Abstract
Two experiments were conducted on broilers to assess the effect of dietary fiber from 00-rapeseed meal (RSM) on phosphorus (P) and calcium (Ca) apparent ileal digestibility (AID) and retention (AR) during the growing (Exp1: 10 to 21 d) or finishing period (Exp2: 21 to 31 d) in diets supplemented or not with microbial phytase. Each experiment involved 144 male Cobb 500 fed one of 8 diets. Fiber content was modulated by incorporating whole RSM, RSM from dehulled rapeseeds, either raw or supplemented with 2 levels of defatted rapeseed hulls. Diets were supplemented or not with 750 phytase units of microbial phytase per kg. Excreta were collected from d 14 to d 17 (Exp1) and from d 27 to d 30 (Exp2) to measure AR. At the end of experiments, digestive tracts were sampled and weighed. The distal ileum and tibias were collected to measure AID and bone mineralization, respectively. Age did not significantly alter the response of birds to the addition of dietary fiber. Inclusion of hulls decreased growth performance (P < 0.05). The weight of the proventriculus-gizzard (PG) increased with the dietary fiber content in Exp1: The decreased weight observed using dehulled RSM was reversed following the inclusion of hulls. In both trials, while the presence of phytase increased the AID of P (P < 0.001) but not Ca, the inclusion of hulls with phytase improved the AID of P and Ca [linear (Lin), P < 0.05]. This effect could depend on the effect of fiber on PG development and physiology. Hulls decreased the moisture content of excreta (P < 0.01), suggesting higher water retention or lower water consumption with fiber. The AR of P was lower than AID of P with hulls, contrary to Ca, suggesting a metabolic imbalance. The decrease of AR together with the decrease of bone characteristics indicates a lack of Ca in diets with hulls and suggests that P and Ca provision should be adapted to the level and the origin of fiber inclusion.
Keywords: broiler, calcium, dietary fiber, phosphorus, rapeseed meal
INTRODUCTION
The increasing availability of rapeseed meal in North America and Europe makes its inclusion of great interest for livestock production. Rapeseed meal represents a suitable protein source for broilers and a sustainable feedstuff, as one kg produced locally has lower environmental impacts than one kg of imported soybean meal (Wilfart et al., 2016). As a result of selection, low glucosinolate and low erucic acid varieties known as 00-rapeseed meal (RSM) are available and have been introduced into broiler diet for many years. However, RSM is still characterized by high dietary fiber and phytate levels, which are known to affect nutrient digestibility (Khajali and Slominski, 2012) and may influence phosphorus (P) availability, although this has not been documented yet. Previous studies reported the effect of RSM or canola meal on nutrient availability, but did not specifically focus on dietary fiber content or the technological processes affecting mineral absorption (de Vries et al., 2014; Mutucumarana et al., 2014). In this regard, dehulling the seed is an interesting process to increase metabolized energy and to reduce the fiber fraction and the phenolic compounds located in the hulls (Amarowicz et al., 2000; Carré et al., 2015).
Historically, dietary fiber has been considered as a diluent, with low energy content and a relatively negative impact on feed intake and nutrient digestibility (Mateos et al., 2014). Dietary fiber, particularly the soluble fraction, reduces nutrient breakdown and digestibility owing to its physicochemical properties, such as viscosity, which limit the contact between the feed matrix and the enzyme (Jha and Berrocoso, 2015). Additionally, fiber sources themselves might bind minerals within their complex matrix and thus decrease their absorption, depending on their properties, such as mineral binding and physical entrapment (Baye et al., 2015). Conversely, recent studies have observed a favorable impact of moderate amounts of fiber on nutrient utilization, linked to an improvement of digestive processes. For example, Jiménez-Moreno et al. (2013a) reported a beneficial effect of 2.5% oat hulls or sugar beet pulp inclusion on nutrient digestibility. As fiber inclusion seems to improve the digestive function of the gizzard (Jiménez-Moreno et al., 2009) and as microbial phytase acts mainly in this compartment (Selle and Ravindran, 2007), fiber may enhance the effect of microbial phytase. Since microbial phytase is known to improve P and also calcium (Ca) digestibility (Qian et al., 1997), the inclusion of fiber could modify the balance between both minerals, which are closely linked. Moreover, the different effects of fiber on morphology and digestibility following the age of broilers cannot be excluded (Jiménez-Moreno et al., 2013b). Therefore, the aim of the present study was to investigate the effects of dietary fiber content on P and Ca digestibility, as well as bone mineralization in RSM rich diets, by using different levels of fiber, with and without microbial phytase during the grower and finisher phases of broilers. Consequently, we hypothesized that 1) dehulling the seed could improve nutrient accessibility by reducing the fiber matrix effect, 2) the hull content could lead to a higher development of the gizzard, thus enhancing mineral solubility and the impact of microbial phytase, and 3) the effects of fiber could be age dependent. Variations of dietary fiber level were obtained by using RSM from raw or dehulled rapeseed, the latter being supplemented with low or high levels of defatted rapeseed hulls.
MATERIALS AND METHODS
Two similar but independent experiments were conducted to study the effect of dietary fiber from RSM incorporated into grower (Exp1: 10 to 21 d) or finisher diets (Exp2: 21 to 31 d). All procedures relative to the use of live birds were approved by the local animal ethics committee and the French Ministry of Higher Education and Research (approval number: 2,015,012,611,348,093_v3). The experiments were conducted at INRA 1295, Unité Expérimentale Pôle d’Expérimentation Avicole de Tours (UE PEAT), approval number 37–175-1, Nouzilly, France.
Animal Management
Day-old Cobb 500 male broiler chicks were obtained from a commercial hatchery. Diets and water were provided ad libitum. The following lighting program was used during the experiments: d 0, 24 light (L); d 1 to d 2, 23L:1 dark (D); d 3 to d 6, 20L:4D; and d 7 to d 31, 18L:6D. Housing temperature began at 31°C and was decreased gradually until 21.5°C was reached at 17 days. At the end of each experiment (21 d and 31 d), all birds were killed by injection of sodium pentobarbital into the occipital sinus.
Experiment 1
Two hundred birds (45.5 ± 2.6 g) were housed in cages (2 birds per cage) equipped with a drinker and an open-trough feeder for 9 days. From d 0 to d 5 and from d 6 to d 9, birds received 2 starter diets successively (S1 and S2, respectively) that met nutrient requirements (NRC, 1994) and differed in the RSM inclusion in order to accustom the chicks to rapeseed (2.5% for S1 and 8% for S2; Table 1). At d 10, chicks were weighed, and the 144 birds closest to the mean weight (287 ± 39.8 g) were allocated randomly and individually to cages until d 21. They received one of the 8 experimental diets (18 animals per diet). Feed intake was recorded individually, and birds were weighed at the beginning and at the end of the experimental period.
Table 1.
Ingredients and chemical composition of starter (S1: 0 to 5 d; S2: 6 to 9 d in Exp1 and Exp2) and grower diets of Exp1 (%, as-fed basis).1
| S1 | S2 | G1- | G1+ | G2- | G2+ | G3- | G3+ | G4- | G4+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients | ||||||||||
| Corn | 58.7 | 56.7 | 56.0 | 55.0 | 54.2 | 53.2 | 55.2 | 54.2 | 56.2 | 55.2 |
| Soybean meal | 34.5 | 30.8 | 25.2 | 25.2 | 26.8 | 26.8 | 25.1 | 25.1 | 23.4 | 23.4 |
| Rapeseed meal | 2.5 | 8.0 | 12.0 | 12.0 | ||||||
| Dehulled rapeseed meal | 8.4 | 8.4 | 8.4 | 8.4 | 8.4 | 8.4 | ||||
| Defatted rapeseed hull | 3.6 | 3.6 | 7.2 | 7.2 | ||||||
| Cornstarch | 3.6 | 3.6 | 7.2 | 7.2 | 3.6 | 3.6 | ||||
| Soybean oil | 0.50 | 1.20 | 1.10 | 1.10 | 1.20 | 1.20 | 1.90 | 1.90 | 2.70 | 2.70 |
| Salt | 0.32 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 |
| Dicalcium phosphate | 2.06 | 1.70 | 0.50 | 0.50 | 0.61 | 0.61 | 0.63 | 0.63 | 0.64 | 0.64 |
| Sodium bicarbonate | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 |
| Calcium carbonate | 0.65 | 0.55 | 0.28 | 0.28 | 0.28 | 0.28 | 0.16 | 0.16 | 0.05 | 0.05 |
| DL-methionine | 0.24 | 0.22 | 0.19 | 0.19 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| L-lysine | 0.07 | 0.09 | 0.11 | 0.11 | 0.10 | 0.10 | 0.13 | 0.13 | 0.15 | 0.15 |
| Phytase2 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Nutritional characteristics, % | ||||||||||
| Dry matter3 | 89.8 | 89.2 | 89.7 | 88.8 | 89.4 | 89.6 | 89.9 | 89.6 | 89.6 | 89.4 |
| Phytase, FTU/kg3 | <100 (0) | <100 (0) | <100 (0) | 1060 (750) | <100 (0) | 920 (750) | <100 (0) | 810 (750) | <100 (0) | 1000 (750) |
| Protein3 | 21.5 (21.5) | 21.3 (21.5) | 19.5 (20.0) | 20.2 (20.0) | 20.0 (20.0) | 20.0 (20.0) | 19.9 (20.0) | 20.1 (20.0) | 19.3 (20.0) | 19.9 (20.0) |
| Fat3 | 3.1 (3.2) | 3.3 (3.8) | 4.7 (4.1) | 5.0 (4.1) | 4.4 (3.8) | 4.0 (3.8) | 4.5 (4.5) | 4.8 (4.5) | 5.7 (5.2) | 5.5 (5.2) |
| Total phosphorus3 | 0.87 (0.77) | 0.80 (0.74) | 0.52 (0.54) | 0.53 (0.54) | 0.54 (0.56) | 0.57 (0.56) | 0.56 (0.55) | 0.58 (0.55) | 0.53 (0.54) | 0.59 (0.54) |
| NPP4 | 0.50 | 0.45 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Calcium3 | 1.12 (1.05) | 0.99 (0.95) | 0.55 (0.53) | 0.55 (0.53) | 0.56 (0.53) | 0.57 (0.53) | 0.57 (0.53) | 0.58 (0.53) | 0.57 (0.53) | 0.58 (0.53) |
| Ca: NPP4,5 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 |
| Crude fiber3 | 3.5 (3.5) | 4.0 (4.0) | 4.6 (4.6) | 3.2 (4.6) | 3.1 (3.4) | 2.9 (3.4) | 3.9 (4.5) | 4.1 (4.5) | 5.2 (5.3) | 4.6 (5.3) |
| NDF3,5 | 11.6 (11.1) | 10.8 (11.9) | 11.8 (12.9) | 12.8 (12.9) | 10.1 (10.8) | 11.0 (10.8) | 12.9 (12.8) | 13.8 (12.8) | 14.4 (14.8) | 13.9 (14.8) |
| ADF3,5 | 4.9 (5.1) | 5.6 (5.8) | 5.9 (6.4) | 6.7 (6.4) | 5.0 (5.3) | 4.9 (5.3) | 6.6 (6.9) | 6.5 (6.9) | 7.7 (8.5) | 7.7 (8.5) |
| ADL3,5 | 0.6 (0.8) | 0.8 (1.2) | 1.0 (1.6) | 1.1 (1.6) | <0.5 (1.1) | 0.6 (1.1) | 1.2 (2.0) | 1.2 (2.0) | 2.0 (3.0) | 1.9 (3.0) |
| Metabolized energy, kcal/kg4 | 2923 | 2930 | 3001 | 3001 | 3014 | 3014 | 2994 | 2994 | 2979 | 2979 |
1Premix (0.40%) was added to all diets: 10,000 IU vitamin A (retinol); 4,000 UI vitamin D3 (cholecalciferol); 80 mg vitamin E (tocopherol); 4 mg vitamin K3 (menadione); 4 mg vitamin B1 (thiamine); 6 mg vitamin B2 (riboflavin); 80 mg vitamin B3 (PP, niacin); 20 mg vitamin B5 (Ca panthotenate); 6 mg vitamin B6 (pyridoxine); 0.2 mg vitamin B8 (biotin, H); 2 mg vitamin B9 (folic acid); 0.02 mg vitamin B12 (cyanocobalamin); 440 mg choline; 40 mg Fe (FeCO3); 16 mg Cu (CuSO4); 64 mg Mn (MnO); 72 mg Zn (ZnSO4); 0.4 mg Co (CoCO3); 2 mg I (Ca(IO3)2); 0.2 mg Se (Na2SeO3); 2.29 g Ca (CaCO3).
TiO2 (0.30%) was added to experimental diets, except for S1 and S2.
2Phytase from Natuphos (10.000 G) was added to the diet in a corn meal mixture (0.75% microbial phytase + 99.25% corn).
3Analysis values, according to Materials and Methods section. Calculated values are in brackets.
4Calculated values.
5NPP = non-phytate phosphorus; NDF = neutral detergent fiber; ADF = acid detergent fiber; ADL = acid detergent lignin.
Experiment 2
Two hundred birds (49.6 ± 2.7 g) were reared in a floor pen for 17 days. They received the S1 diet from d 0 to d 5, the S2 diet from d 6 to d 9, and the grower diet (G0) with 12% RSM from d 10 to d 21 (Tables 1 and 2). At d 17, chicks were weighed, and the 144 chicks closest to the mean weight of 611.9 ± 70.7 g were placed randomly in individual cages. At d 21, broilers received one of the 8 experimental diets until d 31 (18 animals per diet). Feed intake was recorded individually, and birds were weighed at the beginning and at the end of experimental period.
Table 2.
Composition and chemical characteristics of grower (G0; 10 to 20 d) and finisher diets of Exp2 (%, as-fed basis).1
| G0 | F1- | F1+ | F2- | F2+ | F3- | F3+ | F4- | F4+ | |
|---|---|---|---|---|---|---|---|---|---|
| Ingredients | |||||||||
| Corn | 60.1 | 55.6 | 54.6 | 53.3 | 52.3 | 54.3 | 53.3 | 55.3 | 54.3 |
| Soybean meal | 24.1 | 19.5 | 19.5 | 21.6 | 21.6 | 18.7 | 18.7 | 12.7 | 12.7 |
| Rapeseed meal | 12.0 | 15.0 | 15.0 | ||||||
| Dehulled rapeseed meal | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | 10.5 | |||
| Defatted rapeseed hull | 4.5 | 4.5 | 9.0 | 9.0 | |||||
| Cornstarch | 4.5 | 4.5 | 9.0 | 9.0 | 4.5 | 4.5 | |||
| Soybean seed | 2.0 | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 | 8.0 | 8.0 | |
| Soybean oil | 1.20 | 1.70 | 1.70 | 1.70 | 1.70 | 2.70 | 2.70 | 2.80 | 2.80 |
| Salt | 0.30 | 0.31 | 0.31 | 0.30 | 0.30 | 0.31 | 0.31 | 0.30 | 0.30 |
| Dicalcium phosphate | 1.11 | ||||||||
| Monocalcium phosphate | 0.14 | 0.14 | 0.25 | 0.25 | 0.27 | 0.27 | 0.27 | 0.27 | |
| Sodium bicarbonate | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.08 | 0.08 |
| Calcium carbonate | 0.39 | 0.26 | 0.26 | 0.30 | 0.30 | 0.16 | 0.16 | 0.05 | 0.05 |
| DL-methionine | 0.19 | 0.15 | 0.15 | 0.16 | 0.16 | 0.17 | 0.17 | 0.17 | 0.17 |
| L-lysine | 0.13 | 0.11 | 0.11 | 0.10 | 0.10 | 0.13 | 0.13 | 0.16 | 0.16 |
| Phytase2 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Nutritional characteristics, % | |||||||||
| Dry matter3 | 89.9 | 89.8 | 89.7 | 89.8 | 89.4 | 90.0 | 89.6 | 89.8 | 89.3 |
| Phytase, FTU/kg3 | <100 (0) | <100 (0) | 820 (750) | <100 (0) | 780 (750) | <100 (0) | 710 (750) | <100 (0) | 1010 (750) |
| Protein3 | 20.1 (20.0) | 19.2 (19.0) | 19.1 (19.0) | 18.7 (19.0) | 18.8 (19.0) | 18.8 (19.0) | 19.4 (19.0) | 18.9 (19.0) | 19.0 (19.0) |
| Fat3 | 4.4 (3.9) | 5.6 (5.1) | 5.3 (5.1) | 4.8 (4.5) | 4.6 (4.5) | 6.3 (5.7) | 5.6 (5.7) | 6.7 (6.7) | 7.0 (6.7) |
| Total phosphorus3 | 0.80 (0.65) | 0.57 (0.49) | 0.54 (0.49) | 0.56 (0.52) | 0.55 (0.52) | 0.52 (0.50) | 0.56 (0.50) | 0.57 (0.49) | 0.53 (0.49) |
| NPP4,5 | 0.33 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Calcium3 | 0.87 (0.74) | 0.51 (0.42) | 0.49 (0.42) | 0.50 (0.42) | 0.49 (0.42) | 0.50 (0.42) | 0.49 (0.42) | 0.49 (0.42) | 0.49 (0.42) |
| Ca: NPP4 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 | 2.1 |
| Crude fiber3 | 3.8 (4.1) | 4.1 (4.8) | 4.4 (4.8) | 2.9 (3.5) | 2.8 (3.5) | 4.0 (4.5) | 3.9 (4.5) | 4.5 (5.6) | 5.6 (5.6) |
| NDF3,5 | 11.7 (12.5) | 13.3 (13.2) | 13.4 (13.2) | 10.7 (10.6) | 10.7 (10.6) | 12.2 (13.1) | 12.0 (13.1) | 13.8 (15.5) | 14.0 (15.5) |
| ADF3,5 | 6.6 (6.1) | 7.5 (6.7) | 6.6 (6.7) | 4.6 (5.3) | 4.8 (5.3) | 6.2 (7.3) | 6.2 (7.3) | 7.6 (9.2) | 7.6 (9.2) |
| ADL3,5 | 1.4 (1.5) | 1.8 (1.9) | 1.4 (1.9) | 0.9 (1.2) | 0.9 (1.2) | 1.8 (2.4) | 1.8 (2.4) | 2.5 (3.6) | 2.7 (3.6) |
| Metabolized energy, kcal/kg4 | 2960 | 3069 | 3069 | 3076 | 3076 | 3067 | 3067 | 3044 | 3044 |
1Premix (0.40%) was added to all diets: 10,000 IU vitamin A (retinol); 4,000 UI vitamin D3 (cholecalciferol); 80 mg vitamin E (tocopherol); 4 mg vitamin K3 (menadione); 4 mg vitamin B1 (thiamine); 6 mg vitamin B2 (riboflavin); 80 mg vitamin B3 (PP, niacin); 20 mg vitamin B5 (Ca panthotenate); 6 mg vitamin B6 (pyridoxine); 0.2 mg vitamin B8 (biotin, H); 2 mg vitamin B9 (folic acid); 0.02 mg vitamin B12 (cyanocobalamin); 440 mg choline; 40 mg Fe (FeCO3); 16 mg Cu (CuSO4); 64 mg Mn (MnO); 72 mg Zn (ZnSO4); 0.4 mg Co (CoCO3); 2 mg I (Ca(IO3)2); 0.2 mg Se (Na2SeO3); 2.29 g Ca (CaCO3).
TiO2 (0.30%) was added to experimental diets, except for G0.
2Phytase from Natuphos (10,000 G) was added to the diet in a corn meal mixture (0.75% microbial phytase + 99.25% corn).
3Analysis values, according to Materials and Methods section. Calculated values are in brackets.
4Calculated values.
5NPP = non-hytate phosphorus; NDF = neutral detergent fiber; ADF = acid detergent fiber; ADL = acid detergent lignin.
Raw Materials and Experimental Diets
From a single batch of seed (00 variety), whole RSM and dehulled RSM were processed at the OLEAD pilot plant (Pessac, France). The dehulling of seeds was performed prior to oil extraction, and hulls were collected and defatted. Oil was extracted using hexane. Dehulling decreased the dietary fiber level and increased the protein level compared with the raw whole RSM (Table 3).
Table 3.
Analyses of the composition of rapeseed meal, dehulled rapeseed meal, and defatted rapeseed hulls (%, as-fed basis).
| Rapeseed meal | Dehulled rapeseed meal | Defatted rapeseed hulls | |
|---|---|---|---|
| Dry matter | 92.4 | 91.3 | 88.4 |
| Proteins | 31.7 | 37.8 | 19.4 |
| Fat | 5.0 | 1.8 | 1.6 |
| Crude fiber | 16.2 | 9.7 | 26.6 |
| NDF1 | 30.1 | 21.9 | 53.2 |
| ADF1 | 19.6 | 12.7 | 40.7 |
| ADL1 | 8.2 | 4.3 | 21.0 |
| Phosphorus | 0.97 | 1.19 | 0.41 |
| Calcium | 0.70 | 0.62 | 1.15 |
| Glucosinolates, μmol/g | 26.3 | 23.9 | 9.7 |
1NPP = non-hytate phosphorus; NDF = neutral detergent fiber; ADF = acid detergent fiber; ADL = acid detergent lignin.
The experimental diets were based on corn, soybean meal, and/or rapeseed meal obtained from raw or dehulled rapeseeds, the latter being supplemented or not with defatted hulls. All diets were formulated to meet all nutrient requirements of broilers, except for P and Ca, which were marginally deficient in diets without microbial phytase. The level of RSM was adapted to provide similar amounts of protein in the different diets. The dietary fiber content of grower (G) and finisher (F) diets (Exp1 and 2, respectively) was modulated by using whole RSM, dehulled RSM, or dehulled RSM supplemented with 2 levels of defatted hulls. G1 and F1 diets were supplemented with 12 or 15% whole RSM and G2 and F2 diets with 8.4 or 10.5% dehulled RSM. The first level of defatted hulls was selected to obtain the same level of fiber as the G1 and F1 diets, and the second level of defatted hulls doubled the first used. G3 and F3 diets were supplemented with 8.4 or 10.5% dehulled RSM and 3.6 or 4.5% defatted hulls, respectively. G4 and F4 diets were supplemented with 8.4 or 10.5% dehulled RSM and 7.2 or 9.0% defatted hulls, respectively. Diets were supplemented with 0 (−) or 750 FTU (+) microbial phytase/kg (Natuphos® from Aspergillus niger, BASF AG, Ludwigshafen, Germany). All diets had similar levels of Ca and non-phytic P (NPP) with a Ca:NPP ratio of 2:1 for both experiments. Titanium dioxide (TiO2, 0.30%) was added as an indigestible marker, and diets were fed as pellets.
Sample Collection and Chemical Analyses
Total excreta were collected individually between d 14 and d 17 for Exp1 and between d 27 and d 30 for Exp2. The proventriculus-gizzard (PG), the duodenum (from the end of PG to the end of the duodenal loop), the jejunum (from the end of the duodenum to the Meckel's diverticulus), the ileum (from the Meckel's diverticulus to the ceca), and the ceca were excised from 12 animals per diet, and the empty organs were weighed. The digestive content of each segment was collected in addition to the right tibia and the distal ileum, and kept at −20°C until further analysis. The pH was measured on all digestive contents (Mettler Toledo MP220, Greifensee, Switzerland).
All samples were analyzed in duplicate. Dry matter of the diets, freeze-dried excreta, and digestive contents of the stomach were determined after drying at 103°C for 4 hours. The glucosinolate content and fiber characteristics in RSM, dehulled RSM, and defatted rapeseed hulls were determined by HPLC (ISO 9167–1:1992). The crude fiber (CF) content was measured using the Weende method (NF V03–040). Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were obtained according to Van Soest et al. (1991). Phytase activity was measured according to Engelen et al. (1994). Distal ileum content was freeze-dried. Samples were then ashed at 550°C for 8 h in a muffle furnace and solubilized with 16 N nitric acid and 30% hydrogen peroxide on a digestion block to dryness and finally diluted in 0.4 N nitric acid. Calcium and P content were measured on diets, excreta, and ileal digesta, using inductively coupled plasma optical emission spectrometry (ICP OES ThermoscientificTM iCAPTM 7200; method 990.08; AOAC International, 2006). Diets and distal ileum contents were analyzed for TiO2 according to the method of Short et al. (1996). Right tibias were analyzed for bone breaking strength (BS; Model 5543, INSTRON SA, Buc, France) and were then dried at 103°C for 12 h and ashed in a muffle furnace at 550°C for 12 hours.
Calculations and Statistical Analysis
The apparent ileal digestibility (AID) of P and Ca was calculated as follows:
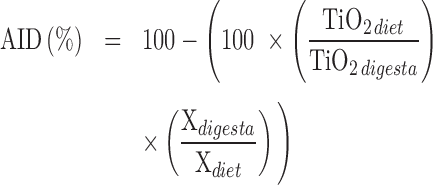 |
where: Xdietand Xdigesta: P or Ca content in the diet (%) and in the digesta (%), respectively; TiO2diet and TiO2digesta: TiO2 content in the diet and the digesta, respectively.
The apparent retention (AR) of Ca and P was calculated as follows:
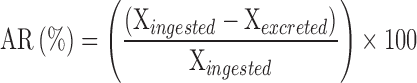 |
where: Xingested: P or Ca intake (g/d), Xexcreted: P or Ca excreted (g/d).
Outliers were determined, and normality of the data was checked using PROC UNIVARIATE for all variables. All data were analyzed using the MIXED procedure of SAS (SAS institute, 1990) as appropriate for a randomized complete block design with the individual broiler as the experimental unit. A block represented 8 consecutive individual cages in which birds received one of the 8 experimental diets. Blocks were used to provide a homogeneous distribution of experimental diets in the room. Consequently, the model included diets as fixed effects, whereas blocks (18 blocks per experiment) were included as random effects. Orthogonal contrasts were performed to separate treatment means. Contrasts were chosen to study: (i): the linear (Lin) effect of microbial phytase (mean of diets without phytase vs. mean of diets with phytase), (ii): the linear effect of dehulled RSM (mean of diets with whole RSM vs. mean of diets with dehulled RSM) with and without microbial phytase, and (iii): the linear and quadratic (Qua) effects of hull content (mean of diets with dehulled RSM vs. mean of diets with the first level of hulls vs. mean of diets with the second level of hulls) with and without microbial phytase. A probability level of P ≤ 0.05 was defined as significant, and a level between 0.1 < P < 0.05 was defined as a tendency. When a significant Lin effect of hull inclusion occurred, a comparison between the highest level (G4 or F4 diets) and the lowest level (G2 or F2 diets) was presented. When a significant Qua effect occurred, the diets compared were specified in brackets.
RESULTS
The use of the dehulling process provided a greater protein content (+19%) and a lower fiber content (−40% CF, −27% NDF, −35% ADF, and −48% ADL; Table 3) in dehulled RSM compared with whole RSM. Concerning the fiber content of experimental diets, results were in accordance, but in general slightly lower than expected values (Tables 1 and 2). Analyzed P concentrations in experimental diets were also in agreement with the expected values. The analyzed Ca concentrations were slightly higher than expected for both experiments. Concerning phytase activity, it was higher than the expected value in Exp1 (1060, 920, 810, and 1,000 FTU/kg in G1+, G2+, G3+, and G4+ diets, respectively). For Exp2, phytase activity was higher than the expected value, except for F3+ (820, 780, 710, and 1,010 for F1+, F2+, F3+, and F4+, respectively). For both experiments, phytase activity was lower than the detection limit of 100 FTU/kg in the diets without supplementation.
Experiment 1
Growth Performance, Mineral Status, and Apparent Ileal Digestibility.
There was no significant effect of microbial phytase on growth performance (Table 4). The inclusion of hulls decreased the final BW and ADG in diets supplemented with phytase (−5%; Lin, P = 0.028 and -6%; Lin, P = 0.025, respectively). A similar trend of decreased ADG was observed in diets without microbial phytase.
Table 4.
Growth performance, bone characteristics, and ileal digestibility of broilers fed diets with 12% whole RSM (G1), 8.4% dehulled RSM (G2), and 8.4% dehulled rapeseed meal with 3.6% (G3) or 7.2% defatted rapeseed hulls (G4) without (−) or with (+) phytase (Exp1: 10 to 21 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.6% hulls | 7.2% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | G1- | G1+ | G2- | G2+ | G3- | G3+ | G4- | G4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Performances | ||||||||||||||||
| BW d 10, g | 288 | 288 | 286 | 292 | 291 | 285 | 288 | 284 | 2.02 | 0.80 | 0.80 | 0.67 | 0.83 | 0.54 | 0.35 | 0.67 |
| BW d 21, g | 862 | 860 | 871 | 891 | 849 | 858 | 845 | 843 | 5.38 | 0.53 | 0.68 | 0.17 | 0.21 | 0.63 | 0.028 | 0.64 |
| ADG, g/d | 53.9 | 53.8 | 55.0 | 56.1 | 52.2 | 53.8 | 52.3 | 52.6 | 0.43 | 0.34 | 0.48 | 0.14 | 0.072 | 0.27 | 0.025 | 0.66 |
| ADFI, g/d | 81.4 | 82.6 | 82.8 | 83.9 | 80.1 | 83.2 | 79.8 | 81.0 | 0.61 | 0.14 | 0.53 | 0.58 | 0.16 | 0.54 | 0.20 | 0.69 |
| FCR | 1.52 | 1.53 | 1.51 | 1.50 | 1.54 | 1.53 | 1.53 | 1.52 | 0.01 | 0.89 | 0.84 | 0.14 | 0.49 | 0.32 | 0.29 | 0.30 |
| Bone characteristics | ||||||||||||||||
| Dry weight, g | 2.33 | 2.41 | 2.36 | 2.44 | 2.40 | 2.42 | 2.41 | 2.35 | 0.02 | 0.51 | 0.69 | 0.75 | 0.60 | 0.87 | 0.30 | 0.73 |
| Ash weight, g DM | 0.85 | 0.91 | 0.86 | 0.90 | 0.88 | 0.89 | 0.86 | 0.85 | 0.01 | 0.18 | 0.78 | 0.74 | 0.92 | 0.58 | 0.14 | 0.63 |
| Ash, % DM | 36.5 | 37.8 | 36.3 | 37.6 | 36.1 | 36.7 | 35.7 | 36.0 | 0.15 | 0.001 | 0.80 | 0.69 | 0.26 | 0.93 | 0.006 | 0.78 |
| BS3, N | 140 | 145 | 138 | 144 | 131 | 140 | 134 | 135 | 1.91 | 0.14 | 0.80 | 0.87 | 0.57 | 0.43 | 0.23 | 0.90 |
1n = 18 per treatment.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
3Bone breaking strength.
Dry weight, ash weight, and BS of the tibia were not affected by treatments. Microbial phytase increased tibia ash content (+0.83 point; P < 0.001), and inclusion of hulls decreased tibia ash content (−1.6 points; Lin, P = 0.006) in birds fed diets supplemented with microbial phytase.
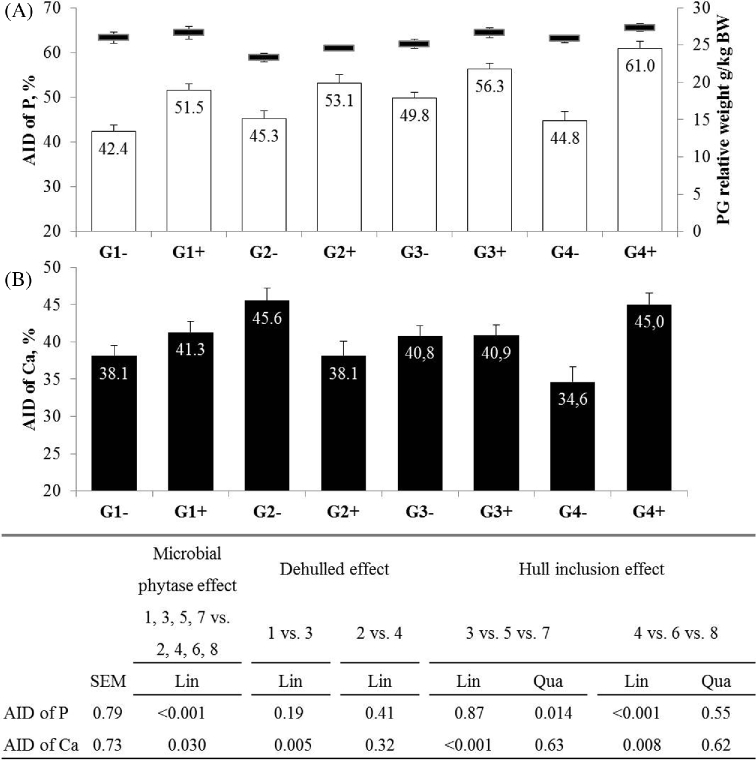
Microbial phytase increased the AID of P (+9.8 points; P < 0.001; Figure 1), but not that of Ca. In the absence of phytase, the use of dehulled seed increased the AID of Ca (+7.1 points; P = 0.005), an effect that was counteracted by the inclusion of hulls (−10.5 points; Lin, P < 0.001). In contrast, in the presence of phytase, the use of dehulled seed did not affect the AID of Ca, but the inclusion of hulls to dehulled RSM increased the AID of Ca (+6.8 points, Lin, P = 0.008). While dehulling did not affect the AID of P, the inclusion of hulls to dehulled RSM altered the AID of P, with a quadratic effect without phytase (50 vs. 45% on average; Qua, P = 0.014) and a linear effect with phytase (+8.5 points; Lin, P = 0.001).
Figure 1.
Apparent ileal digestibility of P with proventriculus-gizzard relative weight in black horizontal lines (A) and apparent ileal digestibility of Ca (B) in Exp1 (10 to 21 d). Values are means with standard error of the mean (n = 18 for AID and n = 12 for PG weight). Standard error of the mean and P-value are presented in the table below the figure. Concerning proventriculus-gizzard weight, all data are presented in Table 5.
Digestive Organ Development and pH of Digesta.
Phytase supplementation significantly increased the weight of PG (+5%; Lin, P = 0.011; Table 5). The use of dehulled RSM decreased the weight of PG with or without phytase (−9% on average; P < 0.05), which was reversed by the subsequent inclusion of hulls (+11% on average; Lin, P < 0.01). The use of dehulled RSM also decreased the weight of the ceca only without phytase (−11%; P = 0.037) and tended to increase it with phytase. The development of the ileum increased following an intermediate inclusion of defatted hulls in RSM from dehulled seeds (12.2 in G3- vs. 13.7 g/kg BW in G2- and G4- on average; Lin, P = 0.024 and Qua, P = 0.001).
Table 5.
Relative empty weight and pH of the digestive segment of broilers fed diets with 12% whole RSM (G1), 8.4% dehulled RSM (G2), and 8.4% dehulled rapeseed meal with 3.6% (G3) or 7.2% defatted rapeseed hulls (G4) without (−) or with (+) phytase (Exp1: 10 to 21 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.6% hulls | 7.2% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | G1- | G1+ | G2- | G2+ | G3- | G3+ | G4- | G4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Weight, g/kg BW | ||||||||||||||||
| Crop | 4.6 | 4.8 | 4.6 | 4.3 | 4.8 | 4.1 | 4.6 | 4.3 | 0.10 | 0.11 | 0.89 | 0.23 | 0.85 | 0.47 | 0.94 | 0.57 |
| PG3 | 26.0 | 26.7 | 23.3 | 24.6 | 25.2 | 26.6 | 25.9 | 27.3 | 0.26 | 0.011 | 0.007 | 0.032 | 0.006 | 0.46 | 0.005 | 0.42 |
| Duodenum | 12.8 | 13.3 | 12.9 | 12.6 | 12.6 | 13.8 | 13.1 | 12.9 | 0.18 | 0.25 | 0.93 | 0.36 | 0.75 | 0.31 | 0.71 | 0.10 |
| Jejunum | 18.9 | 18.5 | 19.3 | 19.1 | 18.1 | 19.3 | 19.3 | 19.7 | 0.23 | 0.60 | 0.67 | 0.53 | 0.94 | 0.14 | 0.54 | 0.86 |
| Ileum | 13.1 | 13.1 | 13.1 | 12.8 | 12.2 | 12.6 | 14.3 | 13.0 | 0.16 | 0.27 | 0.99 | 0.54 | 0.024 | 0.001 | 0.74 | 0.51 |
| Ceca | 5.4 | 4.5 | 4.8 | 5.0 | 4.7 | 4.9 | 4.9 | 5.5 | 0.07 | 0.99 | 0.037 | 0.068 | 0.69 | 0.49 | 0.061 | 0.10 |
| pH | ||||||||||||||||
| PG3 | 2.26 | 2.46 | 2.79 | 2.55 | 2.55 | 2.48 | 2.49 | 2.44 | 0.05 | 0.65 | 0.003 | 0.60 | 0.081 | 0.54 | 0.53 | 0.96 |
| Duodenum | 6.01 | 5.87 | 5.79 | 6.17 | 6.11 | 6.04 | 5.94 | 5.92 | 0.03 | 0.57 | 0.053 | 0.035 | 0.17 | 0.030 | 0.068 | 0.97 |
| Jejunum | 6.12 | 6.11 | 6.09 | 6.08 | 6.05 | 6.06 | 6.04 | 6.03 | 0.01 | 0.72 | 0.32 | 0.50 | 0.20 | 0.63 | 0.16 | 0.96 |
| Proximal ileum | 6.94 | 7.08 | 7.01 | 7.04 | 7.0 | 7.0 | 6.98 | 6.94 | 0.04 | 0.61 | 0.52 | 0.81 | 0.85 | 1.00 | 0.49 | 0.95 |
| Ceca | 6.14 | 6.24 | 5.99 | 6.33 | 6.27 | 6.27 | 6.08 | 6.03 | 0.04 | 0.27 | 0.37 | 0.61 | 0.60 | 0.11 | 0.088 | 0.54 |
1n = 12 per treatment.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
3Proventriculus-gizzard.
Without phytase, the use of dehulled RSM increased the pH of PG (+0.53 point; P = 0.003) and tended to decrease the pH of the duodenum. Inclusion of dehulled seed increased the pH of the duodenum with phytase (+0.30 point; P = 0.035). Inclusion of hulls tended to decrease pH in the PG without phytase and tended to decrease the pH in the duodenum and the ceca with phytase. A Qua effect occurred with inclusion of hulls for the duodenum pH without phytase (6.11 in G3- vs. 5.87 in G2- and G4- on average; Qua, P = 0.030).
Balance Trial Between d 14 and d 17.
Feed intake and excreta weight were not affected by the diets (Table 6). Use of dehulled RSM increased excreta moisture significantly without phytase (+1.8 points; P = 0.010) and only numerically with phytase. The inclusion of hulls counteracted this effect. A similar profile was observed for DM digestibility, which increased with the use of dehulled RSM (+1.8 points on average; P < 0.01) and decreased following inclusion of hulls (−2.4 points and -4 points on average without and with phytase, respectively; Lin, P < 0.001).
Table 6.
Feed intake, excreta characteristics, and retention of P and Ca of broilers, between d 14 and d 17, fed diets with 12% whole RSM (G1), 8.4% dehulled RSM (G2), and 8.4% dehulled rapeseed meal with 3.6% (G3) or 7.2% defatted rapeseed hulls (G4) without (−) or with (+) phytase (Exp1: 10 to 21 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.6% hulls | 7.2% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | G1- | G1+ | G2- | G2+ | G3- | G3+ | G4- | G4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Feed intake, g/d DM | 82 | 84 | 83 | 82 | 82 | 81 | 82 | 82 | 0.7 | 0.90 | 0.80 | 0.44 | 0.91 | 0.98 | 0.97 | 0.78 |
| Excreta | ||||||||||||||||
| Weight, g/d | 106 | 109 | 109 | 105 | 105 | 114 | 106 | 109 | 4.14 | 0.33 | 0.62 | 0.48 | 0.54 | 0.71 | 0.42 | 0.12 |
| Humidity, % | 74.8 | 75.4 | 76.6 | 76.5 | 75.6 | 76.0 | 73.7 | 74.6 | 0.20 | 0.21 | 0.010 | 0.12 | <0.001 | 0.53 | 0.008 | 0.54 |
| DM digestibility | 68.5 | 68.7 | 70.2 | 70.6 | 68.4 | 68.0 | 66.2 | 66.6 | 0.18 | 0.56 | 0.002 | 0.001 | <0.001 | 0.69 | <0.001 | 0.22 |
| P intake, mg/d | 423 | 453 | 445 | 466 | 459 | 471 | 440 | 483 | 4.27 | <0.001 | 0.17 | 0.45 | 0.74 | 0.25 | 0.30 | 0.85 |
| Excreta P, mg/d | 216 | 207 | 218 | 191 | 220 | 217 | 233 | 227 | 2.94 | 0.031 | 0.88 | 0.12 | 0.15 | 0.60 | <0.001 | 0.38 |
| Ca intake, mg/d | 450 | 469 | 463 | 474 | 470 | 471 | 471 | 476 | 4.24 | 0.27 | 0.44 | 0.81 | 0.63 | 0.84 | 0.90 | 0.81 |
| Excreta Ca, mg/d | 221 | 213 | 226 | 193 | 219 | 214 | 273 | 226 | 3.06 | 0.014 | 0.67 | 0.094 | 0.31 | 0.23 | 0.005 | 0.66 |
1n = 18 per treatment.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
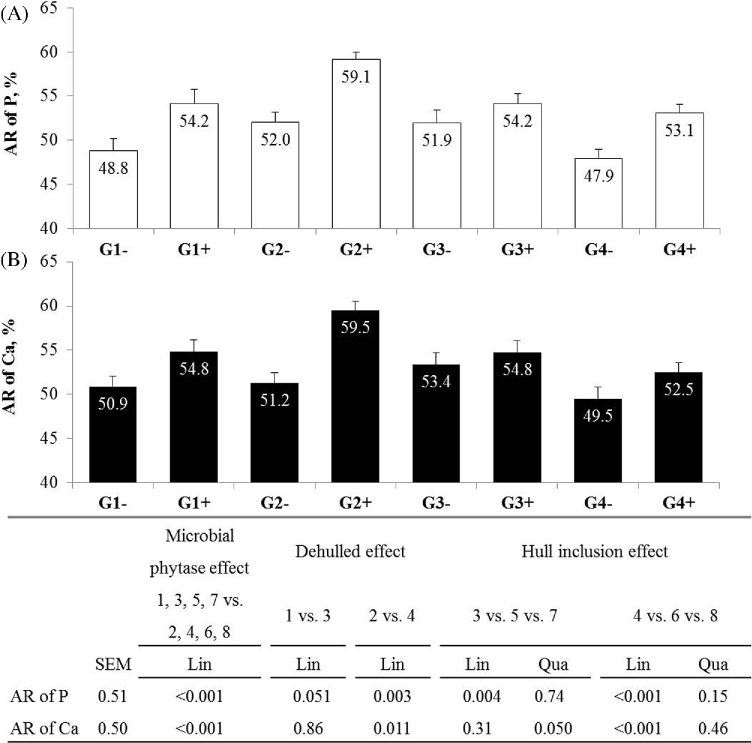
Microbial phytase increased P intake (+6%; P < 0.001), but not Ca intake, while it decreased excreted P (−5%; P = 0.031) and Ca (−7%; P = 0.014). Consequently, the AR of P and Ca increased (+5.2 and +4.0 points, respectively; P < 0.001; Figure 2). Inclusion of dehulled seed increased the AR of P (55.1 vs. 51.6% on average; P = 0.051 and P = 0.003 without and with phytase, respectively) and Ca (+4.7 points; P = 0.011 with phytase). Without phytase, inclusion of hulls decreased AR of P (−4.4 points; Lin, P = 0.004) and of Ca. With phytase, inclusion of hulls increased excreta P and Ca (+18% on average, Lin, P < 0.01), resulting in a lower AR of P and Ca (−6.4 points on average; Lin, P < 0.001).
Figure 2.
Apparent retention of P (A) and Ca (B) in Exp1 (10 to 21 d). Values are means with standard error of the mean (n = 18). Standard error of the mean and P-value are presented in the table below the figure.
Experiment 2
Growth Performance, Mineral Status, and Apparent Ileal Digestibility.
Inclusion of dehulled seed tended to decrease ADFI without phytase and increased ADG with phytase (Table 7). A quadratic trend occurred for higher ADG with the inclusion of hulls without phytase. With phytase, inclusion of hulls decreased the final BW (−4%, Lin, P = 0.042), ADG (−12%, Lin, P = 0.001), and ADFI (−10%, Lin, P = 0.001).
Table 7.
Growth performance, bone characteristics, and ileal digestibility in broilers fed diets with 15% whole RSM (F1), 12% dehulled RSM (F2), and 12% dehulled rapeseed meal with 4.5% (F3) or 9% defatted rapeseed hulls (F4) without (−) or with (+) phytase (Exp2: 21 to 31 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.5% hulls | 9.0% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | F1- | F1+ | F2- | F2+ | F3- | F3+ | F4- | F4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Performances | ||||||||||||||||
| BW 21 d, g | 939 | 948 | 955 | 948 | 951 | 939 | 953 | 960 | 4.34 | 0.96 | 0.33 | 0.98 | 0.89 | 0.84 | 0.52 | 0.33 |
| BW 31 d, g | 1807 | 1825 | 1796 | 1862 | 1811 | 1798 | 1758 | 1792 | 8.40 | 0.12 | 0.73 | 0.25 | 0.25 | 0.23 | 0.042 | 0.34 |
| ADG, g/j | 90.1 | 90.1 | 87.2 | 95.4 | 90.6 | 88.2 | 84.6 | 84.3 | 0.84 | 0.37 | 0.31 | 0.080 | 0.38 | 0.073 | 0.001 | 0.55 |
| ADFI, g/d | 138.2 | 139.6 | 131.1 | 144.1 | 137.2 | 131.3 | 134.0 | 129.8 | 1.111 | 0.60 | 0.079 | 0.26 | 0.49 | 0.19 | 0.001 | 0.13 |
| FCR | 1.54 | 1.53 | 1.51 | 1.51 | 1.52 | 1.49 | 1.56 | 1.49 | 0.01 | 0.11 | 0.32 | 0.60 | 0.10 | 0.59 | 0.49 | 0.65 |
| Tibia characteristics | ||||||||||||||||
| Dry weight, g | 5.05 | 5.40 | 5.33 | 5.46 | 5.31 | 5.32 | 5.10 | 5.40 | 0.04 | 0.005 | 0.071 | 0.33 | 0.15 | 0.51 | 0.65 | 0.052 |
| Ash weight, g DM | 1.74 | 1.88 | 1.79 | 1.96 | 1.76 | 1.8 | 1.68 | 1.82 | 0.02 | <0.001 | 0.36 | 0.14 | 0.071 | 0.56 | 0.018 | 0.070 |
| Ash, % DM | 33.9 | 34.9 | 33.6 | 35.4 | 33.2 | 33.4 | 33.0 | 33.0 | 0.19 | 0.021 | 0.60 | 0.40 | 0.40 | 0.92 | 0.004 | 0.11 |
| BS3, N | 171 | 181 | 167 | 175 | 157 | 170 | 141 | 166 | 2.40 | 0.011 | 0.68 | 0.55 | 0.087 | 0.83 | 0.31 | 0.92 |
1n = 18 per treatment.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
3Bone breaking strength.
Microbial phytase increased tibia dry weight (+4%, P = 0.005), ash weight (+8%; P < 0.001), ash content (+0.7 point; P = 0.056), and BS (+9%; P = 0.011). The use of dehulled seed without phytase tended to increase tibia dry weight. Without phytase, the use of hulls tended to decrease tibia ash weight and BS. With phytase, inclusion of hulls decreased tibia ash weight (−7%; Lin, P = 0.018 and Qua, P = 0.070) and ash content (–2.1 points; Lin, P = 0.004), and a quadratic trend was observed for dry weight.
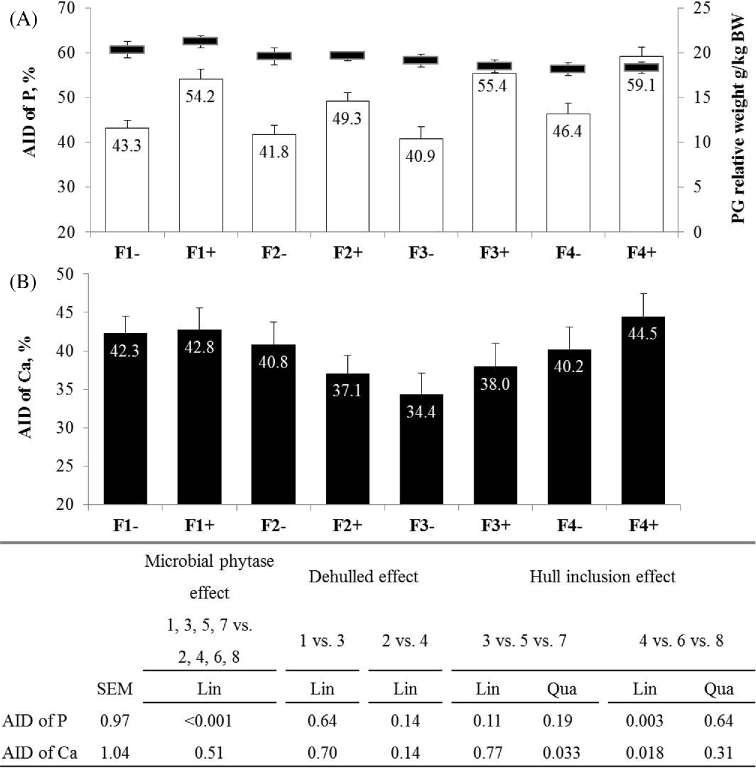
Microbial phytase increased the AID of P (+11.3 points; P < 0.001; Figure 3) but not of Ca. Without phytase, a quadratic trend occurred towards a lower AID of Ca with inclusion of hulls (34.4% in F3- vs. 40.5% in F2- and F4- on average; Qua, P = 0.033). With phytase, inclusion of hulls increased AID of P (+9.9 points; Lin, P = 0.003) and Ca (+8.2 points; Lin, P = 0.018).
Figure 3.
Apparent ileal digestibility of P and relative weight of proventriculus-gizzard in horizontal black lines (A) and apparent ileal digestibility of Ca (B) in Exp2 (21 to 31 d). Values are means with standard error of the mean (n = 18 for AID and n = 12 for PG weight). Standard error of the mean and P-value are presented in the table below the figure. Concerning proventriculus-gizzard weight, all data are presented in Table 8.
Digestive Organ Development and pH of Digesta.
With phytase, a trend towards a higher empty weight of the duodenum and PG occurred (Table 8). Without phytase, inclusion of dehulled seed decreased the weight of the duodenum (−14%; P = 0.006) and of the ileum (−9%, P = 0.011), and the same trend occurred for the jejunum. Without phytase, the use of hulls tended to increase the weight of the jejunum. With phytase, the use of hulls increased the weight of the duodenum (+13%; Lin, P = 0.022).
Table 8.
Relative empty weight and pH of the digestive segment of broilers fed diets with 15% whole RSM (F1), 12% dehulled RSM (F2), and 12% dehulled rapeseed meal with 4.5% (F3) or 9% defatted rapeseed hulls (F4) without (−) or with (+) phytase (Exp2: 21 to 31 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.5% hulls | 9.0% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | F1- | F1+ | F2- | F2+ | F3- | F3+ | F4- | F4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Weight, g/kg BW | ||||||||||||||||
| Crop | 4.1 | 4.3 | 5.0 | 4.5 | 4.5 | 4.7 | 5.4 | 5.4 | 0.19 | 0.93 | 0.23 | 0.83 | 0.59 | 0.23 | 0.25 | 0.71 |
| PG3 | 20.4 | 21.3 | 19.6 | 19.7 | 19.1 | 18.5 | 18.2 | 18.3 | 0.28 | 0.084 | 0.49 | 0.16 | 0.19 | 0.80 | 0.24 | 0.64 |
| Duodenum | 8.1 | 7.9 | 7.0 | 7.4 | 7.5 | 8.2 | 7.6 | 8.3 | 0.11 | 0.060 | 0.006 | 0.19 | 0.14 | 0.55 | 0.022 | 0.22 |
| Jejunum | 13.7 | 13.3 | 12.8 | 13.0 | 13.2 | 12.7 | 13.7 | 13.4 | 0.13 | 0.31 | 0.074 | 0.51 | 0.085 | 0.91 | 0.47 | 0.31 |
| Ileum | 10.7 | 10.1 | 9.8 | 9.7 | 10.0 | 10.3 | 10.2 | 10.1 | 0.09 | 0.49 | 0.011 | 0.34 | 0.30 | 0.81 | 0.35 | 0.23 |
| Ceca | 2.7 | 2.7 | 2.7 | 2.7 | 2.6 | 2.7 | 2.8 | 2.9 | 0.04 | 0.86 | 0.99 | 0.96 | 0.85 | 0.36 | 0.27 | 0.74 |
| pH | ||||||||||||||||
| PG3 | 2.67 | 2.92 | 2.93 | 2.90 | 3.18 | 3.25 | 2.66 | 3.03 | 0.05 | 0.089 | 0.18 | 0.94 | 0.16 | 0.024 | 0.48 | 0.10 |
| Duodenum | 5.84 | 5.92 | 5.43 | 5.83 | 5.40 | 5.57 | 5.65 | 5.44 | 0.04 | 0.15 | 0.012 | 0.59 | 0.16 | 0.26 | 0.010 | 0.63 |
| Jejunum | 5.94 | 6.02 | 5.97 | 5.98 | 5.92 | 5.97 | 5.90 | 5.91 | 0.01 | 0.15 | 0.54 | 0.48 | 0.18 | 0.70 | 0.20 | 0.58 |
| Proximal ileum | 7.51 | 7.81 | 7.33 | 7.68 | 7.52 | 7.68 | 7.42 | 7.46 | 0.04 | 0.008 | 0.26 | 0.39 | 0.59 | 0.27 | 0.17 | 0.43 |
| Ceca | 6.14 | 6.30 | 6.23 | 6.46 | 6.20 | 6.29 | 6.25 | 6.30 | 0.03 | 0.024 | 0.43 | 0.16 | 0.80 | 0.71 | 0.16 | 0.42 |
1n = 12 per treatments.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
3Proventriculus-gizzard.
Microbial phytase increased the pH of the proximal ileum (+0.21 points; P = 0.008) and of the ceca (+0.13 points; P = 0.024) and a trend towards a higher pH of the PG occurred. Without phytase, the use of dehulled seed decreased the pH of the duodenum (5.43 vs. 5.84; P = 0.012). The pH of PG increased following an intermediate supplementation of hulls (3.18 in F3- vs. 2.80 on average for F2- and F4-; Qua, P = 0.024). With phytase, supplementation of hulls decreased the duodenum pH (−0.39 point; Lin, P = 0.010).
Balance Trial Between d 27 and d 30
The use of dehulled seed tended to decrease feed intake in diets without phytase, and hull supplementation decreased it in diets with phytase (−12%; Lin, P = 0.001; Table 9). Microbial phytase tended to increase excreta weight. The use of dehulled seed increased DM digestibility without phytase (+2.6 points; P = 0.008) and increased excreta weight and moisture content of excreta with phytase (+16% and +3.4 points, respectively; P < 0.01). Without phytase, inclusion of hulls decreased DM digestibility (−6.3 points; Lin, P < 0.001), and the same trend occurred for excreta moisture content. With phytase, supplementation of hulls decreased the weight of excreta (−17%, Lin, P = 0.005), the moisture content (−5.3 points, Lin, P < 0.001), and the DM digestibility (−4.0 points, Lin, P < 0.001).
Table 9.
Feed intake, excreta characteristics, and retention of P and Ca of broilers, between d 27 and d 30, fed diets with 15% whole RSM (F1), 12% dehulled RSM (F2), and 12% dehulled rapeseed meal with 4.5% (F3) or 9% defatted rapeseed hulls (F4) without (−) or with (+) phytase (Exp2: 21 to 31 d).1
| RSM | Dehulled RSM | P-value2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.5% hulls | 9.0% hulls | Microbial phytase effect | Dehulled effect | Hull inclusion effect | ||||||||||||
| Diets | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1,3,5,7 vs. 2,4,6,8, | 1 vs. 3 | 2 vs. 4 | 3 vs. 5 vs. 7 | 4 vs. 6 vs. 8 | |||
| Description | F1- | F1+ | F2- | F2+ | F3- | F3+ | F4- | F4+ | SEM | Lin | Lin | Lin | Lin | Qua | Lin | Qua |
| Feed intake, g DM | 181 | 177 | 170 | 186 | 175 | 173 | 164 | 164 | 1.63 | 0.35 | 0.089 | 0.13 | 0.29 | 0.14 | 0.001 | 0.63 |
| Excreta characteristics | ||||||||||||||||
| Weight, g | 256 | 246 | 233 | 286 | 243 | 255 | 239 | 238 | 4.02 | 0.099 | 0.14 | 0.009 | 0.71 | 0.62 | 0.005 | 0.62 |
| Humidity, % | 78.9 | 78.8 | 79.5 | 82.2 | 77.9 | 79.0 | 77.2 | 76.9 | 0.33 | 0.16 | 0.62 | 0.004 | 0.062 | 0.65 | <0.001 | 0.62 |
| DM digestibility, % | 70.6 | 71.4 | 73.2 | 72.4 | 69.7 | 69.7 | 66.9 | 68.1 | 0.28 | 0.51 | 0.008 | 0.30 | <0.001 | 0.70 | <0.001 | 0.51 |
| P intake, mg | 838 | 830 | 799 | 840 | 827 | 839 | 754 | 802 | 7.65 | 0.12 | 0.19 | 0.73 | 0.14 | 0.049 | 0.23 | 0.51 |
| Excreta P, mg | 438 | 390 | 379 | 417 | 461 | 423 | 442 | 420 | 6.93 | 0.21 | 0.024 | 0.31 | 0.025 | 0.027 | 0.92 | 0.85 |
| Ca intake, mg | 842 | 809 | 807 | 803 | 761 | 775 | 703 | 738 | 7.82 | 0.82 | 0.20 | 0.83 | <0.001 | 0.80 | 0.029 | 0.85 |
| Excreta Ca, mg | 344 | 312 | 306 | 335 | 433 | 396 | 403 | 378 | 6.42 | 0.13 | 0.068 | 0.27 | <0.001 | <0.001 | 0.057 | 0.035 |
1n = 18 per treatment.
2Contrast analysis: linear effect of microbial phytase; linear effect of dehulled rapeseed seed without and with microbial phytase; linear and quadratic effects of the inclusion of hulls without and with microbial phytase.
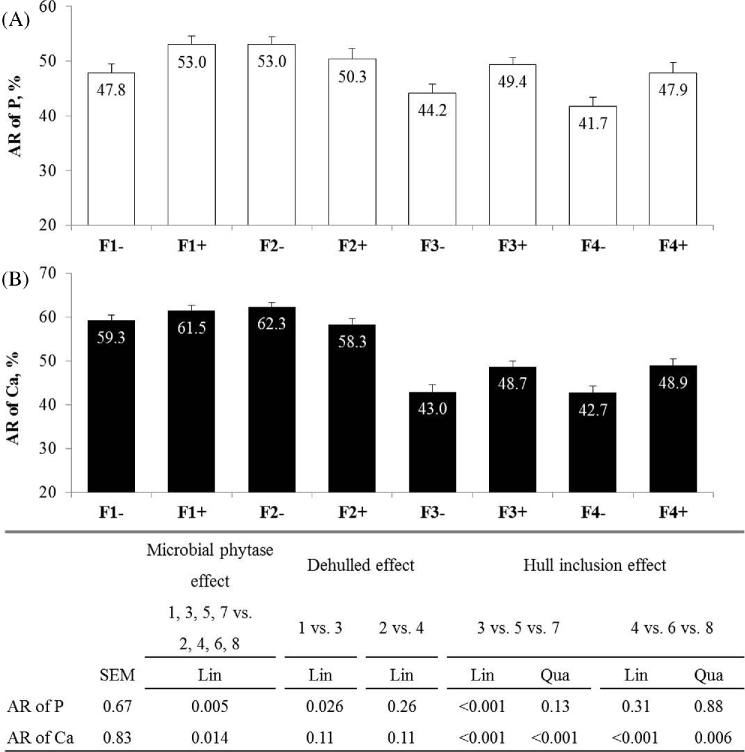
Microbial phytase increased the AR of P (+3.6 points, P = 0.005; Figure 4) and Ca (+2.8 points; P = 0.024). Without phytase, the use of dehulled seed decreased excreta P and tended to decrease excreta Ca (−14%, P = 0.024 for P and P = 0.068 for Ca) and increased the AR of P (+4.9 points; P = 0.026). A Qua effect also occurred for higher P intake with the supplementation of hulls (827 in F3- vs. 778 mg/j in F2- and F4- on average; Qua, P = 0.049), and a plateau was observed for excreta P (Lin, P = 0.025 and Qua, P = 0.027), for excreta Ca and for AR of Ca (Lin, Qua, P < 0.001) with the supplementation of hulls. For the same treatments, a decrease occurred for AR of P (−11.1 points, Lin, P < 0.001) and for Ca intake (−13%, Lin, P < 0.001). With phytase, supplementation of hulls decreased Ca intake (−8%; Lin, P = 0.029) and AR of Ca (−9.4 points; Lin, P < 0.001 and Qua, P = 0.006), and a Qua effect for higher excreta Ca occurred (396 in G3+ vs. 356 mg/j in G2+ and G4+ on average; Lin, P = 0.057 and Qua, P = 0.035).
Figure 4.
Apparent retention of P (A) and Ca (B) in Exp2 (21 to 31 d). Values are means with standard error of the mean (n = 18). Standard error of the mean and P-value are presented in the table below the figure.
DISCUSSION
Higher fiber content, through hull supplementation, reduced the positive impact of phytase on growth performance. The dehulling process probably permitted enzymes to access nutrients more easily. Rapeseed fiber may modify the physicochemical characteristics of the digesta, modulating the retention time and increasing bulk volume or water retention, thus leading to a greater feeling of satiety (Hetland et al., 2005; Mateos et al., 2013). However, recent studies have reported a beneficial effect of moderate amounts of dietary fiber on growth performance. Jiménez-Moreno et al. (2016) observed an increase of 4.2, 5.0, and 4.1 g of ADG with the inclusion of 5% oat hulls, rice hulls, or sunflower hulls, respectively (7.3% NDF on average in diets). The discrepancy observed in our results could be explained by the higher level of fiber, with NDF values of 14.8 and 15.5% in Exp1 and Exp2, respectively. Indeed, according to Mateos et al. (2014), there is a threshold beyond which dietary fiber has negative effects on growth performance. The nature and the level of fiber also modulate these responses. In addition, the Ca:NPP ratio used in this experiment was expected to optimize growth performance in conventional diets (Rousseau et al., 2016) and was consequently not responsible for the decrease observed.
Moreover, we observed a decrease of DM digestibility with hull inclusion, which is in line with the results of growth performance as reported by previous authors (Walugembe et al., 2014). The moisture content of the excreta decreased as the level of hulls increased. This result is in agreement with van der Hoeven-Hangoor et al. (2014) who showed that adding insoluble fiber in the form of oat hulls to a wheat-based diet reduced excreta and litter moisture content. Conversely, Jimenéz-Moreno et al. (2016) observed that increasing insoluble fiber from 2.5 to 5% in the form of oat hulls, rice hulls, or sunflower hulls led to a higher water intake and moisture content of the excreta. Although we did not measure water intake, a lower water consumption or greater water reabsorption by broilers fed diets supplemented with hulls cannot be excluded. Water intake is indeed highly correlated with feed intake, but diet composition might affect this relationship (Carré et al., 2013). On the other hand, the cecum is the main digestive segment for electrolyte and water absorption (Thomas and Skadhauge, 1989), and an active one might have occurred concurrently with fermentation. These results are of particular interest regarding the current need to improve litter quality and limit footpad lesions, as a positive correlation exists between the litter moisture and the incidence of pododermatitis (Taira et al., 2014).
Hull inclusion resulted in a higher relative empty gizzard weight at d 21, whereas the use of dehulled seed decreased it. An enlargement of the gizzard is observed when hulls, wood shavings, or large cereal particles are included in the diet, especially when those particles are composed of insoluble fiber (Svihus, 2011; Jiménez-Moreno et al., 2013b). Sacranie et al. (2017) reported an increase of 65% of the gizzard weight with oat hull inclusion compared with cellulose inclusion, illustrating the effect of a native fiber compared with an isolated source. Surprisingly, this effect was not observed at d 31 when the small intestine was more affected by dietary fiber content, especially the duodenum, which was heavier with the inclusion of hulls. Previous works have reported similar results and assumed that the source of insoluble fiber could influence organ development differentially (González-Alvarado et al., 2008). The gradual increase of RSM incorporation for all birds with the pre-starter, the starter, and the grower phases (from 2.5 to 12%) probably resulted in a greater development of the gizzard at d 21. This previous adaptation could have masked the effect of fiber on the gizzard in the later phase.
The amount, the retention time, and the characteristics of the feed are known to alter the digestive pH, mostly in the gizzard (Svihus, 2011). Our values were below the average of 3.5, but were in accordance with the range of 1.9 to 4.5 reported by Svihus (2011). Only small differences of the digestive pH were observed between diets in both experiments. Even in small amounts, the presence of dietary fiber may have led to higher retention time and HCl secretions (Mateos et al., 2013; Svihus, 2014). At the same time, high-fiber diets may have increased duodenal refluxes (Sacranie et al., 2012). Consequently, the basic components from bile and pancreatic juices may have neutralized the pH of the gizzard in the case of high-fiber diets.
Insoluble fiber improved the development of the gizzard and also the AID of Ca and P in birds supplemented with microbial phytase. The effect of microbial phytase on P and Ca digestibility has been demonstrated in previous studies in broilers (Sebastian et al., 1996; Qian et al., 1997; Dilger et al., 2004). The present study highlighted a positive interaction between dietary fiber and microbial phytase (Figures 1 and 3), which is consistent with the observations of Shang et al. (2015), showing a better use of P in diets supplemented with 0.5% fructooligosaccharides and 500 FTU of microbial phytase per kg of feed (+11.5 points). The acidic pH of the PG promotes mineral solubilization and phytate hydrolysis, as the pH optimum of the phytase is between 2.5 and 5.5 (Dersjant-Li et al., 2015). The higher functionality and retention time of the gizzard have probably favored these processes, thus delivering higher levels of inorganic and soluble P and Ca for absorption in the small intestine (Adedokun and Adeola, 2013). As the pH of the duodenum remains low, it can prevent the formation of Ca-P complexes and ensures optimal phytase activity. In addition, the lower concentration of limestone in diets supplemented with hulls also may have favored phytate-P hydrolysis in the digestive tract (Tamim and Angel, 2003). It is worth noting that the presence of hulls in diets without phytase limited the AID of Ca. This might be explained by the higher feed passage rate through the small intestine, which could limit the time for absorption.
In contrast to AID, AR of P was gradually reduced as hull supplementation increased in diets supplemented with phytase. The improvement in AID of P with hulls was higher than for Ca. Consequently, a metabolic imbalance between P and Ca could have occurred, leading to higher urinary P excretion (Leytem et al., 2008). Indeed, plasma Ca is maintained in a narrow range through a system of hormonal regulation, whereas plasma P is less regulated. An explanation could be that low plasma Ca stimulates the release of parathyroid hormone (PTH), which is known to stimulate tubular reabsorption of Ca and reduce reabsorption of P (Proszkowiec-Weglarz & Angel, 2013). This is consistent with the observation that P absorbed in the small intestine in relation to phytase addition was not retained, but was probably filtered by the glomerulus and excreted in the urine (Berndt et al., 2005). This suggests that the Ca:NPP ratio, and probably microbial phytase addition, should be adapted according to the level of fiber in the diet.
Mutucumarana et al. (2014) observed higher values of AID and AR of P than in the present experiments (61.8 and 64.5%, respectively) in 24- to 28-day-old broilers fed a diet containing canola meal. This discrepancy can be related to the fact that their birds were fed semi-synthetic diets with very low levels of P. Consequently, regulation processes may have occurred with an increase in the absorption rate of P (Viveros et al., 2002). They did not observe any effect on AID of P, but a significant decrease of the coefficients of AR of P with the inclusion of canola meal (0.697 vs. 0.539 points for diet with 13.5 and 54.0% canola meal, respectively), which is in agreement with our results. Fermentation of dietary fiber is known to decrease luminal pH and induce production of short chain fatty acids (SCFA) and an enlargement of ceca (Jozefiak et al., 2004). Therefore, a specific effect of bacterial P cannot be excluded and may explain the excretion of P. The microbial community has a specific requirement in P, as previously reported by Tilocca et al. (2016). Indeed, P is involved in several functions required for microbiota activity and is excreted in the feces simultaneously with bacteria (Metzler and Monsenthin, 2008; Metzler-Zebeli et al., 2010). Consequently, the P used by microbiota is lost for the host and participates in the lower retention of P in diets with hulls. The higher inclusion of hulls rich in dietary fiber might have increased fermentations in the ceca and the microbial community. This is in line with the enlargement of the ceca and the decrease of the cecal pH (mostly at d 21).
On the other hand, the higher AR of Ca compared with AID might also suggest absorption of Ca in the ceca. The absorption of Ca in the large intestine of rats has been known for a long time, and TRPV6 as well as vitamin D receptor expression have been measured in the cecum and colon (Karbach and Feldmeier, 1993; Christakos et al., 2014). Moreover, a recent in vitro study showed a significant increase of Ca absorption through a laying hen's colonic epithelium, when a SCFA mixture was added with a molasses-oligofructose mix (+36.3 nmol/min/cm² for Ca; Gultemirian et al., 2014). The lack of Ca in the metabolic compartment might have enhanced Ca absorption in the large intestine, probably through the absorption of SCFA, as suggested in rats by Trinidad et al. (1996). These hypotheses request further researches, but the cecum appears to be a homeostatic compartment, able to absorb Ca if necessary in order to maintain the balance.
In conclusion, the present data suggested that the inclusion of fiber, in the form of rapeseed hulls, results in beneficial effects on the development of the gastrointestinal tract. The improvement of gizzard development and functionality led to a better microbial phytase effect on P and Ca digestibility, whatever the age of the birds. The use of dehulled RSM seems to improve nutrient accessibility, but this aspect should be investigated further. However, the levels of fiber inclusion from rapeseed meal, in the form of whole seeds or hulls, must be carefully adjusted to avoid any deterioration of feed intake or growth performance. In addition, the current results pointed out the need to adapt the Ca:NPP ratio according to the level and source of fiber and the microbial phytase addition, in order to limit extra urinary losses of P due to homeostatic regulation.
ACKNOWLEDGEMENTS
The authors are grateful to MiXscience, Terres Inovia, and Terres Univia for their financial support, N. Besné, O. Callut, I. Jottreau, P. Ganier, F. Mercerand, and H. Rigoreau from UE PEAT for the animal care, and M. Couty, J. Jiménez, and M. Leconte from URA and Y. Jaguelin and C. Perrier from PEGASE (INRA, Saint-Gilles, France) for their help with laboratory analyses.
REFERENCES
- Adedokun S. A., Adeola O.. 2013. Calcium and phosphorus digestibility: Metabolic limits. J. Appl. Poult. Res. 22:600–608. [Google Scholar]
- Amarowicz R., Naczk M., Shahidi F.. 2000. Antioxydant activity of crude tannins of canola and rapeseed hulls. J. Am. Oil. Chem. Soc. 77:957–961. [Google Scholar]
- AOAC 2006. Method 990.08: Metals in Solid Wastes. In Official Methods of Analysis of AOAC International. 18th ed. Assoc.. Association of Official Analytical Chemists, Arlington, VA. [Google Scholar]
- Baye K., Guyot J. P., Mouquet-River C.. 2015. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 57:949–957. [DOI] [PubMed] [Google Scholar]
- Berndt T. J., Schiavi S., Kumar R.. 2005. “Phosphatonins” and the regulation of phosphorus homeostasis. Am. J. Physiol. Renal Physiol. 289:F1170–F1182. [DOI] [PubMed] [Google Scholar]
- Carré B., Lessire M., Juin H.. 2013. Prediction of metabolisable energy value of broiler diets and water excretion from dietary chemical analyses. Animal. 7:1246–1258. [DOI] [PubMed] [Google Scholar]
- Carré P., Quinsac A., Citeau M., Fine F.. 2015. A re-examination of the technical feasibility and economic viability of rapeseed dehulling. OCL 22:D304. [Google Scholar]
- Christakos S., Lieben L., Masuyama R., Carmeliet G.. 2014. Vitamin D endocrine system and the intestine. Bonekey Rep. 3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., Partridge G.. 2015. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95:878–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries S., Putsjens A. M., Kabel M. A., Kwakkel R. P., Gerrits W. J. J.. 2014. Effects of processing technologies and pectolytic enzymes on degradability of nonstarch polysaccharides from rapeseed meal in broilers. Poult. Sci. 93:589–598. [DOI] [PubMed] [Google Scholar]
- Dilger R. N., Onyango E. M., Sands J. S., Adeola O.. 2004. Evaluation of microbial phytase in broiler diets. Poult. Sci. 83:962–970. [DOI] [PubMed] [Google Scholar]
- Engelen A. J., van der Heeft F. C., Randsdorp P. H. G., Smits E. L. C.. 1994. Simple and rapid determination of phytase activity. J. AOAC Int. 77:760–764. [PubMed] [Google Scholar]
- González-Alvarado J. M., Jiménez-Moreno E., Valencia D. G., Lázaro R., Mateos G. G.. 2008. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci. 87:1779–1795. [DOI] [PubMed] [Google Scholar]
- Gultemirian M. L., Corti H. R., Chaia A. P., Apella M. C.. 2014. Fermentation in vitro of a mixture of dietary fibers and cane molasses by the cecal microbiota: Application on mineral absorption through the laying hen's colonic epithelium. Anim. Feed Sci. Technol. 191:76–82. [Google Scholar]
- Hetland H., Svihus B., Choct M.. 2005. Role of insoluble fiber on gizzard activity in layers. J. Appl. Poult. Res. 14:38–46. [Google Scholar]
- Jha R., Berocoso J. D.. 2015. Review: Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 9:1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J. M., de Coca-Sinova A., Lázaro R., Mateos G. G.. 2009. Effects of source of fibre on the development and pH of the gastrointestinal tract of broilers. Anim. Feed Sci. Technol. 154:93–101. [Google Scholar]
- Jiménez-Moreno E., Frikha M., de Coca-Sinova A., Lázaro R. P., Mateos G. G.. 2013a. Oat hulls and sugar beet pulp in diets for broilers 1. Effects on growth performance and nutrient digestibility. Anim. Feed Sci. Technol. 182:33–43. [Google Scholar]
- Jiménez-Moreno E., Frikha M., de Coca-Sinova A., Lázaro R. P., Mateos G. G.. 2013b. Oat hulls and sugar beet pulp in diets for broilers. 2. Effects on the development of the gastrointestinal tract and on the structure of the jejunal mucosa. Anim. Feed Sci. Technol. 182:44–52. [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J. M., de Coca-Sinova A., Mateos G. G.. 2016. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult. Sci. 95:41–52. [DOI] [PubMed] [Google Scholar]
- Jozefiak D., Rutkowski A., Martin S. A.. 2004. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 113:1–15. [Google Scholar]
- Karbach U., Feldmeier H.. 1993. The cecum is the site with the highest calcium absorption in rat intestine. Dig. Dis. Sci. 38:1815–1824. [DOI] [PubMed] [Google Scholar]
- Khajali F., Slominski B. A.. 2012. Factors that affect the nutritive value of canola meal for poultry. Poult. Sci. 91:2564–2575. [DOI] [PubMed] [Google Scholar]
- Leytem A. B., Willing B. P., Thacker P. A.. 2008. Phytate utilization and phosphorus excretion by broiler chickens fed diets containing cereal grains varying in phytate and phytase content. Anim. Feed Sci. Technol. 146:160–168. [Google Scholar]
- Mateos G. G., Serrano M. P., Berrocoso J., Perez-Bonilla A., Lázaro R.. 2013. Improving the utilization of raw materials in poultry feeding: New technologies and inclusion levels. XXIV World's Poultry Congress; Salvador de Bahía, Brazil, pp. 1–13. [Google Scholar]
- Mateos G. G., Jiménez-Moreno E., Guzman P., Saldaña B., Lázaro R.. 2014. Role of fiber in broiler diets - Friend or foe. 5th international broiler nutritionists conference, New Zeland. [Google Scholar]
- Metzler B. U., Monsenthin R.. 2008. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian Australas. J. Anim. Sci. 21:603–615. [Google Scholar]
- Metzler-Zebeli B. U., Hooda S., Monsenthin R., Gänzle M. G., Zijlstra R. T.. 2010. Bacterial fermentation affects net mineral flux in the large intestine of pigs fed diets with viscous and fermentable nonstarch polysaccharides. J. Anim. Sci. 88:3351–3362. [DOI] [PubMed] [Google Scholar]
- Mutucumarana R. K., Ravindran V., Ravindran G., Cowieson A. J.. 2014. Measurement of true ileal digestibility and total tract retention of phosphorus in corn and canola meal for broiler chickens. Poult. Sci. 93:412–419. [DOI] [PubMed] [Google Scholar]
- NRC 1994. Nutrient Requirements of Poultry. 9th ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Proszkowiec-Weglarz M., Angel R.. 2013. Calcium and phosphorus metabolism in broilers: Effect of homeostatic mechanism on calcium and phosphorus digestibility. J. Appl. Poult. Res. 22:609–627. [Google Scholar]
- Qian H., Kornegay E. T., Denbow D. M.. 1997. Utilization of phytate phosphorus and calcium as influenced by microbial phytase, cholecalciferol, and the calcium: Total phosphorus ratio in broiler diets. Poult. Sci. 76:37–46 [DOI] [PubMed] [Google Scholar]
- Rousseau X., Valable A. S., Létourneau-Montmiy M. P., Même N., Godet E., Magnin M., Nys Y., Duclos M. J., Narcy A.. 2016. Adaptative response of broilers to dietary phosphorus and calcium restrictions. Poult. Sci. 95:2849–2860. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Svihus B., Denstadli V., Moen B., Iji P. A., Choct M.. 2012. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poult. Sci. 91:693–700. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Adiya X., Mydland L. T., Svihus B.. 2017. Effect of intermittent feeding and oat hulls to improve phytase efficacy and digestive function in broiler chickens. Br. Poult. Sci. 58:442–451. [DOI] [PubMed] [Google Scholar]
- Sebastian S., Touchburn S. P., Chavez E. R., Lague P. C.. 1996. The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper and zinc in broiler chickens fed corn-soybean diets. Poult. Sci. 75:729–736. [DOI] [PubMed] [Google Scholar]
- Selle P. H., Ravindran V.. 2007. Microbial phytase in poultry nutrition. Anim. Feed. Sci. Tech. 135:1–41. [Google Scholar]
- Shang Y., Rogiewicz A., Patterson R., Slominski B. A., Kim W. K.. 2015. The effect of phytase and fructooligosaccharide supplementation on growth performance, bone quality, and phosphorus utilization in broiler chickens. Poult. Sci. 94:955–964. [DOI] [PubMed] [Google Scholar]
- Short F. J., Gorton P., Wiseman J., Boorman K. N.. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Tech. 59:215–221. [Google Scholar]
- Svihus B. 2011. The gizzard: Function, influence of diet structure and effects on nutrient availability. Worlds Poult. Sci. J. 67:207–223. [Google Scholar]
- Svihus B. 2014. Function of the digestive system. J. Appl. Poult. Res. 23:306–314. [Google Scholar]
- Tamim N. M., Angel R.. 2003. Phytate phosphorus hydrolysis as influenced by dietary calcium and micro-mineral source in broiler diets. J. Agric. Food Chem. 51:4687–4693. [DOI] [PubMed] [Google Scholar]
- Taira K., Nagai T., Obi T., Takase K.. 2014. Effect of litter moisture on the development of footpad dermatitis in broiler chickens. J. Vet. Med. Sci. 76:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. H., Skadhauge E.. 1989. Water and electrolyte transport by the avian ceca. J. Exp. Zool. B Mol. Dev. Evol. 252:95–102. [DOI] [PubMed] [Google Scholar]
- Tilocca B., Witzig M., Rodehutscord M., Seifer J.. 2016. Variation of phosphorous accessibility causing changes in microbiome functions in the gastrointestinal tract of chickens. PLoS ONE 11:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad T. P., Wolever T. M., Thompson L. U.. 1996. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am. J. Clin. Nutr. 63:574–578. [DOI] [PubMed] [Google Scholar]
- van der Hoeven-Hangoor E., Rademaker C. J., Paton N. D., Verstegen M. W. A., Hendriks W. H.. 2014. Evaluation of free water and water activity measurements as functional alternatives to total moisture content in broiler excreta and litter samples. Poult. Sci. 93:1782–1792. [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., Lewis A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. [DOI] [PubMed] [Google Scholar]
- Viveros A., Brenes A., Arija I., Centeno C.. 2002. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activites in broiler chicks fed different levels of phosphorus. Poult. Sci. 81:1172–1183. [DOI] [PubMed] [Google Scholar]
- Walugembe M., Rothschild M. F., Persia M. E.. 2014. Effects of high fiber ingredients on the performance, metabolizable energy and fiber digestibility of broiler and layer chicks. Anim. Feed. Sci. Tech. 188:46–52. [Google Scholar]
- Wilfart A., Espagnol S., Dauguet S., Tailleur A., Gac A., Garcia-Launay F.. 2016. ECOALIM : A dataset of environmental impacts of feed ingredients used in French animal production. PLoS ONE 11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]