Abstract
Objective
To investigate the association between asymptomatic intracranial atherosclerosis and cognitive impairment in the Atherosclerosis Risk in Communities (ARIC) cohort.
Methods
ARIC participants underwent high-resolution 3T magnetic resonance angiography and a neuropsychology battery and neurologic examination adjudicated by an expert panel to detect mild cognitive impairment (MCI) and dementia. We adjusted for demographic and vascular risk factors in weighted logistic regression analysis, accounting for stratified sampling design and attrition, to determine the association of intracranial atherosclerotic stenosis (ICAS) with cognitive impairment.
Results
In 1,701 participants (mean age 76 ± 5.3, 41% men, 71% whites, 29% blacks) with adequate imaging quality and no history of stroke, MCI was identified in 578 (34%) and dementia in 79 (4.6%). In white participants, after adjustment for demographic and vascular risk factors, ICAS ≥50% (vs no ICAS) was strongly associated with dementia (odds ratio [OR] 4.1, 95% confidence interval [CI] 1.7–10.0) and with any cognitive impairment (OR 1.7, 95% CI 1.1–2.8). In contrast, no association was found between ICAS ≥50% and MCI or dementia in blacks, although the sample size was limited and estimates were imprecise.
Conclusion
Our results suggest that asymptomatic ICAS is independently associated with cognitive impairment and dementia in whites.
The relation between cognitive impairment and intracranial atherosclerosis is increasingly recognized.1–4 There is growing evidence that intracranial atherosclerosis is associated with clinical and pathologic manifestations of Alzheimer disease (AD).5–12 Experimental13–18 and clinical studies13,19–21 support the hypothesis that cerebral ischemia contributes to AD pathology. Intracranial atherosclerotic stenosis (ICAS) may also be associated with cognitive impairment and AD by causing cerebral hypoperfusion.5–7,22 However, these studies were limited to small clinical case-control series5,6 or autopsy studies without clinical data.7
To study the association of ICAS with cognitive impairment, we performed a cross-sectional study using cognitive screening and high-resolution magnetic resonance angiography (MRA) in participants of the Atherosclerosis Risk in Communities (ARIC) study cohort.
Methods
Study population
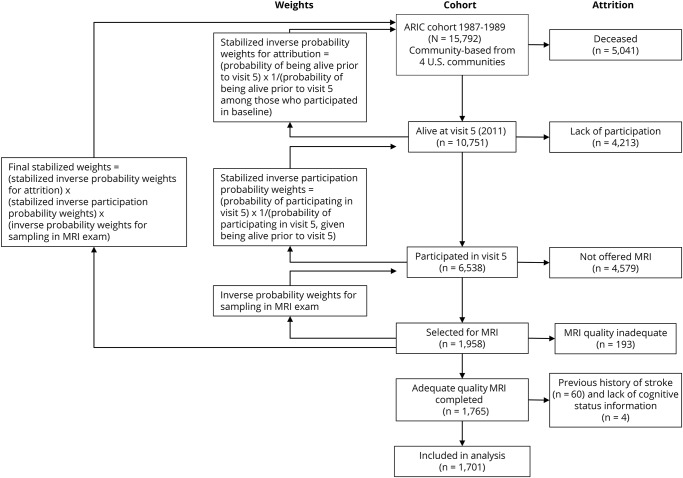
The ARIC study cohort recruited 15,792 participants 45 to 64 years of age from 1987 to 1989 from 4 randomly selected US communities: Forsyth County, North Carolina; Jackson, MS; suburban Minneapolis, MN; and Washington County, Maryland. Follow-up examinations occurred in 1990 to 1992, 1993 to 1995, and 1996 to 1998. A fifth examination was conducted from 2011 to 2013. Among the 10,036 participants alive at the time of the fifth visit, 6,538 (65%) were evaluated. Among those who had no contraindications, MRA was offered to those who had received an ARIC brain MRI scan in 2004 to 2006 (offered to 573, completed in 433), those with low current cognitive test scores and large declines on the longitudinally administered tests (offered to 1,047, completed in 664),23 and an age-stratified random sample of the remaining individuals (offered to 1,202, completed in 861). The goal was to acquire MRA scans in ≈2,000 participants.
We excluded all participants who had history of clinical strokes (n = 60) and those with a lack of cognitive status information (n = 4).
Standard protocol approvals, registrations, and patient consents
Institutional Review boards approved the study at each site, and participants provided written informed consent.
MRA protocol and image analysis
A total of 1,958 participants completed a standard MRA protocol. Details of the MRI protocol, image analysis, quality control, and reliability have been reported.24 All MRI scans were performed on 3.0T Siemens scanners. High-resolution vascular sequences included a 3-dimensional time-of-flight MRA acquired in a transverse plane through the circle of Willis, centered to include the distal vertebral artery segments inferiorly and the middle cerebral artery branches superiorly. Repeat MRI scans were performed in 102 participants with a target interval of 13 weeks to establish interscan reliability. MRIs were analyzed by certified readers at the MRI reading center without knowledge of the participant characteristics. Studies with poor image quality or poor protocol adherence (n = 193) were excluded from this analysis. MRA images for the remaining participants had adequate or excellent quality for ICAS identification in the vessels of interest and were included in the current analysis.
Vessel segments analyzed included the supraclinoid and cavernous segments of the internal carotid artery, middle cerebral artery (M1–M4 segments), anterior cerebral artery (A1–A3 segments), intracranial segments of the vertebral artery, basilar artery, and posterior cerebral artery (P1–P3 segments). For each territory, the ordinal degree of narrowing was recorded for the most stenotic plaque measured on time-of-flight MRA images with criteria established in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial.25 The κ statistics for agreement were 0.68 (intrareader), 0.63 (interreader), and 0.60 (interscan) for ordinal ICAS24 and were similar between races. Because our objective was to study the association of ICAS with cognitive impairment, vessel segments noted to have plaque but without any measurable stenosis were not included. We identified the most stenotic segment of each vessel territory analyzed and used the severity of the most stenotic lesion in each participant to determine the association of ICAS with cognitive impairment. We made an effort to accurately classify stenosis in clinically significant and commonly used ordinal categories (no detectable stenosis, <50%, 51%–70%, 71%–99%, and occlusion).26 For this analysis, we grouped ≥50% stenosis into 1 category to achieve meaningful statistical power.
Demographic and clinical risk factors
Demographic covariates included age (continuous), sex, and race (white or black). Because most of the black population was from a single study center (Jackson, MS), race was excluded when the model was adjusted for study center. Education status was classified as <12, 12, and >12 grades. Clinical risk factors were assessed at the time of visit 5 examination and included history of cigarette smoking (current, past, or never), use of antihypertensive or cholesterol-lowering medications, body mass index (kilograms per meter squared), prevalent myocardial infarction, systolic blood pressure, plasma low-density lipoprotein cholesterol, plasma high-density lipoprotein cholesterol, and plasma triglycerides. We defined hypertension as blood pressure ≥140/90 mm Hg or use of antihypertensive medications and diabetes mellitus as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, use of hypoglycemic medications, or self-reported physician diagnosis of diabetes mellitus. Distribution of ICAS across demographic and clinical variables has been previously reported.26
Depressive symptoms were measured at visit 5 with the 11-item Center for Epidemiologic Studies Depression Scale (CES-D). The total score can range from 0 to 22. Only 3 participants had a total score ≥16 (cutoff for clinical depression). We used a median of 2 as the cutoff, i.e., ≤2 vs ≥3. A sports index was calculated on the basis of the intensity (light, moderate, or heavy) and duration of leisure time activity as a measure of physical activity.27 The data from these 2 codons (130 and 176) were combined to generate APOE genotypes.28
For volumetric analysis of white matter hyperintensity (WMH), leukoaraiosis voxels were automatically separated out from the CSF and normal brain on axial fluid-attenuated inversion recovery images.29 Infarcts were manually identified and excluded to calculate the WMH volume. Because all patients with clinical strokes were excluded from the analysis, any infarct noted on MRI was considered a silent infarct.
Cognitive impairment
A detailed description of the cognitive assessments performed at ARIC visit 5 and the criteria used to define mild cognitive impairment (MCI) and dementia have been published.23 Briefly, all study participants completed extensive cognitive testing, and an additional subset, including those with evidence of some cognitive impairment on the basis of Mini-Mental State Examination score and decline in 5 cognitive domains, underwent a neurologic examination and completed additional questionnaires, including the Clinical Dementia Rating interview, the Functional Activities Questionnaire (completed by an informant), the Neuropsychiatric Inventory, and the Hachinski ischemic score (completed by study staff). Using this information, a committee of neurologists and neuropsychologists reviewed the results of cognitive testing and clinical data and provided a syndromic and etiologic diagnosis based on established criteria.30,31 Syndromic categories included no cognitive impairment, MCI, and dementia. Etiologic categories included pure AD, all AD (AD with or without other etiologies), pure cerebrovascular disease–related dementia, and all cerebrovascular dementia (cerebrovascular dementia with or without other etiologies). In addition, standardized domain scores were calculated for the memory, language, and executive function domains. A global z score was created by averaging these 3 domain-specific z scores. These scores were then scaled so that 1 unit equaled 1 SD of that score.32
Statistical analysis
Weights based on the inverse probability of being sampled for the MRI examination from visit 5 were applied to adjust for the stratified sampling design (figure). Because the presence of both ICAS and cognitive impairment is likely to cause attrition by reducing survival and participation among survivors, we calculated stabilized inverse survival probability weights for attrition based on demographic and vascular risk factors at visit 1 for survival up to visit 5, and we stabilized inverse participation probability weights for attrition based on demographic variables at visit 1 for participation in visit 5. The product of these 2 stabilized sampling weights and the stratified sampling design weights was used to minimize the selection bias for the analysis. These weights were used for all statistical analyses. We used logistic regression analysis with robust standard errors33 to study the association of ICAS (≥50% stenosis vs no detectable stenosis) in any of the vessel segments with outcome variable classified as no cognitive impairment (reference category), any cognitive impairment (MCI or dementia), MCI only, and dementia only. For etiologic categories, no cognitive impairment was used as the reference category. Additional sensitivity analysis was performed for domain scores (continuous) as outcome with linear regression. The analysis was adjusted with confounding variables in the following models: model 1: age, sex, race, and education; model 2: model 1 plus education, body mass index, diabetes mellitus, systolic blood pressure, use of antihypertension medicine, triglycerides (milligrams per deciliter), low-density lipoprotein (milligrams per deciliter), high-density lipoprotein (milligrams per deciliter), total cholesterol, cholesterol medication, cigarette smoking, and alcohol consumption; and model 3: model 2 plus myocardial infarction.
Figure. Attrition and use of sampling weights to adjust for selection bias.
ARIC = Atherosclerosis Risk in Communities.
Additional confounding variables—depressive symptoms, sports index, APOE polymorphisms, silent infarcts, and WMH volume—were tested by adding these variables to model 2. Because most of the black population was from a single study center (Jackson, MS), race was excluded when the model was adjusted for study center.
Results
The mean ± SD age of the 1,701 participants with adequate neuroimaging studies was 76 ± 5 years; 700 (41%) were men; 1,208 (71%) were white; 490 (29%) were black; and 3 were categorized as other races. MCI, dementia, and no cognitive impairment were diagnosed in 578 (34%), 79 (4.6%), and 1,044 (61.4%) participants, respectively.
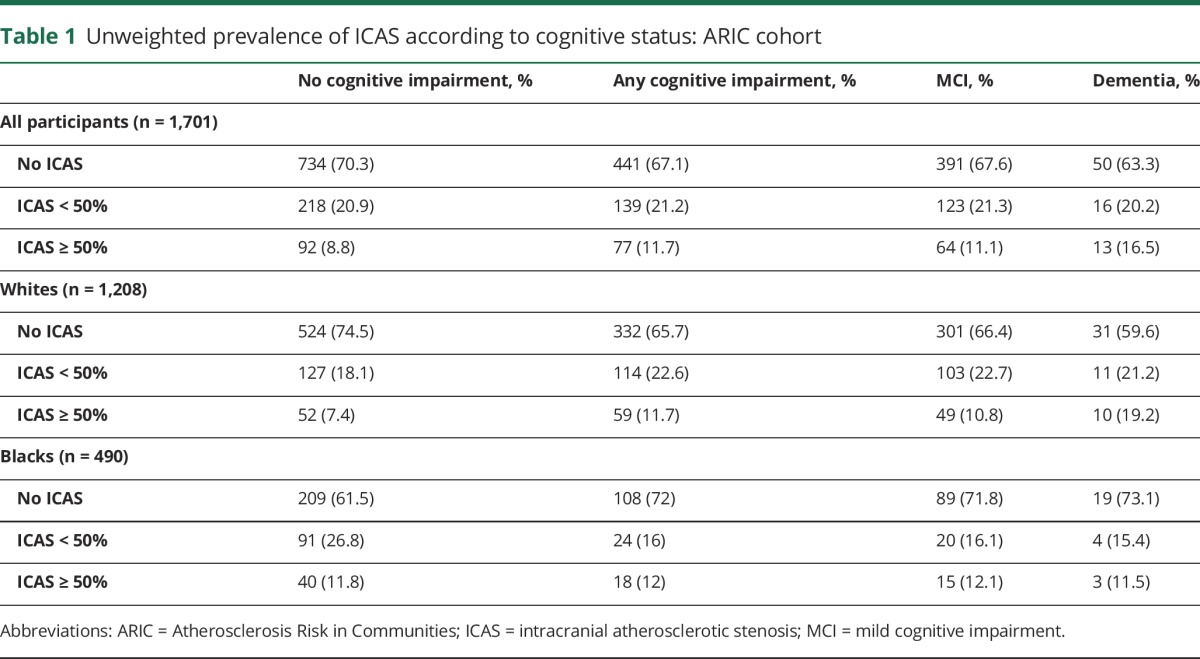
Table 1 cross-tabulates the 3 categories of cognitive status with severity of ICAS. ICAS ≥50% was more prevalent in participants with dementia and MCI than in those with no cognitive impairment. In whites, with increasing severity of cognitive impairment (dementia, MCI, and no cognitive impairment), there was a trend toward a higher prevalence of ICAS ≥50. No such trend was identified in black participants.
Table 1.
Unweighted prevalence of ICAS according to cognitive status: ARIC cohort
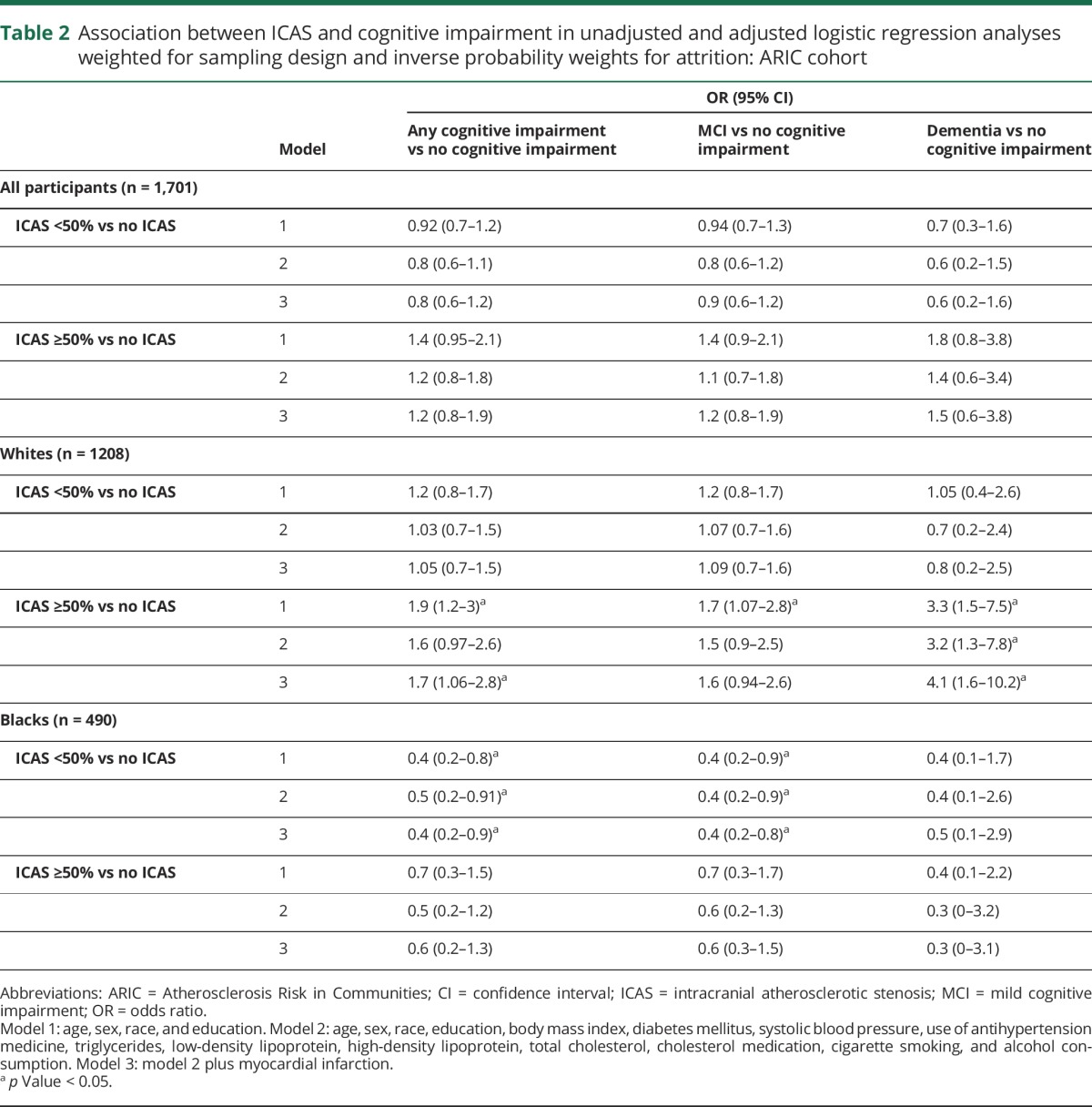
Results of the multivariable-adjusted analysis to study the association of ICAS with cognitive impairment are shown in table 2. Among white participants, ICAS ≥50% was associated with dementia after adjustment for demographic and clinical variables (model 2). After inclusion of history of myocardial infarction in the model (model 3), there was a higher prevalence of ICAS ≥50% in those with MCI, with a stronger association in those with dementia. Among blacks, ICAS ≥50% was not significantly associated with cognitive impairment. Additional multivariate models using study center instead of race, history of hypertension instead of use of antihypertensive medications, and use of statins instead of use of any cholesterol-lowering medications did not change the results.
Table 2.
Association between ICAS and cognitive impairment in unadjusted and adjusted logistic regression analyses weighted for sampling design and inverse probability weights for attrition: ARIC cohort
The significant association of ICAS ≥50% (vs no ICAS) with dementia (vs no cognitive impairment) noted in whites in model 2 remained unchanged after adjusted for APOE genotype (odds ratio [OR] 3.3, 95% confidence interval [CI] 1.3–8.5), continuous CES-D (OR 3.3, 95% CI 1.3–7.9), dichotomized CES-D (OR 3.2, 95% CI 1.3–7.7), sports index (OR 2.9, 95% CI 1.2–7.4), WMH volume (OR 3.02, 95% CI 1.2–7.4), and silent infarcts (OR 3.0, 95% CI 1.2–7.1). The results of similar analysis of MCI vs no cognitive impairment (in all participants, whites, and blacks) and dementia vs no cognitive impairment (in all participants and blacks) remained statistically nonsignificant.
Analysis for an association of syndromic cognitive impairment with ICAS ≥50% is included in tables e-1 and e-2 (links.lww.com/WNL/A301). In white participants, there was an association between ICAS ≥50% and all AD after adjustment for demographic risk factors (model 1), but this association was attenuated and no longer statistically significant after the addition of vascular risk factors to the model. The association between cerebrovascular disease and ICAS ≥50% was significant in all models for whites. There was no association between any syndromic dementia diagnosis and ICAS ≥50% in blacks. Additional sensitivity analysis with cognitive domain scores as outcome and adjusted for model 2 covariates demonstrated a significant association of ICAS ≥50% (vs no ICAS) with memory (β = −0.25, p < 0.05), executive function (β = −0.13, p < 0.05), and global domains (β = −0.20, p < 0.05), but not with language domain (β = −0.08, p > 0.05), in whites. Similar analysis demonstrated no significant association in blacks.
Discussion
We found that white participants with ICAS ≥50%, compared to those without ICAS, had 4-times higher odds of dementia, even after adjustment for demographic and vascular risk factors.
Multiple autopsy studies have identified a higher occurrence of intracranial atherosclerosis among patients with pathologic findings of AD.5,7–9 Because vascular anatomy is deformed in autopsy specimens, most of the autopsy studies used measures of atherosclerotic plaque burden instead of luminal narrowing. Even though these studies were adjusted only for age, sex and APOE and grading of intracranial atherosclerosis severity in cadaveric studies differs from measurements in our study, the associations between severity of intracranial atherosclerosis and AD noted in these studies support our findings.
ICAS is often used as a surrogate for intracranial atherosclerosis, but atherosclerotic plaque may exist without any luminal stenosis.34 Quantification of ICAS severity allows us to study the relationship between hemodynamic compromise and cognitive impairment. Some clinical studies have suggested that hypoxia may play a role in the genesis of pathologic findings of AD, including β-amyloid,19,20 neuritic plaques,21 and cortical microinfarcts.13 ICAS can also cause atherothrombotic embolization, and spontaneous cerebral microemboli have been shown to be associated with both vascular dementia and AD.35 Other studies have followed up patients with cognitive impairment and noted cognitive decline in those with ICAS.10,11 Results of these studies suggest that the association between ICAS and cognitive impairment noted in our study is causative.
One interesting finding of our study is that adjustment for vascular risk factors led to minimal change in the strong association between ICAS and dementia. Finding an association between ICAS and dementia in an analysis unadjusted for vascular risk factors, as was used in autopsy studies, is expected because vascular risk factors are associated with both dementia1,2 and ICAS.3 Previous studies that adjusted for vascular risk factors are limited. A case-control study of 55 participants noted an association between ICAS detected by transcranial Doppler and AD.6 Another study used MRA in 278 participants and noted an association between cognitive impairment and ICAS that was independent of vascular risk factors.12 Recently, in a larger case-control study of 424 participants, ICAS was strongly associated with dementia (OR 4.7) after adjustment for vascular risk factors.4 This association was attenuated by WMH volume, and those investigators considered WMH a mediator between ICAS and dementia. This is in contrast to our study in which the association between ICAS and dementia remained significant even after adjustment for WMH volume. We believe this difference is the result of a larger sample size in our study, especially considering that the association between WMH and ICAS is controversial.36 Identification of an association between ICAS and dementia that is independent of vascular risk factors is an important finding in that it raises the possibility that ICAS is a possible direct pathway for vascular risk factors to cause dementia.
There was a strong association of ICAS with cognitive impairment in whites in our study, but we did not identify an association in blacks. Strokes in blacks are known to be more attributable to ICAS than other etiologies.37 In addition, the incidence38 and prevalence39 of dementia are higher in elderly blacks compared to whites. It is possible that compared to whites more blacks with ICAS had strokes and dementia and thus were unable to participate in the study. It is also possible that some of this selection bias remained unaccounted for despite our use of selection weights. One small autopsy study of 39 black and 81 white patients with AD reported results contradictory to our finding.40 On the basis of the identification of severe intracranial atherosclerosis in 2 black participants and 2 white participants, this study concluded a higher prevalence of severe intracranial atherosclerosis in blacks than whites with AD. There was no difference in mild and moderate atherosclerosis. Considering the study design difference of the use of living participants in our study, it is possible that both studies are actually complementary. Blacks with ICAS and dementia may have shorter survival, and thus, the incidence of this association is higher in autopsies and lower in cross-sectional studies. Our sample size of black participants was also small, and an association of a smaller magnitude than what is noted in whites would not be identifiable. We adjusted for major vascular risk factors; however, these differences between races could be the result of other unexplored factors such as cultural differences or discrimination in the health care setting.
This was a cross-sectional study, so a causative association between ICAS and cognitive impairment cannot be established from this analysis. However, ICAS secondary to dementia will be an implausible explanation of this association. Although we attempted adjustment for all potential cross-sectional confounders, the cross-sectional nature of this study cannot rule out confounding risk factors earlier in life. In addition, because of a lack of information, we may have missed some other confounders (e.g., overall dietary quality). The study was also limited by the possibility of uncontrolled selection bias. Although the ARIC cohort originally was community based, if participants with ICAS and dementia had low survival and those who survived had low participation rates in visit 5, then the OR for this association will be artifactually lowered. We noted an increase in the association between ICAS and cognitive impairment in whites after adjusting for selection bias, suggesting that there was some selection bias affecting this association before this adjustment. We probably were unable to completely eliminate the selection bias despite our selection-weighted analysis. We did not identify a significant association between ICAS ≥50% and cognitive impairment in blacks, probably because of a smaller sample size and a lack of power. It is also possible that measurements are less reliable in blacks, and this would have contributed to the lack of significant association.
We did not measure ICAS as a continuous variable and are unable to check whether the association between ICAS and cognitive impairment is linear. Another limitation of our analysis was the use of OR as a measure of association. Because of the high prevalence of MCI, the OR would overestimate relative risk. However, the prevalence of dementia was only 4.6%, so the OR would be a good estimator of the relative risk for dementia.
We found a strong association of ICAS with dementia in whites. This finding supports a potential role of ICAS in the development of dementia. Because the association was independent of demographic and vascular risk factors, the role of ICAS in identifying the population at risk of dementia needs to be further investigated in longitudinal studies.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- CES-D
Center for Epidemiologic Studies Depression Scale
- CI
confidence interval
- ICAS
intracranial atherosclerotic stenosis
- MCI
mild cognitive impairment
- MRA
magnetic resonance angiography
- OR
odds ratio
- WASID
Warfarin-Aspirin Symptomatic Intracranial Disease
- WMH
white matter hyperintensity
Author contributions
M. Fareed K. Suri: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, study supervision, obtaining funding. Jincheng Zhou: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision. Ye Qiao: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, contribution of vital reagents/tools/patients, acquisition of data, study supervision. Haitao Chu: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Adnan I. Qureshi: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval. Thomas Mosley: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Rebecca F. F. Gottesman: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Lisa Wruck: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval. A. Richey Sharrett: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, review approval of final manuscript. Alvaro Alonso: drafting/revising the manuscript, accepts responsibility for conduct of research and will give final approval, study supervision. Bruce A. Wasserman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding.
Study funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the NIH under awards R01HL105626 and R01HL105930. The ARIC study is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 from the NHLBI and the National Institute of Neurologic Disorders and Stroke and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
Disclosure
M. Suri, J. Zhou, Y. Qiao, H. Chu, A. Qureshi, T. Mosley, R. Gottesman, L. Wruck, R. Sharrett, and A. Alonso report no disclosures relevant to the manuscript. B. Wasserman reports ownership interest in the 3-dimensional black-blood MRI technique used (patent pending, 13/822, 111). Go to Neurology.org/N for full disclosures.
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement 2015;11:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014;383:984–998. [DOI] [PubMed] [Google Scholar]
- 4.Hilal S, Xu X, Ikram MK, et al. Intracranial stenosis in cognitive impairment and dementia. J Cereb Blood Flow Metab 2017;37:2262–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roher AE, Esh C, Kokjohn TA, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol 2003;23:2055–2062. [DOI] [PubMed] [Google Scholar]
- 6.Roher AE, Garami Z, Alexandrov AV, et al. Interaction of cardiovascular disease and neurodegeneration: transcranial Doppler ultrasonography and Alzheimer's disease. Neurol Res 2006;28:672–678. [DOI] [PubMed] [Google Scholar]
- 7.Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 2007;113:13–21. [DOI] [PubMed] [Google Scholar]
- 8.Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 2012;135:3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology 2005;64:494–500. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Wang Y, Li J, Deng J, Zhou H. Intracranial artery stenosis and progression from mild cognitive impairment to Alzheimer disease. Neurology 2014;82:842–849. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Song IU, Jeong DS, Lee KS. Clinical effect of cerebrovascular atherosclerosis on cognition in Alzheimer's disease. Arch Gerontol Geriatr 2016;63:55–58. [DOI] [PubMed] [Google Scholar]
- 12.Hilal S, Saini M, Tan CS, et al. Intracranial stenosis, cerebrovascular diseases, and cognitive impairment in Chinese. Alzheimer Dis Assoc Disord 2015;29:12–17. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto Y, Yamamoto T, Kalaria RN, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 2012;123:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhiyou C, Yong Y, Shanquan S, et al. Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer's disease. Neurochem Res 2009;34:1226–1235. [DOI] [PubMed] [Google Scholar]
- 15.Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-beta. Am J Pathol 2010;177:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada M, Ihara M, Okamoto Y, et al. The influence of chronic cerebral hypoperfusion on cognitive function and amyloid beta metabolism in APP overexpressing mice. PLoS One 2011;6:e16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Im DS, An YS, Hong JM, Gwag BJ, Joo IS. Chronic cerebral hypoperfusion in a mouse model of Alzheimer's disease: an additional contributing factor of cognitive impairment. Neurosci Lett 2011;489:84–88. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Gu J-H, Dai C-L, et al. Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice. Front Aging Neurosci 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zetterberg H, Mortberg E, Song L, et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid beta levels in humans. PLoS One 2011;6:e28263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid beta. Acta Anaesthesiol Scand 2013;57:82–88. [DOI] [PubMed] [Google Scholar]
- 21.Huang KL, Lin KJ, Ho MY, et al. Amyloid deposition after cerebral hypoperfusion: evidenced on [(18)F]AV-45 positron emission tomography. J Neurol Sci 2012;319:124–129. [DOI] [PubMed] [Google Scholar]
- 22.Kalback W, Esh C, Castano EM, et al. Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer's disease. Neurol Res 2004;26:525–539. [DOI] [PubMed] [Google Scholar]
- 23.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst) 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016;280:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 26.Suri MFK, Qiao Y, Ma X, et al. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population: the Atherosclerosis Risk in Communities study. Stroke 2016;47:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 28.Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol 2006;164:342–348. [DOI] [PubMed] [Google Scholar]
- 29.Rost NS, Sadaghiani S, Biffi A, et al. Setting a gold standard for quantification of leukoaraiosis burden in patients with ischemic stroke: the Atherosclerosis Risk in Communities Study. J Neurosci Methods 2014;221:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross AL, Power MC, Albert MS, et al. Application of latent variable methods to the study of cognitive decline when tests change over time. Epidemiology 2015;26:878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 34.Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke 2016;47:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purandare N, Burns A, Daly KJ, et al. Cerebral emboli as a potential cause of Alzheimer's disease and vascular dementia: case-control study. BMJ 2006;332:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topakian R. Conflicting evidence on the association of white matter hyperintensities with large-artery disease. Eur J Neurol 2015;22:4–5. [DOI] [PubMed] [Google Scholar]
- 37.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 2005;111:1327–1331. [DOI] [PubMed] [Google Scholar]
- 38.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- 39.Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: the South Florida Program on Aging and Health. Ann Epidemiol 2003;13:472–478. [DOI] [PubMed] [Google Scholar]
- 40.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015;85:528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]