Abstract
Objective
To examine sun exposure and multiple sclerosis (MS) over the life course (ages 5–15 and 16–20 years, every 10 years thereafter).
Methods
Cases with MS (n = 151) and age-matched controls (n = 235) from the Nurses' Health Study cohorts completed summer, winter, and lifetime sun exposure history questionnaires. Cumulative ambient ultraviolet (UV)-B (based on latitude, altitude, cloud cover) exposure before MS onset was expressed as tertiles. Seasonal sun exposure was defined as low vs high hours per week (summer [≤9 vs >10 h/wk]; winter [≤3 vs >4 h/wk]). Relative risks (RRs) and 95% confidence intervals (CIs) were estimated via conditional logistic regression with adjustment for body mass index, ancestry, smoking, and vitamin D supplementation.
Results
Most participants were white (98%); the mean age at MS onset was 39.5 years. Living in high (vs low) UV-B areas before MS onset was associated with a 45% lower MS risk (adjusted RR 0.55, 95% CI 0.42–0.73). Similar reduced risks (51%–52%) for medium or high exposure were observed at ages 5 to 15 years and at 5 to 15 years before MS onset (adjusted p < 0.05). At age 5 to 15 years, living in a high (vs low) UV-B area and having high (vs low) summer sun exposure were associated with a lower MS risk (RR 0.45, 95% CI 0.21–0.96).
Conclusion
Living in high ambient UV-B areas during childhood and the years leading up to MS onset was associated with a lower MS risk. High summer sun exposure in high ambient UV-B areas was also associated with a reduced risk.
Multiple sclerosis (MS) is a chronic, life-changing disease in which the CNS becomes the target of an individual's own immune defense system. The causes of MS are unknown. Previous studies have reported associations between lower latitudes or low past sunlight exposure and increased MS risk.1–5 Indirect evidence stems from ecologic studies of ultraviolet (UV)-B radiation, but these typically examined prevalent rather than incident cases.6 Sun exposure, specifically skin exposed to UV-B radiation (290–315 nm), is a major source of vitamin D in humans. There is evidence supporting low vitamin D as a risk factor for increased MS incidence.7–9 However, sunlight exposure itself may convey independent effects,10 possibly mediated through other immunomodulatory pathways. A major challenge exists in measuring past sunlight exposure, limiting the ability to examine its potential role in MS. For example, few have been able to assess the seasonal timing (summer, winter) or the nature of such exposure (e.g., general outdoor exposure or direct sun itself). These may be important to better understand the potential role of sunlight in the pathogenesis of MS. Furthermore, while many studies report early childhood and adolescence as periods of susceptibility, most have not assessed environmental exposures much beyond these periods. Yet, timing is key when planning feasible, effective intervention studies to mitigate MS risk.1,11 In addition, while vitamin D deficiency is common and measurable in individuals with MS,12 whether it is related to reduced sun exposure once a person develops MS is not well understood.
We examined the association between prior sunlight exposure and MS risk. We used both objective and self-reported measures and explored the timing of exposure by season (summer, winter) and age. Finally, we described sun-related practices and how they might change over the life course in MS.
Methods
Study participants
We conducted a nested case-control study within the Nurses' Health Study (NHS; n = 121,700) and NHS II (n = 116,671) cohorts. The NHS (established in 1976) enrolled nurses 30 to 55 years of age from 11 US states, and the NHS II (established 1989) enrolled nurses 25 to 42 years of age from 14 states. All participants complete biennial questionnaires regarding lifestyle factors and medical conditions, including MS, which was confirmed by either the treating or study neurologist (via medical record review). Within the cohorts, there is a set of birth year– and cohort- (NHS or NHS II) matched cases with MS and controls (183 incident cases and 438 controls).
Self-reported outdoor exposure before MS onset and over the life course–related information
In 2012 to 2013, cases and controls were invited to participate in an additional study on “the effect of environmental and personal exposures on the risk of developing [either] MS [for cases with MS] or chronic diseases [for healthy controls]” using a prepiloted questionnaire13 adapted for MS. All cases were aware of their MS diagnosis when completing the questionnaires. Up to 2 reminders were sent. Sun- and outdoor exposure–relevant questions were asked over the life course (ages 5–15, 16–20, 21–30, 31–40, 41–50, and ≥51 years), including time spent outdoors in summer and winter, time spent in direct sunlight (in “minimal clothing”), sunburn episodes, use of sunscreen, and place of main residence (town/city and state). Natural skin and hair color (at age 20 years) and tendency to tan or burn as a child or adolescent were also collected (table e-1, links.lww.com/WNL/A313).
Ambient UV-B before MS onset: From early life through adulthood
For each age or time period, ambient UV-B was quantified with a composite measure of UV-B based on latitude, altitude, and cloud cover, estimated according to each individual's reported state of residence during that period (collected via the questionnaires, as above), and expressed in Robertson-Berger units ×10−4.14 Sometimes called UV flux but referred to here as UV-B or ambient UV-B, this has been used to assess the contribution of ambient UV-B to the onset of other chronic conditions.14
Cumulative average ambient UV-B was estimated for each individual during each age or time period (including at birth) leading up to MS onset or the equivalent time for controls. The last age category examined was 31 to 40 years because beyond 40 years of age, there were too few individuals still before MS onset to assess. In addition, two 5- and 10-year windows, covering up to 15 years before onset, were examined.
Continuous variables, i.e., UV-B estimates and time in direct sunlight, were categorized as low, medium, or high on the basis of tertiles derived from the relevant age- or time period–specific control group. For the categorical summer and winter exposures, high vs low represented ≥10 vs <10 h/wk and ≥4 vs <4 h/wk, respectively, derived from the original 8-response question (table e-1, links.lww.com/WNL/A313), with groupings informed by the controls' data distribution.
Ambient UV-B and self-reported outdoor exposure before MS onset combined
Because outdoor exposure may differ on the basis of an individual's ambient UV-B and together these might be relevant in altering MS risk, the 2 were combined, before MS onset only (ages 5–15 through 31–40 years), expressed as low, intermediate, or high, with low representing the lowest categories (tertiles) for both outdoor exposure and ambient UV-B, high representing the reverse, and intermediate including all remaining combinations. A similar approach was used when combining ambient UV-B (tertiles) with the summer or winter high/low categories; all possible combinations are given in table 1.
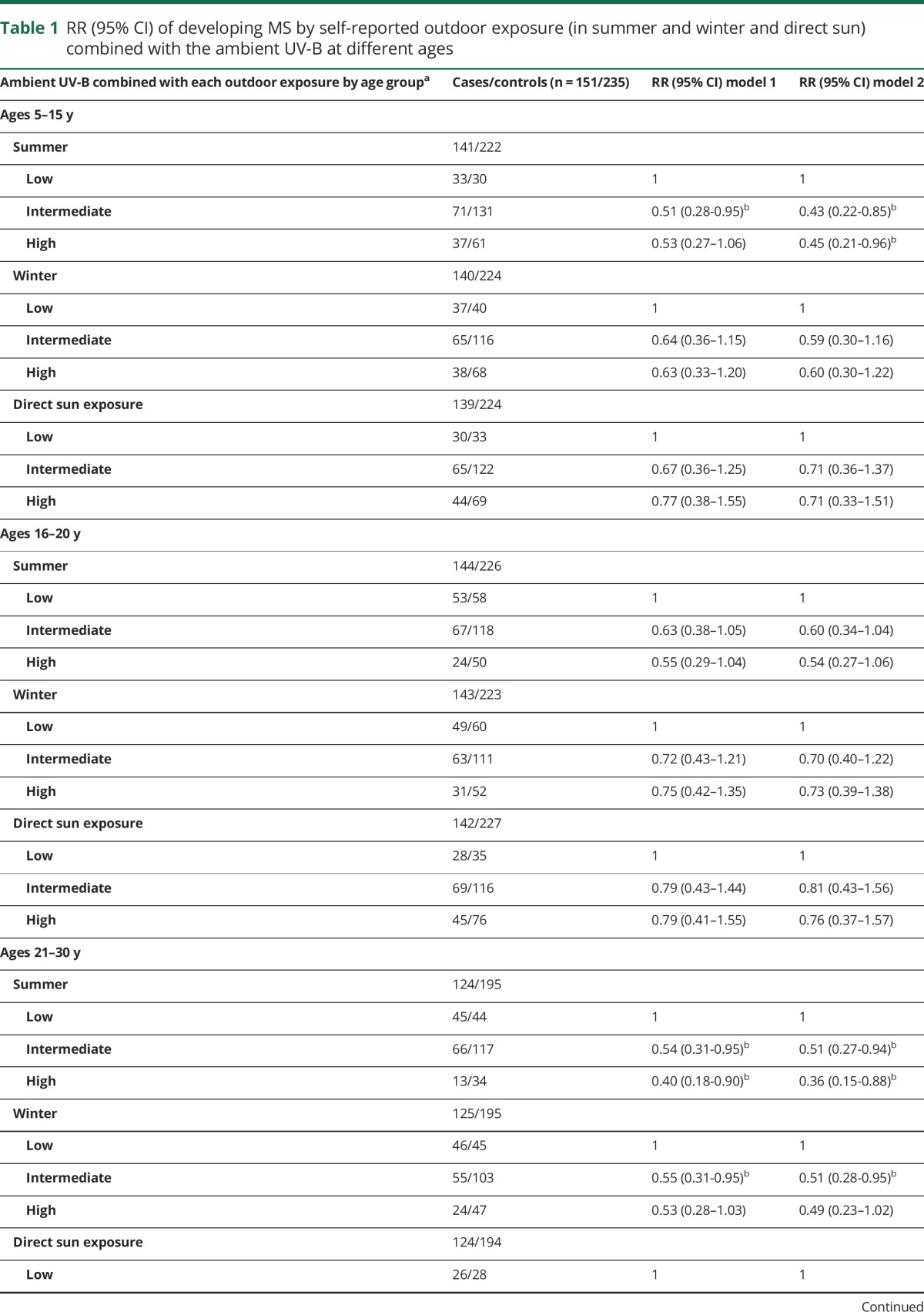
Table 1.
RR (95% CI) of developing MS by self-reported outdoor exposure (in summer and winter and direct sun) combined with the ambient UV-B at different ages

Cohort characteristics and covariates
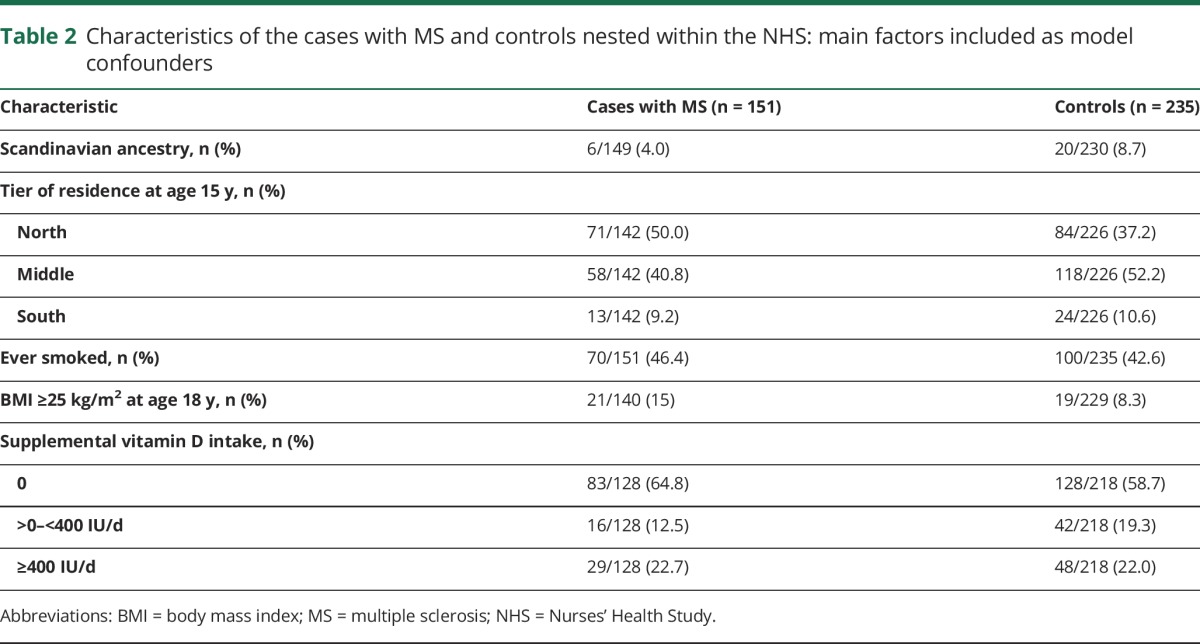
Additional cohort characteristics and potential confounders were collected via the biennial questionnaires that all cohort participants complete and included ancestry (NHS 1992, NHS II 1989; grouped as Scandinavian, southern European, all other whites, other)15; state of residence at 15 years of age (NHS 1992, NHS II 1993; categorized into latitude north, middle, south, as previously described16); body mass index (BMI) at 18 years of age calculated from reported height on baseline questionnaires and weight (NHS 1980, NHS II 1989; grouped as <18.5, 18.5 to <25, ≥25 kg/m2)17; smoking status (ever vs never)18; and supplemental intake of vitamin D on baseline dietary questionnaires (NHS 1986, NHS II 1991; 0, >0–<400, ≥400 IU).7
Statistical analyses
Data were examined primarily before MS onset (up to ages 31–40 years, after which most had developed MS) and secondarily over the full life course (from ages 5–15 through ≥51 years), irrespective of the MS onset date. For the pre-MS onset years, a case's data were included only if MS onset had yet to be reached; e.g., the age group of 16 to 20 years included only those yet to develop MS, along with their matched controls.
For both approaches, conditional logistic regression was used to examine the association between time outdoors, ambient UV-B, and MS. Cases with at least 1 birth year– and cohort-matched control were included (model 1). Models were additionally adjusted for ancestry, BMI at age 18 years, smoking, and vitamin D supplement use (model 2, see covariates above). A third model (model 3) was also adjusted for place of residence at age 15 years, although not for ambient UV-B to avoid overadjustment, because (current) place of residence was used to estimate this exposure. Findings were reported as unadjusted and adjusted relative risks (RRs) with 95% confidence intervals (CIs). The individual age-specific risk estimates for ambient UV-B before MS onset were also pooled with the use of random-effects models, with the Q statistic to assess heterogeneity. Analyses were conducted with SAS version 9.3 (SAS Institute Inc, Cary, NC).
Standard protocol approvals, registrations, and patient consents
The relevant Institutional ethics boards approved the study; completion of the questionnaire was considered consent to participate.
Results
Study participants
From 621 women contacted, 90% (556) responded (167 cases with MS and 389 controls), and 16 cases had no matched control, leaving 151 cases with MS and 235 matched controls (67 had exactly 1 control, 84 had 2 controls). Characteristics are shown in table 2 and table e-2, links.lww.com/WNL/A313. Most self-identified as white (n = 377, 98%) with fair to medium skin (361, 94%). The mean MS onset age was 39.5 years (SD 8.93 years); 90% (136 of 151) had developed MS by age 50 years.
Table 2.
Characteristics of the cases with MS and controls nested within the NHS: main factors included as model confounders
Ambient UV-B before MS onset: From early life through adulthood
Higher ambient UV-B levels were associated with a 36% to 45% reduced risk of MS across all age groups combined (table 3). For specific age group exposure, increased ambient UV-B levels at ages 5 to 15 and 31 to 40 years were associated with statistically significant 51% and 65% reduced risks of MS, respectively. Although significance was not reached for the remaining age groups after full model adjustment (model 2), a similar direction of effect was observed (table 3).
Table 3.
RR (95% CI) of developing MS by ambient UV-B exposure (low, medium, high) at different ages

Cumulative ambient UV-B in the years leading up to MS onset was inversely associated with MS risk, but findings were not significant for all time periods (table e-3, links.lww.com/WNL/A313); higher ambient UV-B in the 5 to10 years before MS onset was associated with the lowest risk of MS (56% reduced risk; fully adjusted RR 0.44, 95% CI 0.22–0.85, medium vs lowest tertiles, model 2, table e-3). Reduced risks were observed in the remaining windows except the 5 years directly before MS onset, which failed to reach significance (models 1 and 2, table e-3).
Self-reported outdoor exposure before MS onset and over the life course for cases and controls
Before MS onset only: Ages 5 to 15 years to 31 to 40 years
Reported time spent outdoors in the summer and winter or in direct sun before MS onset showed no significant associations with MS risk (all p > 0.05, data not shown), aside from 1 age group (age 5–15 years) and for direct sun exposure only, but this failed to reach significance after additional adjustments (p > 0.05, model 3, table e-4, links.lww.com/WNL/A313).
However, when outdoor sun exposure was considered in the context of ambient UV-B, a different pattern emerged; when both were high, relative to low, the risk of MS decreased (table 1). For those 5 to 15 years of age, high summer outdoor exposure in areas of high ambient UV-B was associated with a statistically significant 55% lower risk of MS (table 1). However, although winter sun exposure in high ambient UV-B areas was also inversely associated with MS risk, the associations did not reach statistical significance (table 1). Similar relationships were observed for winter and sun (in minimal clothing) and across remaining age groups, although after additional model adjustments, not all reached significance (model 2, table 1).
Over the life course: Irrespective of the date of MS onset
While cases with MS and controls did not differ significantly in the earlier years for sun and outdoor exposure (from ages 16–20 through 31–40 years, all p > 0.05 after model adjustments, data not shown), reduced exposure was observed after the fourth decade of life for cases relative to controls (table e-5, links.lww.com/WNL/A313). By ≥51 years of age, cases with MS were 49% to 56% less likely than controls to report medium or high sun exposure (relative to low; adjusted RR 0.51, 95% CI 0.28–0.94 and adjusted RR 0.44, 95% CI 0.24–0.83, respectively, table e-5). Other sun-related phenotypic characteristics (hair, skin color, tendency to tan or burn) were similar for cases and controls (table e-2), as was sunscreen use over the life course (figure e-1, links.lww.com/WNL/A312). Episodes of severe sunburn were similar for cases and controls in childhood, decreasing for both over the life course, although perhaps more so for cases (figure e-2).
Discussion
Our findings support the hypothesis that low ambient UV-B exposure is associated with an increased MS risk.2 Living in areas of higher ambient UV-B before MS onset was associated with a 45% reduced risk of MS. Specifically during childhood and adolescence, the associated reduced risk was >50%. However, this window extended beyond these early years and into adulthood, possibly up to the fourth decade of life, in addition to the years leading up to MS onset. For those 31 to 40 years of age yet to develop MS and for the 5 to 10 and 5 to 15 years before MS onset (i.e., adulthood for many study participants), even a moderate ambient UV-B was associated with a 55% to 65% reduced risk of MS compared to those living in areas with low ambient UV-B. There was also a suggestion of reduced sun exposure after MS onset, which included reduced time outdoors in summer and winter and reduced direct sun exposure, which could have implications for the health of those living with MS.
Our study approach and findings are novel and have important implications. First, our measure of ambient UV-B penetrance (UV-B flux) enabled quantification of potential UV-B exposure over several years. This facilitated exploration across age groups and time periods and may be of greater biological relevance than 1 measure at 1 point in time such as at birth or when latitude alone is used.14 Consequently, we were able to identify windows of susceptibility beyond childhood and into adulthood, as well as the years leading up to MS onset. UV-B flux also takes into consideration actual UV-B penetrance to earth, potentially increasing the accuracy of UV-B exposure. Its reliance on place of residence may also minimize recall errors or bias, and others have shown ambient UV-B to be a good proxy for personal UV exposure.19 Our findings may facilitate the design of intervention studies aimed at mitigating MS risk. Second, by examining outdoor exposure over the life course, we found possible sun avoidance practices and reduced outdoor exposure in both summer and winter for individuals once they developed MS; this may have a negative effect on MS progression and disease activity,20,21 as well as having broader health implications.22 It remains possible that outdoor self-reported exposure reflects a marker of exercise, although we adjusted for BMI at 18 years of age. Recall bias may influence self-reported time spent in the sun, especially at younger ages, but it is unlikely to affect report of place of residence during specific ages. Thus, recall bias seems an unlikely explanation for the ambient UV-B findings. A study strength was the ability to demonstrate consistency across the different measures of UV-related and outdoor exposure, although chance findings are possible with multiple comparisons and our sample size was modest. However, cases and controls were selected (nested) from the same source cohorts (the NHS), minimizing selection bias. Finally, most blood samples were procured after MS diagnosis; therefore, we were unable to adjust for serum vitamin D levels, which may be of value in future studies.
Identifying the potential windows of exposure susceptibility for MS risk is important for the design of prospective intervention studies aimed at mitigating MS risk. Our findings both confirm and extend prior observations. Lower sun or outdoor exposure in childhood and adolescence has been reported to increase MS risk by others, including investigators in Tasmania, Australia (6–15 years of age2), Norway (16–18 years3 and 16–20 years of age in Troms and Finnmark4), and Italy (Sardinia, Ferrara province, and San Remo, birth–5 years of age3) and a monozygotic twin cohort from the United States5 (childhood years were not defined). We show that this at-risk period may extend well into adulthood and include the years leading up to, but still distal to, MS onset (i.e., did not include the 5 years immediately prior). These observations also support the hypothesis that other, presumably environmental, factors such as exposure to infection work in synergy with UV-B during these periods to trigger MS.1,11,23
The relationship between time spent outdoors during childhood and MS risk became apparent only when considered in the context of an individual's ambient UV-B environment; a 55% lower risk for MS was associated with more time spent outdoors in summer when residing in areas of high ambient UV-B. Previous studies reporting low sun exposure in early childhood as increasing MS risk have involved cohorts from small catchment areas living at similar latitudes (e.g., Tasmania or specific regions in Norway or Italy2–4). Conversely, our participants included those living across the United States (spanning 50 states during the study follow-up), hence at a range of latitudes and ambient UV-B levels. These findings are important for study design, whether observational or interventional, but especially if multisite or across multiple latitudes.
The strongest association between higher outdoor exposure and lower MS risk was during the summer rather than winter months, and more time outside in summer appeared more important than time spent in the sun (in minimal clothing). Together, these findings support previous observations that the effect could be mediated by vitamin D7,8 synthesis (via exposed skin) and via sunlight itself10 (if vitamin D synthesis was the overriding factor, sun exposure in minimal clothing might be expected to have a larger effect). Others also found an association with higher summer outdoor exposure and lower MS risk, including investigators in northern Norway, Tasmania, and regions in Italy.2–4 However, researchers in the last 2 areas also found significant associations in winter, with more time outdoors associated with lower MS risk, which might reflect the extended periods over which the temperature and UV-B are both sufficient to stimulate vitamin D production on exposed skin.3,24
Findings were suggestive of sun avoidance practices, particularly in later life, in established MS (regardless of ambient UV-B). Less time spent outdoors in summer and winter and in direct sun was observed for the cases with MS relative to controls. We were unable to find another study examining the outdoor exposures of patients with MS over the life course. However, these observations would be consistent with others reporting lower serum vitamin D levels12,25 and lower skin cancer risks in individuals after MS onset.26 Less time outdoors is likely a consequence of MS, related to heat intolerance and reduced mobility, ability to work, and hence opportunity for outdoor exposure(s), and has implications for the health of individuals with MS.12 Reduced exposure of skin to sunlight is associated with low serum vitamin D levels and potential negative health consequences, both specific to MS (e.g., more rapid disability progression, higher disease activity, and earlier conversion from relapsing remitting to secondary progressive MS20,21,27) and possibly nonspecific28 (e.g., mortality risk29). In addition, human melatonin production relies on exposure to outdoor light (in summer or winter). Melatonin also affects circadian rhythm and immune regulation and may contribute to disease activity in MS.30,31 Our observations also have implications for study design and interpretation; enrollment of cases with prevalent MS and controls to assess differences in serum biomarkers (such as vitamin D or melatonin) or skin cancer risk may lead to erroneous conclusions regarding these exposures and MS risk.25,26
Our findings provide evidence that the relationship between higher ambient UV-B, higher summer outdoor exposure, and lower MS risk can occur across age groups, including the years leading up to MS onset. Reduced sun exposure was evident in later life for patients with MS, which may have health consequences.
Acknowledgment
The authors thank Leslie Unger and Allison Gordon for data cleaning and entry and Thomas Duggan for facilitating data coding.
Glossary
- BMI
body mass index
- CI
confidence interval
- MS
multiple sclerosis
- NHS
Nurses' Health Study
- RR
relative risk
- UV
ultraviolet
Author contributions
Study concept (H.T., K.L.M., A.A.) and study design (all authors); acquisition of data (H.T., K.L.M., A.A.); data analyses (F.Z.) and data interpretation (all authors); critical revision of the manuscript for important intellectual content (all authors).
Study funding
This study was funded by the Martha Piper Research Fund, University of British Columbia, Canada, and supported by the US NIH (grants UM1 CA186107, UM1 CA176726). Helen Tremlett is funded as a Canada Research Chair.
Disclosure
H. Tremlett is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. She currently receives research support from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, and the Multiple Sclerosis Scientific Research Foundation. In addition, in the last 5 years, she has received research support from the Multiple Sclerosis Society of Canada (Don Paty Career Development Award), the Michael Smith Foundation for Health Research (Scholar Award), and the UK MS Trust; speaker honoraria and/or travel expenses to attend conferences from the Consortium of MS Centres (2013), the National MS Society (2012, 2014, 2016), ECTRIMS (2012, 2013, 2014, 2015, 2016), the Chesapeake Health Education Program, US Veterans Affairs (2012), Novartis Canada (2012), Biogen Idec (2014), and American Academy of Neurology (2013, 2014, 2015, 2016). All speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by her research group. F. Zhu reports no disclosures relevant to the manuscript. A. Ascherio receives grant funding from the NIH, the Department of Defense, and the National Multiple Sclerosis Society; has participated on a medical advisory board for Bayer Healthcare; and has received honoraria for speaking at scientific symposia from Almirall, Roche, Sanofi Aventis, and Serono. K. Munger reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012;8:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Mei IA, Ponsonby AL, Dwyer T, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003;327:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornevik K, Riise T, Casetta I, et al. Sun exposure and multiple sclerosis risk in Norway and Italy: the EnvIMS study. Mult Scler 2014;20:1042–1049. [DOI] [PubMed] [Google Scholar]
- 4.Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007;254:471–477. [DOI] [PubMed] [Google Scholar]
- 5.Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007;69:381–388. [DOI] [PubMed] [Google Scholar]
- 6.Orton SM, Wald L, Confavreux C, et al. Association of UV radiation with multiple sclerosis prevalence and sex ratio in France. Neurology 2011;76:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60–65. [DOI] [PubMed] [Google Scholar]
- 8.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 9.Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology 2012;79:2140–2145. [DOI] [PubMed] [Google Scholar]
- 10.Lucas RM, Ponsonby AL, Dear K, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011;76:540–548. [DOI] [PubMed] [Google Scholar]
- 11.Pakpoor J, Ramagopalan S. Evidence for an association between vitamin D and multiple sclerosis. Curr Top Behav Neurosci 2015;26:105–115. [DOI] [PubMed] [Google Scholar]
- 12.van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol 2007;254:581–590. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses' Health Study. Int J Epidemiol 2006;35:1514–1521. [DOI] [PubMed] [Google Scholar]
- 14.Arkema EV, Hart JE, Bertrand KA, et al. Exposure to ultraviolet-B and risk of developing rheumatoid arthritis among women in the Nurses' Health Study. Ann Rheum Dis 2013;72:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernan MA, Zhang SM, Lipworth L, Olek MJ, Ascherio A. Multiple sclerosis and age at infection of common viruses. Epidemiology 2001;12:301–306. [DOI] [PubMed] [Google Scholar]
- 16.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–1718. [DOI] [PubMed] [Google Scholar]
- 17.US NIH. Body mass index categories. Available at: nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm. Accessed November 15, 2017.
- 18.Simon KC, van der Mei IA, Munger KL, et al. Combined effects of smoking, anti-EBNA antibodies, and HLA-DRB1*1501 on multiple sclerosis risk. Neurology 2010;74:1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahoon EK, Wheeler DC, Kimlin MG, et al. Individual, environmental, and meteorological predictors of daily personal ultraviolet radiation exposure measurements in a United States cohort study. PLoS One 2013;8:e54983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 2014;71:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald KC, Munger KL, Kochert K, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol 2015;72:1458–1465. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 23.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010;9:599–612. [DOI] [PubMed] [Google Scholar]
- 24.Engelsen O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010;2:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandit L, Ramagopalan SV, Malli C, D'Cunha A, Kunder R, Shetty R. Association of vitamin D and multiple sclerosis in India. Mult Scler 2013;19:1592–1596. [DOI] [PubMed] [Google Scholar]
- 26.Goldacre MJ, Seagroatt V, Yeates D, Acheson ED. Skin cancer in people with multiple sclerosis: a record linkage study. J Epidemiol Community Health 2004;58:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muris AH, Rolf L, Broen K, Hupperts R, Damoiseaux J, Smolders J. A low vitamin D status at diagnosis is associated with an early conversion to secondary progressive multiple sclerosis. J Steroid Biochem Mol Biol 2016;164:254–257. [DOI] [PubMed] [Google Scholar]
- 28.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015;162:109–122. [DOI] [PubMed] [Google Scholar]
- 30.Farez MF, Mascanfroni ID, Mendez-Huergo SP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015;162:1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson G, Rodriguez M. Multiple sclerosis: the role of melatonin and N-acetylserotonin. Mult Scler Relat Disord 2015;4:112–123. [DOI] [PubMed] [Google Scholar]


