleus and cell cytoplasm was estimated by enzymatic cholesterol assay based on fluorometric method17, and the fluorescence was read at 530 nm (excitation wavelength) and at 580 nm (emission wavelength). The organelle-specific cholesterol concentration was expressed per gram of prostate tissue mass.
Protein estimation: The protein concentration was determined using Bradford assay18.
Statistical analysis: The data, IOD, were represented as mean±SD. Student's t test was applied between two groups to compare the mean values.
Results
Routine serum PSA (data not shown) was estimated for each individual as screening test of prostatic carcinoma in affected individual. Significantly high value was obtained only in those having carcinoma. Significantly low level was found with control and sham controls. Only marginal increase in serum PSA level was seen in BPH.
Low-density lipoprotein receptor (LDLR) expression: The IHC findings (Fig. 1) showed that there was a significant increase in the expression of LDLR in case of carcinogenic or benign tumours (P<0.001) compared to sham control. The estimated IOD of the DAB stain taken by LDLR protein represented its state of expression. The increased expression of LDLR is expected to transfer more cholesterol into prostate cells.
Fig. 1.
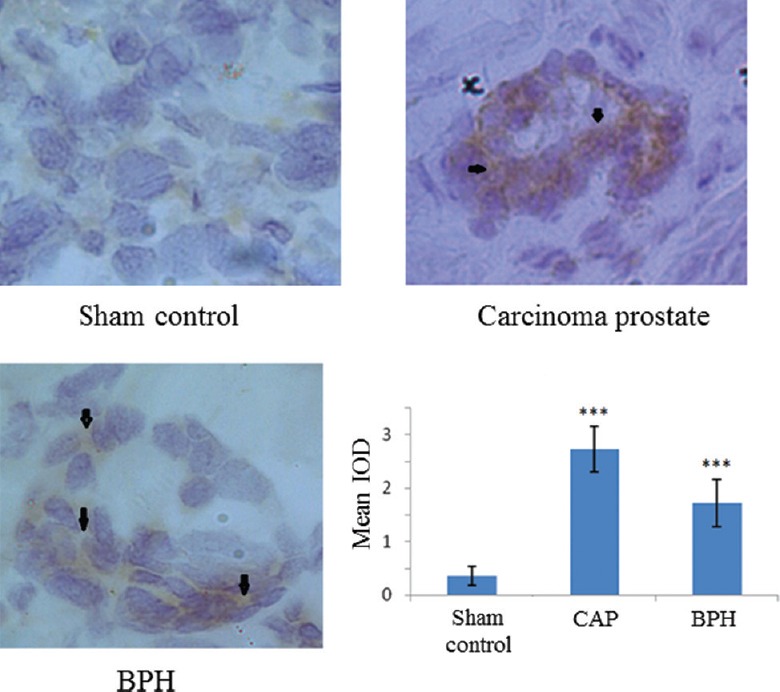
Low-density lipoprotein receptor (LDLR) protein expression by immunohistochemistry (magnification ×200). DAB stain (diaminobenzidine; brown colour) depicts a significant increase in LDLR expression in carcinogenic and non-carcinogenic (benign) tumours. Arrows indicate areas of higher expression. In the graph each bar shows the mean±standard deviation of 25 samples, represented as IOD (integral optical density) from the respective category. ***P<0.001 compared to sham control.
Peripheral-type benzodiazepine receptor (PBR) expression: The IHC findings (Fig. 2) showed a significant increase in the expression of PBR in case of carcinogenic (5-7 fold) or benign tumours (3-4 fold). Staining of the cell nucleus showed a preferentially increased PBR expression around nuclear periphery of cancerous cells as compared to the expression found in prostate cells collected from unaffected site of the patients undergoing radical cystectomy for carcinoma bladder. The increased expression of PBR around nucleus was presumably due to transfer of more cytoplasmic cholesterol into cell nucleus.
Fig. 2.
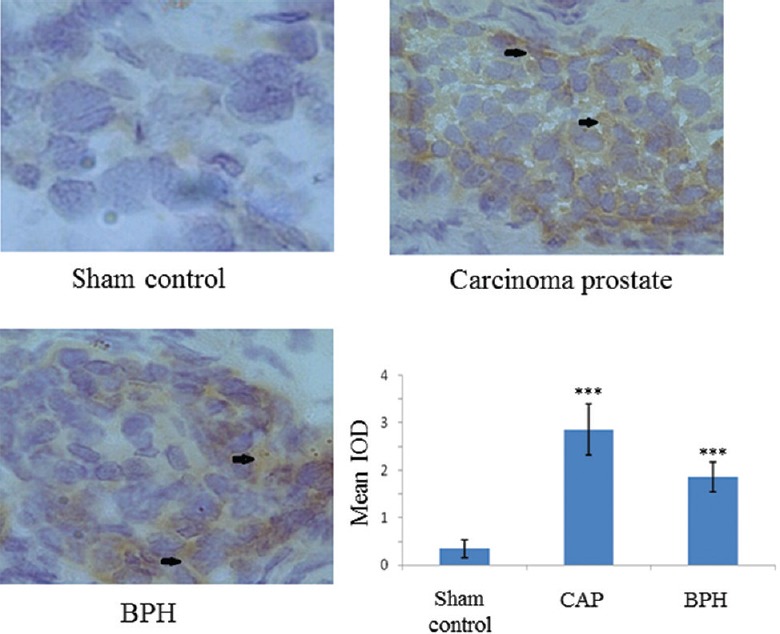
Peripheral-type benzodiazepine receptor (PBR) protein expression by immunohistochemistry (magnification ×200). DAB stain (brown colour) depicts a significant increase in PBR expression in carcinogenic and non-carcinogenic (benign) tumours. Arrows indicate areas of higher expression. In the graph each bar shows the mean±standard deviation of 25 samples, represented as IOD. ***P<0.001 compared to sham control.
Cholesterol content in cell nucleus and cell cytoplasm of the prostatic tissue: Nuclear and cytoplasmic fractions were separated using ultracentrifugation, and the purity of the isolated nuclear and cytoplasmic fractions obtained from normal as well as CAP samples were determined (Fig. 3). DNA and LDH were chosen as site-specific markers for cell nucleus and cytoplasm. The optimal purity of nucleus and cytoplasm was decided on the relative ratio of DNA:LDH in each respective compartment following the standard reported protocol16. DNA was found more in nucleus (Fig. 3A) and LDH (Fig. 3B) in the cell cytoplasm.
Fig. 3.
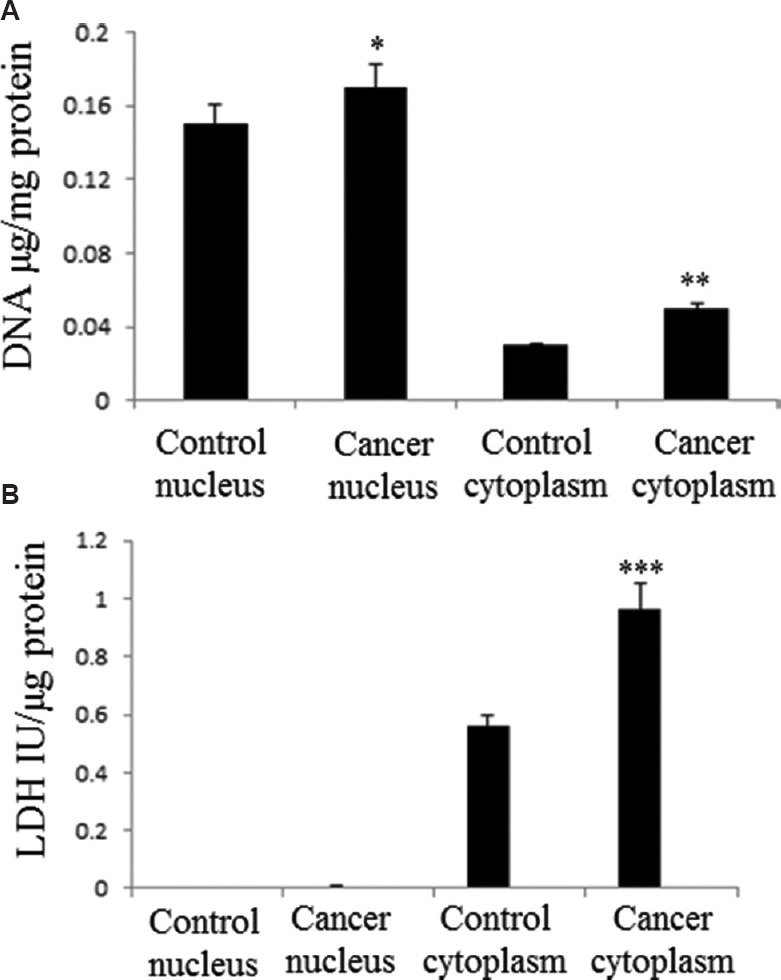
Marker assay in the isolates of ultracentrifugation. (A) DNA (nuclear marker), and (B) lactate dehydrogenase, LDH (cytosolic marker) were measured to determine the purity of the fractions. Each bar shows the mean±standard deviation of 25 samples. P*<0.05, **<0.01, ***<0.001 compared to respective control.
Significant increase of the cholesterol content in cell nucleus and cell cytoplasm of prostate cancer tissue was observed (Fig. 4). The concentration of nuclear cholesterol in CAP was found to be two-fold higher compared to sham control. The fold increase of cholesterol concentration in cell cytoplasm was comparatively less than that found in the nuclear compartment; this was probably because cytoplasmic cholesterol was drawn away by cell nucleus for its intranuclear needs.
Fig. 4.
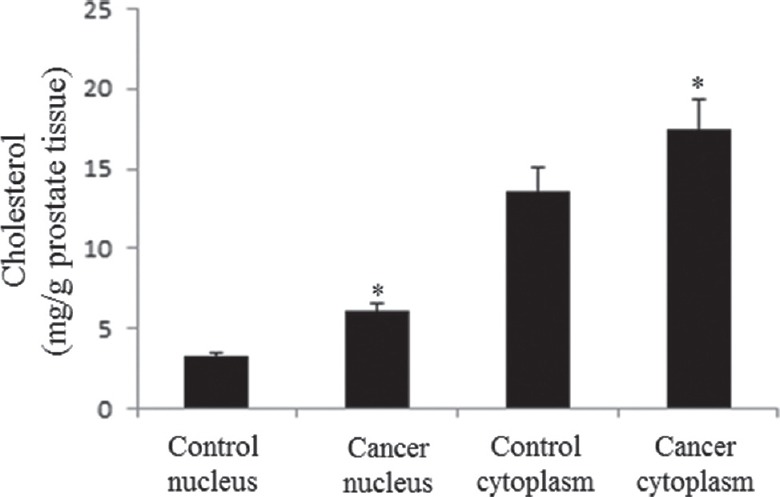
Measurement of cholesterol content. The concentration of cholesterol in cell nucleus and cytoplasm was found higher in cancer prostate tissue as compared to control prostate. The data show the mean±standard deviation (mg cholesterol/g prostate tissue) of 25 samples. *P<0.05 compared to respective control. Nuclear-cholesterol ratio in cancer prostate tissue to control prostate: 1.8.
Expression and nuclear localization of cyclin E: Figure 5 shows nuclear localization of cyclin E, as detected using FITC (green) tagged anti-cyclin E antibody and the nuclear stain propidium iodide (red) and their colocalization.
Fig. 5.

Nuclear localization of cyclin E by confocal microscopy. Cyclin E was labelled by fluorescein isothiocyanate (green) and nucleus was stained with propidium iodide (red) (Magnification ×400). The merged image (yellow-coloured) shows the nuclear localization of cyclin E.
Expression of cyclin E in prostate tissue: Cyclin E is a cell cycle protein. There was a significant increase (IOD value) in the expression of cyclin E in case of tumours and particularly high in CAP (Fig. 6).
Fig. 6.
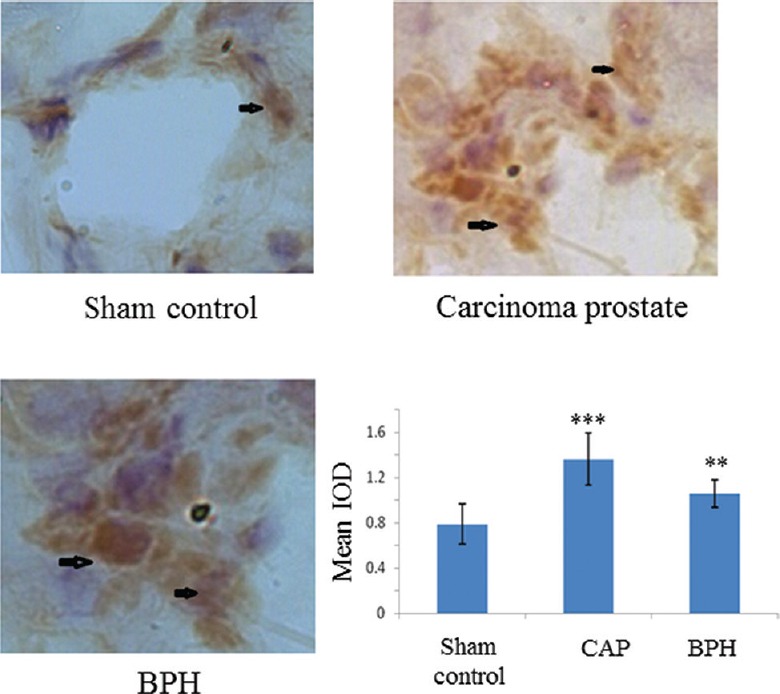
Expression of cyclin E on prostate tissue detected by immunohistochemistry (magnification ×200) of cyclin E protein. The IOD of DAB stain taken by cyclin E protein shows a significant increase of cyclin E expression in carcinogenic and non-carcinogenic (benign) tumours. Increased expression was observed in the carcinoma prostate group. Arrows indicate areas of higher expression. Each bar shows the mean±standard deviation of 25 samples. P**<0.01, ***<0.001 compared to sham control.
Discussion
An earlier study has shown higher activity of HMG-CoA reductase, the regulatory enzyme of cholesterol biosynthetic pathway, in rapidly dividing tumour cells19. High expression of LDLR, a cholesterol transporting protein on surface membrane, has also been reported on various cancer cells20. In addition, the well-known feedback regulatory mechanism for the maintenance of intracellular cholesterol pool21 was also found to be obviated in cancer cells6.
Besides, this newly growing consensus on the role of cholesterol in cell nucleus had got solicited by the following observations: (i) presence of cholesterol with the chromosomal DNA14,22, (ii) existence of sphingomyelin-bound cholesterol (esterified cholesterol) in cell nucleus23, and (iii) during liver regeneration, sphingomyelin-bound cholesterol goes down and more free cholesterol is generated23; these reports raised an inquisition regarding the use of cholesterol in cell cycle process and especially about any of its secret role in DNA synthesis/replication of proliferative tissues.
Although the aspects of LDLR expression, loss of feedback regulation of intracellular cholesterol homoeostasis, existence of highly expressed cholesterol transporter on nuclear membrane have been studied by several groups of investigators, the reports are mostly fragmentary. A significant increase of expression of LDLR was observed in cancer cells as compared to those in control and affected BPH cells. The clearance of extracellular cholesterol was expected to be high by the cells expressing higher LDLR on their cell surface. The net utilization of plasma cholesterol by the prostate tissue was not expected to be substantial enough to cause a significant change in cholesterol levels in systemic circulation as was reported earlier from our laboratory2. A moderate rise in cholesterol concentration was observed in cell cytoplasm of cancer cells in contrast to the observed cholesterol concentration of control cells.
PBR, a cholesterol channel, was found on the surface of mitochondrial24,25 and nuclear membrane26. The expression of PBR was found higher in cancer cells as compared to normal ones. In the same cancer cells, comparatively, more cholesterol concentration was detected in cell nucleus against their normal counterpart, which suggested the plausibility of transfer of more cholesterol from cell cytosol through PBR.
Cyclin E is a cell cycle protein and found in the cell nucleus. A higher expression of this protein is expected in highly proliferative cells to keep the nucleus continually in an active replication phase. In our study, cyclin E level was found more in cancer prostate than control or BPH. Since earlier reports claimed the role of nuclear cholesterol in cell proliferation, our study showed higher cyclin E level in cholesterol-filled nucleus, implicating a possible role of cholesterol in the process of cell multiplication.
It has been shown that elevated cholesterol levels in prostate cancer cells are a result of an aberrant regulation of cholesterol metabolism27. A fall in blood cholesterol levels was reflected only upon massive utilization of plasma cholesterol by solid/floating tissues - a scenario previously reported in case of haematological carcinoma28,29. Normal prostate gland is very small in size. However, when it increases in size reaching a critical mass, it can probably utilize enough circulatory cholesterol which in turn can contribute to a fall in the total blood cholesterol levels.
The ratio of the cyclin E expression (cyclin E is a cell cycle protein and remain expressed in dividing cells allowing cells to enter from G1 to S phase) in prostate cancer cells to that of their control counterpart was found exactly identical with the ratio of nuclear cholesterol in cancer cells to that of their corresponding control. The numerical value of this ratio was 1.8. This indicated that in cancer prostate nucleus, the cyclin E expression went up almost twice of its normal expression with twice the cholesterol concentration in the same cell nucleus as compared to control cells. Increase of cholesterol concentration in cell nucleus might be a stimulus for cell division. The ultimate source of the cholesterol found in cell nucleus was the cytoplasmic pool which was maintained by the influx of extracellular cholesterol through LDLR. Therefore, the net expression of LDLR in prostate cancer cells takes the pivotal role in supplying cholesterol to the cell nucleus. More work is needed to specify the mechanism responsible for stimulating cell division by cholesterol component of the nucleus. Besides its normal use in cell membrane, future studies on the role of cholesterol in DNA replication and the following new cell formation or cell proliferation would be a new perspective of cholesterol in cellular metabolism and homoeostasis.
In conclusion, our results showed high expression of cholesterol transport proteins viz., LDLR and PBR in prostate cancer tissue. Cholesterol concentration and cyclin E were also higher in cancer tissue than the control counterpart. Our findings indicated that cholesterol might have an intriguing role in the mechanism of cell proliferation. Since cancer tissue possesses highly proliferative cells, demand for cholesterol is apparently more in cancer cells as compared to its content in respective control tissue.
Acknowledgment
The study was partially supported by the funds from Institute grant of AIIMS, New Delhi, and from the contingency of MD thesis scholarship award of the first author (GS) from the Indian Council of Medical Research, New Delhi, India.
Footnotes
Conflicts of Interest: None.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 2.Singh G, Sankanagoudar S, Dogra PN, Mathur SR, Chandra NC. A study on lipid profile in prostate carcinoma patients admitted in AIIMS, New Delhi. J Biomed Pharm Res. 2014;3:49–51. [Google Scholar]
- 3.Dessì S, Batetta B, Spano O, Pulisci D, Anchisi C, Pani P, et al. Serum lipoproteins during bone marrow hyperplasia after phenylhydrazine administration in rats. Int J Exp Pathol. 1990;71:671–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Henriksson P, Eriksson M, Ericsson S, Rudling M, Stege R, Berglund L, et al. Hypocholesterolaemia and increased elimination of low-density lipoproteins in metastatic cancer of the prostate. Lancet. 1989;2:1178–80. doi: 10.1016/s0140-6736(89)91790-x. [DOI] [PubMed] [Google Scholar]
- 5.YuPeng L, YuXue Z, PengFei L, Cheng C, YaShuang Z, DaPeng L, et al. Cholesterol levels in blood and the risk of prostate cancer: A meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev. 2015;24:1086–93. doi: 10.1158/1055-9965.EPI-14-1329. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91:41–5. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Murai T. The role of lipid rafts in cancer cell adhesion and migration. Int J Cell Biol. 2012;2012:763283. doi: 10.1155/2012/763283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babina IS, Donatello S, Nabi IR, Hopkins AM. Lipid rafts as master regulators of breast cancer cell function. In: Gunduz M, Gunduz E, editors. Breast cancer-carcinogenesis, cell growth and Signalling Pathways. London: InTech; 2011. [Google Scholar]
- 9.Wu X, Daniels G, Lee P, Monaco ME. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014;2:111–20. [PMC free article] [PubMed] [Google Scholar]
- 10.Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012;12:751–9. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albi E, Magni MV. The presence and the role of chromatin cholesterol in rat liver regeneration. J Hepatol. 2002;36:395–400. doi: 10.1016/s0168-8278(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–35. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- 14.Hardwick M, Fertikh D, Culty M, Li H, Vidic B, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: Correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999;59:831–42. [PubMed] [Google Scholar]
- 15.Ramakrishnan G, Rana A, Das C, Chandra NC. Study of low-density lipoprotein receptor regulation by oral (steroid) contraceptives: Desogestrel, levonorgestrel and ethinyl estradiol in JEG-3 cell line and placental tissue. Contraception. 2007;76:297–305. doi: 10.1016/j.contraception.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Chandra NC, Spiro MJ, Spiro RG. Identification of a glycoprotein from rat liver mitochondrial inner membrane and demonstration of its origin in the endoplasmic reticulum. J Biol Chem. 1998;273:19715–21. doi: 10.1074/jbc.273.31.19715. [DOI] [PubMed] [Google Scholar]
- 17.Robinet P, Wang Z, Hazen SL, Smith JD. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J Lipid Res. 2010;51:3364–9. doi: 10.1194/jlr.D007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–17. [PubMed] [Google Scholar]
- 20.Niendorf A, Nägele H, Gerding D, Meyer-Pannwitt U, Gebhardt A. Increased LDL receptor mRNA expression in colon cancer is correlated with a rise in plasma cholesterol levels after curative surgery. Int J Cancer. 1995;61:461–4. doi: 10.1002/ijc.2910610405. [DOI] [PubMed] [Google Scholar]
- 21.Horton JD, Goldstein JL, Brown MS. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albi E, Viola Magni MP. The role of intranuclear lipids. Biol Cell. 2004;96:657–67. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Albi E, Cataldi S, Rossi G, Magni MV. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J Hepatol. 2003;38:623–8. doi: 10.1016/s0168-8278(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–7. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–8. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 26.Venturini I, Zeneroli ML, Corsi L, Avallone R, Farina F, Alho H, et al. Up-regulation of peripheral benzodiazepine receptor system in hepatocellular carcinoma. Life Sci. 1998;63:1269–80. doi: 10.1016/s0024-3205(98)00388-9. [DOI] [PubMed] [Google Scholar]
- 27.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–85. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 28.Tatidis L, Gruber A, Vitols S. Decreased feedback regulation of low density lipoprotein receptor activity by sterols in leukemic cells from patients with acute myelogenous leukemia. J Lipid Res. 1997;38:2436–45. [PubMed] [Google Scholar]
- 29.Mazzone T, Basheeruddin K, Ping L, Frazer S, Getz GS. Mechanism of the growth-related activation of the low density lipoprotein receptor pathway. J Biol Chem. 1989;264:1787–92. [PubMed] [Google Scholar]


