Abstract
Patient: Male, 60
Final Diagnosis: Atypical gelsolin amyloidosis
Symptoms: Cranial nerve palsy • proximal muscle weakness
Medication: —
Clinical Procedure: —
Specialty: Hematology
Objective:
Rare disease
Background:
Gelsolin amyloidosis is a very rare systemic disease presenting with a pathognomonic triad of corneal lattice dystrophy, cutis laxa, and polyneuropathy. The disease is mostly restricted to a Finnish population with known mutations (G654A, G654T) in exon 4 of the gelsolin gene. The mutations lead to errors in protein processing and folding, and ultimately leads to deposition of an amyloidogenic fragment in the extracellular space, causing the symptoms of disease.
Case Report:
We present a case of gelsolin amyloidosis in a male of African descent with an atypical clinical presentation including fevers, skin rash, polyneuropathy, and anemia. Gelsolin amyloidosis was diagnosed based on mass spectrometry of tissue samples. Importantly, a novel mutation in the gelsolin gene (C1375G) in exon 10 was found in this patient. His atypical presentation can possibly be attributed to the presence of a novel mutation in the gelsolin gene as the likely underlying cause of the syndrome. PCR primers were used to amplify the gelsolin gene from genomic DNA. Purified PCR products were then shipped to Eton Biosciences (San Diego, CA) for sequencing.
Conclusions:
This study carries several important lessons relevant to the practice of medicine. First, the differential diagnosis for multisystem disease presentations should always include amyloidosis. Second, despite what has been uncovered about the molecular biology of disease, there is always more that can be discovered. Finally, further work to verify the link between this mutation and the clinical syndrome is still needed, as are effective treatments for this disease.
MeSH Keywords: Amyloid Neuropathies, Familial; Amyloidosis, Familial; Gelsolin
Background
Amyloidosis is a group of diseases that are characterized by a pathologic deposition of misfolded proteins (in a beta sheet type structure) in the extracellular space. Currently, there are about 25 different proteins known to cause amyloidosis [1]. Gelsolin amyloidosis is a rare subtype that was first described by a Finnish ophthalmologist, Jouko Meretoja, in 1969 [2,3]. It has also been referred to as AGel amyloidosis, Meretoja syndrome, Meretoja amyloidosis, familial amyloidosis Finnish type, and hereditary gelsolin amyloidosis. Despite its rarity, over the years the genetics and pathogenesis have been elucidated. Gelsolin amyloidosis is an autosomal dominant and monogenic disease with 100% penetrance. Initially thought to be restricted to Finnish bloodlines, a few cases in other countries (with and without Finnish descent) have been reported with known, and sometimes novel, mutations in Asia, Mexico, Japan, the US, Canada, and Iran. By 2012, approximately 1000 gene defect carriers had been identified within the Finnish population [4–8].
Gelsolin is a protein produced from a 70-kb gene located on chromosome 9 in the q32-34 region, and it contains 17 exons [4,5,7]. Gelsolin is an actin-binding protein that plays an integral part in cytoplasmic actin filament organization (assembly and disassembly) [7,9]. It is an 81–83 kDa protein with 6 homologous subdomains (G1–G6) [10]. Gelsolin regulation of the actin cytoskeleton is modulated by calcium binding (activation) and by phosphoinositide signaling (deactivation) [10–12]. There are 2 forms of gelsolin produced as a result of alternative splicing of the same gene. These include a plasma gelsolin (83 kDa) protein and a cytosolic gelsolin (81 kDa) protein [12]. Cytosolic gelsolin facilitates the transition of fibrillary actin into soluble actin intracellularly [7]. Plasma gelsolin scavenges the blood for actin filaments released following cellular injury. Plasma gelsolin caps the ends of these free actin filaments and thus prevents aberrant polymerization. In addition, plasma gelsolin prevents an increase in blood viscosity and direct actin fibril toxicity [9]. Other extracellular functions of plasma gelsolin include modulating the inflammatory responses and binding of bioactive lipids. Furthermore, it is hypothesized that plasma gelsolin plays a protective role in nongelsolin-amyloid-associated toxicity, as has been described in, for example, Alzheimer’s disease [7]. Gelsolin is produced heterogeneously throughout the body but the major source derives from skeletal muscle cells. Plasma gelsolin concentrations ranges from 200 to 300 ug/mL in the serum [13].
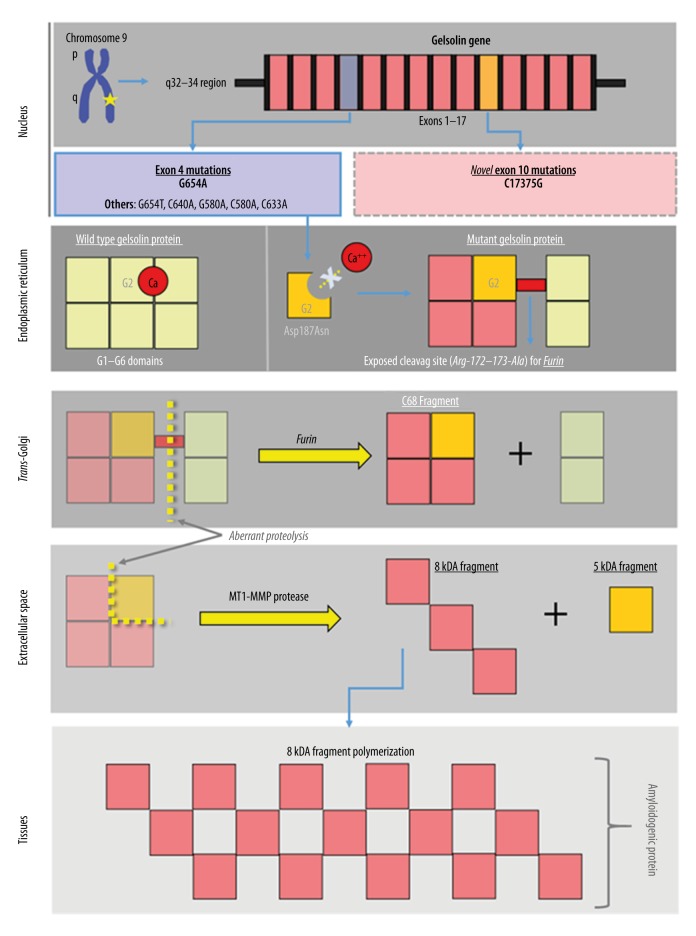
All mutations known to cause gelsolin amyloidosis arise from a single-point mutation in exon 4 that results in a gain-of-toxic function state (Figure 1). The most common nucleotide substitution observed involves a substitution of the nucleic acid guanine for adenine, and is referred to as G654A. The change in the nucleic acid causes an amino acid substitution – aspartic acid to asparagine – at residue 187 of the G2 domain. It is also referred to as D187N or asp187asn [10]. By having asparagine in that specific location, the protein structure becomes altered because of its inability to bind to calcium [7,12]. When the misfolded protein arrives in the trans-Golgi network, the abnormal protein configuration exposes a novel cleavage site for furin (an alpha-gelsolinase). This cleavage site is located in the G2 domain at the arg-172-173-ala interface [10]. The major product following furin cleavage consists of a 68-kDA protein referred to C68 (C-terminal 68-kDa fragment). The C68 protein fragment is then secreted into the extracellular space.
Figure 1.
Pathogenesis of gelsolin amyloidosis, showing key steps in the formation of amyloidogenic protein from the genetic point mutations in gelsolin gene.
In the extracellular space, the C68 fragment is cleaved again by a membrane type-1 matrix metalloproteinase (MT1-MMP). Consequently, the C68 fragment is broken down into smaller fragments referred to as 8 kDa and 5 kDa. These smaller fragments, particularly the 8 kDa, are the pathological end-products of the G654A mutation. These fragments are the amyloidogenic components in the disease pathology. They are insoluble fragments that can further polymerize and aggregate, and become systemically deposited into tissues. Over time, the accumulation of these polymerized protein fragments causes cellular and tissue architecture disruption. Eventually, the deposition leads to the degeneration of tissues, and organ dysfunction ensues [7]. Interestingly, the absence of the wild-type gelsolin protein is believed to confer no significant pathology, mostly because the wild-type gelsolin protein and its inherent function are considered redundant in the human body [7].
The other, but less common, nucleotide substitutions include G654T, G640A, G580A, C580A, and C633A. These mutations also form an aberrant gelsolin protein and subsequent amyloidogenic protein fragments with similar clinical consequences.
Historically, gelsolin amyloidosis has been characterized by a pathognomonic triad of clinical manifestation that include corneal lattice dystrophy, cutis laxa, and polyneuropathies. These findings arise from pathological deposition of the gelsolin amyloid proteins into these tissues. Most organs are affected at some level [4]. Irrespective of disease burden and the level of organ dysfunction, patients with gelsolin amyloidosis appear to have a normal lifespan.
Corneal lattice dystrophy occurs when gelsolin amyloid is deposited into the anterior and middle stroma of the eye. Ophthalmological symptoms are commonly the first signs manifested in the evolution of the disease. The onset of these symptoms begins in the late third and early fourth decades of life. Initial complaints consist of progressive eye dryness, eye irritation, and sometimes eye pain. Later in the disease course, impaired vision can occur. Furthermore, corneal ulcers, cataracts, and, less commonly, glaucoma can also develop [4].
Examination of the cornea can demonstrate comma-shaped and filamentous opacities assorted in a lattice-type of arrangement. Also appreciated is a decrease in corneal sensitivity and an absent corneal reflex. This is believed to be secondary to amyloid deposition in corresponding nerves [4,5]. Eye dryness is attributed to amyloid deposition in the ciliary body, consequently altering normal aqueous humor flow. Management is symptomatic and mostly focuses on maintaining adequate eye lubrication. In certain circumstances, a corneal transplantation can be considered if the disease burden is severe.
Neuropathy is mostly along large nerve fibers and is associated with sensory deficits only. The distribution mostly consists of cranial nerves and peripheral nerves, with occasional autonomic nerve involvement. Patients often present with a constellation of pathology such as: facial nerve paresis, polyneuropathy, impaired hearing, ataxia, dysarthria, and orthostatic hypotension [4]. These symptoms often appear early in the fourth decade of life.
Cutis laxa is a deposition of gelsolin amyloid into the basement membrane of the dermis [4]. The amyloid causes disruption of the dermal architecture, affecting normal function of the collagen and elastin components. This produces a reduction in elasticity and resilience of the skin, which manifest clinically as loosened skin. Drooping eyelids are a common initial presentation. This process typically starts in the middle of the fourth decade of life [4]. Amyloid deposits can also be found in sweat glands, causing skin dryness, and deposits in hair follicles can cause alopecia. The microvasculature can be involved, leading to abnormal cutaneous bruising. Management of these symptoms is usually supportive. In severe cases, cosmetic and reconstructive procedures are possible, particularly when symptoms affect the face.
Other manifestation from systemic gelsolin amyloid deposition have been described and include: cardiomyopathy, arrhythmias, sleep apnea, and end-stage renal disease. Of note, the development of a nephrotic syndrome has been specifically associated with a homozygous mutation involving G580A and C633A [5].
Gelsolin amyloidosis can be initially diagnosed by immunohistochemical methods showing Congo-red positivity (40–80% sensitivity and specificity) on a subcutaneous fat aspirate sample. Next, the subtype of amyloid should be further delineated using laser microdissection and mass spectrometry. These methods have 100% specificity and sensitivity for the amyloid subtypes [1,14]. When attempting to identify an underlying mutation, the initial step is obtaining a comprehensive family history. In the absence of next-generation sequencing, a focus on the more common point mutations, G654A and G654T, in the gelsolin gene is a reasonable start.
Currently, there is no specific treatment available for gelsolin amyloidosis. Until recently, most therapeutic approaches have been focused on symptomatic management of the disease. Now that the underlying pathogenesis of the disease is better understood, there is growing interest in molecularly targeting key steps in the pathogenesis. Research with animal models has shown a decrease in amyloid deposition in tissues by blocking various proteolytic steps in the formation of the amyloidogenic fragments [15,16]. One example is by preventing the formation of the amyloidogenic fragments (8 kDa and 5 kDa) through inhibition of the MT1-MMP proteolysis using nanobodies [15]. Alternatively, there is ongoing work also using nanobodies to prevent the initial furin proteolysis step and downstream cascade [16]. Despite this progress, no human studies or clinical trials have been performed to date.
Material and Methods
Genomic DNA was purified from a muscle tissue biopsy using the GeneJET Gel Extraction and DNA Cleanup Micro Kit. The genomic DNA was amplified by PCR using the Thermo Scientific Maxima Hot Start PCR kit and the various primers designed to amplify the exons of the gelsolin gene. The PCR products were evaluated by agarose gel electrophoresis. All PCR primers were synthesized by MWG Operon (Huntsville, AL). Purified PCR product was shipped to Eton Biosciences (San Diego, CA) for sequencing.
Case Report
Our patient was a 60-year-old male of African descent with a past medical history of gastroesophageal reflux disease who presented with a 5-month history of gradually worsening symptoms. His symptoms included diffuse joint pain and joint swelling, a diffuse rash, headaches, and generalized weakness. He also reported subjective fevers, chills, profuse night sweats, and an unintentional 30-pound weight loss.
The rash was pruritic and initially started on his face and spread throughout his body. The headaches were associated with bilateral eye pain, eye swelling, double vision, and mild vision blurriness. The muscle weakness was most pronounced in his lower extremities and was bilateral, graded at 3/5 in flexion and extension of the hip, 4-/5 in flexion and extension of the knee, and 4-/5 in dorsiflexion and plantarflexion of the ankle. He also complained of dysphagia for solids and liquids.
On the initial exam, the patient was alert and oriented but was febrile and appeared mildly toxic. The patient had diffuse alopecia and adjacent scarring. A polymorphous rash was noted on the face, back, and all extremities. There were areas of hypopigmentation that were superimposed by areas of hyperpigmentation. In addition, sporadic eczematous-like macules and patches were noted in the extremities and hips. Multiple joints (hands, elbows, and shoulders) were edematous and tender to palpation. There was symmetrical facial weakness of the upper and lower face. Decreased facial sensation was most pronounced along the cranial nerve distribution of V2 and V3. Additionally, there was profound proximal and distal muscle weakness compounded by decreased muscle bulk and increase muscle tone. A bilateral positive Babinski reflex was noted (i.e., extension of the hallux with plantar stimulation) and hyperreflexive joint reflexes.
Laboratory exam results showed normocytic anemia and thrombocytopenia. The chemistry panel was only notable for elevated liver enzymes. A peripheral blood smear did not show evidence of schistocytes, dysplasia, or blasts, but was notable for thrombocytopenia, normochromic, normocytic anemia, and mild leukopenia. See Table 1 for laboratory values.
Table 1.
Initial laboratory values on admission.
| Lab | Lab values | Reference range |
|---|---|---|
| White blood cells | 4.8 | 4.8–11.8 bil/L |
| Hemoglobin | 10.3 | 11–16 g/dL |
| Mean corpuscular volume | 82.8 | 77–96 fl |
| Platelets | 55 | 140–340 bil/L |
| Erythrocyte sedimentation rate | 55 | 0–20 mm/hr |
| C-reactive protein | 1.4 | 0.0–0.8 mg/dL |
| Lactate dehydrogenase | 373 | 74–290 U/L |
| Thyroid-stimulating hormone | 1.19 | 0.800–7.700 uIU/mL |
| Creatinine | 0.8 | 0.7–1.3 mg/dL |
| Aspartate transaminase | 278 | 0–35 U/L |
| Alanine transaminase | 105 | 0–45 U/L |
| Total bilirubin | 0.5 | 0.0–1.0 mg/dL |
| Aldolase | 5.8 | <7.7 U/L |
| CK-MB | 325 | 41–266 U/L |
| Troponin | 0.01 | ≤0.03 ng/mL |
A magnetic resonance imaging (MRI)scan of the head and spine, for the initial presenting symptoms, was unremarkable. All other imaging obtained was unremarkable as well.
The initial clinical impression pointed towards a possible mixed connective tissue disease such as a dermatomyositis or a systemic lupus flare. An extensive rheumatological panel was negative. A left finger punch biopsy showed perivascular dermatitis without vasculitis. Dermal mucin deposition was identified, which suggested a possible connective tissue disorder. An extensive and comprehensive infectious disease workup that encompassed viral, bacterial, and fungal studies, was negative. There were no clear signs or symptoms of malignancy identified. No monoclonal gammopathy was identified.
The patient began to report mild muscle pain in the proximal lower extremities. Creatinine kinase and aldolase levels were normal. An MRI showed subcutaneous edema and myositis particularly in the anterior compartment muscles of the left thigh. Subsequently, biopsies of the left quadriceps muscle showed focal myopathy and denervation atrophy (severe, type II). Over the course of the hospitalization, several other biopsies were obtained that included bone marrow, liver, and a left axillary lymph node. All biopsies were negative for malignancy or amyloid deposition.
Although creatinine kinase and aldolase levels were normal, the patient’s clinical picture and initial pathologic findings were suggestive of seronegative dermatomyositis. As such, the patient was started on high-dose parenteral steroids and immunosuppressive therapy with oral hydroxychloroquine, but without clinical improvement.
The patient died shortly after being transferred to the medical intensive care unit for worsening respiratory distress and severe sepsis, both of uncertain etiology. Prior to the patient’s death, the individual with medical power of attorney had declined further escalation of care, and later declined an autopsy. Postmortem, the final pathology studies from the muscle biopsy, which had been sent to an outside reference lab, demonstrated gelsolin amyloidosis. Prior to his death, a gingival mucosal biopsy was also obtained, which also showed focal amyloid deposition in the blood vessels. However, in the gingival specimen no further subtyping of the amyloid protein was requested. The patient’s clinical course is outlined in Table 2.
Table 2.
Timeline of key events during hospitalization.
| Hospital day | Hospital clinical event |
|---|---|
| Day 1 | Admission and neurology consult |
| Day 2 | Infectious disease consulted |
| Day 3 | Dermatology consult and skin biopsy performed |
| Day 6 | Hematology and Oncology consulted for pancytopenia |
| Day 8 | Rheumatology consulted |
| Day 12 | Liver Biopsy performed, MRI of muscles performed |
| Day 22 | Muscle Biopsy performed |
| Day 33 | Bone marrow biopsy performed |
| Day 34 | Transferred to intensive care unit for respiratory distress |
| Day 35 | Intubated, Imaging showed questionable intraperitoneal air |
| Day 36 | Transthoracic echocardiogram with ejection fraction of 65% |
| Day 38 | Nephrology consulted for oliguria and acute kidney injury |
| Day 42 | Patient expired |
| Day 44 | Final muscle biopsy showed Gelsolin amyloidosis and a gingival mucosal biopsy showed amyloid deposition |
In our laboratory, we purified genomic DNA from the patient’s original muscle biopsy tissue. Then, we utilized known polymerase chain reaction (PCR) primers to amplify and sequence exon 4 of the gelsolin gene. This exon is known to harbor all the known genetic mutations previously described in hereditary gelsolin amyloidosis. The results, unexpectedly, demonstrated a wild-type exon 4 sequence. We then used PCR primers that were designed to amplify, and later sequence, the remaining 16 exons of the gelsolin gene. A homozygous missense mutation, C>G1375, was identified within exon 10. This mutation leads to an amino acid sequence change of proline 459 to arginine within the gelsolin domain 4. Gelsolin domain 4 is a type I calcium-binding domain that interacts with the other calcium binding domains of the gelsolin protein. Intact domain 4 structure is likely critical to the ability of gelsolin protein to fold into an appropriate conformation and function normally. Of note, clinically relevant gelsolin gene mutations outside of the exon 4 have not previously been described.
Discussion
Outside of the Finnish population, gelsolin amyloidosis is an exceedingly rare disease. Currently, there are no known published reports that have linked this subtype of amyloidosis with an individual of African descent. Because of the circumstances of the case and post hoc diagnosis, we were unable to directly probe his family history prior to his death. Furthermore, no known living relatives were ever identified.
In this case, the diagnosis of gelsolin amyloidosis was made postmortem. As a result, we could only rely on prior documentation to sort out clues. His presentation was complex, with common diseases considered in his differential diagnosis. Interestingly, of the classical clinical symptoms that could be present in his age group, the patient only presented with a peripheral sensory neuropathy, which was most pronounced in the face. The patient also had involvement of upper motor neurons with neurologic deficits. Our patient, however, did not exhibit the common manifestations of cutis laxa or corneal lattice dystrophy as classically described among the Finnish population. Most of his acute symptoms and clinical findings were thought to be consistent with dermatomyositis. The patient presented with proximal muscle weakness, dysphagia, polyarthritis, Gottron’s papules, alopecia, a diffuse heliotrope eruption, and myositis. The diagnosis was further supported by finding dermal mucin deposits and elevated serum inflammatory markers. Interstitial lung disease and progressive muscle weakness can possibly explain the worsening respiratory distress of the patient. The trigger for the dermatomyositis also remains unclear since there was no inciting event, no evidence of infection, and no malignancy identified. Currently, there is no reported link between dermatomyositis and gelsolin amyloidosis in the medical literature. Despite this, non-specific amyloidosis has been previously associated with dermatomyositis [17]. Nonetheless, it is unclear if his gelsolin amyloidosis alone contributed to his relatively rapid clinical decline and death.
Another important aspect of this case is that sequencing of the gelsolin gene in our patient identified a novel C>G1375 transversion leading to a change in the amino acid sequence of gelsolin domain 4. This mutation was not previously described in the SNP database. On the previously published crystal structure, domain 4 was identified as a type I calcium-binding site, and disruption of this domain would likely disrupt the ability of other domains to interact and bind calcium as well. Since calcium binding is required for the conformational changes in the gelsolin protein, it is likely that the mutant gelsolin conformation is disrupted. This may allow for unmasking of the furin cleavage site, which ultimately leads to the production of the amyloidogenic fragments thought to cause the clinical symptoms of this disease. In addition, this mutation may open up gelsolin for proteolytic cleavage by other enzymes. It is tempting to speculate that the atypical clinical spectrum observed in our patient is due to the different location of the mutation. Regardless, mutation sequence prediction modeling does suggest that this mutation would lead to disease and have significant effects on multiple domains of the gelsolin protein [18]. Taken together, this information suggests that the mutation is likely functional and possibly clinically relevant. Given the lack of availability of tissue for further studies, however, it is impossible to perform additional germline analysis. Further studies are required to fully elucidate the effect of this mutation on gelsolin amyloidosis. Specific in vitro functional assays may shed light on the pathologic processing of this mutant protein and may provide additional evidence as to its clinical relevance to the syndrome described in this case report.
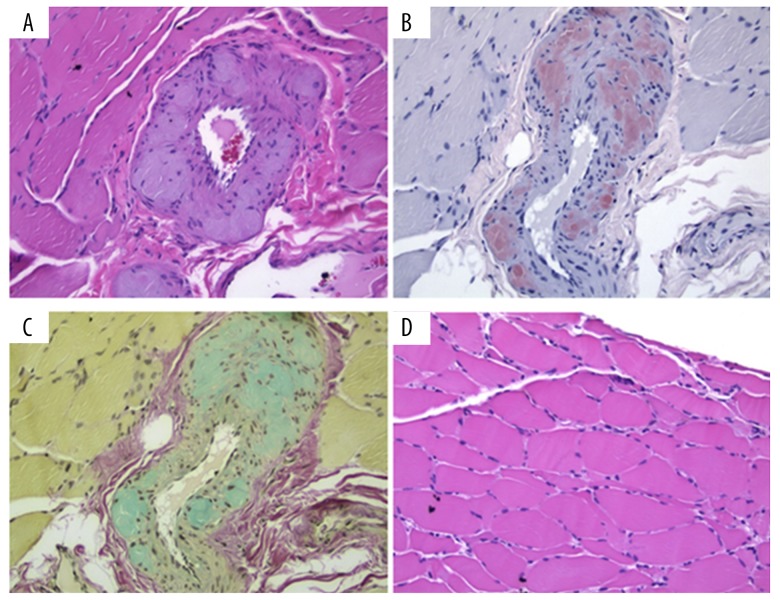
In our case, there was proven mucosal gingival involvement by amyloid (data not shown) and suspected involvement of the liver, heart, and kidney, which suggests an already existing and widespread amyloidogenic protein deposition. In further support of the widespread amyloidogenic protein deposition, the previously described blood vessel changes found in gelsolin amyloid angiopathy were also identified in our patient [19]. The major pathological changes in the arteries are shown in Figure 2A (H&E stain) thickened vascular wall with accumulation of abundant nodular AGel fibrils, which disrupt the tunica media with disorganization and replacement of vascular smooth muscle cells. Additional elastin stain shows that the lamina elastica interna becomes fragmented and diminished (not shown). Congo red and sulfated Alcian blue stains demonstrate amyloidosis in the vascular wall (Figure 2B, 2C). Some degree of type II myocyte atrophy is also seen (Figure 2D).
Figure 2.
Pathology from skeletal muscle biopsy showing gelsolin amyloid involvement. (A) Nodular vascular amyloid deposition, (B) Vascular amyloid staining strongly with Congo red, (C) Amyloid deposits positive for Sulfated Alcian Blue, and (D) Randomly scattered atrophic myofibers.
Therefore, the presence of a distinct systemic inflammatory syndrome coupled with widespread amyloid deposition is the most plausible explanation for this patient’s multiorgan dysfunction and ultimate demise. Although this syndrome is not completely overlapping with that described for typical gelsolin amyloidosis, it is likely that since the mutation we identified is distinct from those described previously, the clinical syndrome would be distinct as well.
Conclusions
Our case report is the first to present an elderly male of African descent presenting with gelsolin amyloidosis. His clinical presentation was atypical for this rare disease, presenting with fever, rash, multiorgan failure, polyneuropathy, and cytopenias, without evidence of cutis laxa or corneal lattice dystrophy. Importantly, a novel mutation in the gelsolin gene was identified. This mutation is likely pathogenic and is the probable cause of this atypical clinical syndrome. This complicated presentation illustrates the importance of thinking of rare diseases and atypical presentations of diseases, especially in cases where diagnosis is delayed. Amyloidosis, for example, should be considered in any patient presenting with multisystem pathology. In addition, it is important to note that although much is known about the molecular pathologic changes that give rise to disease, much remains to be discovered. Finally, although this novel mutation is important, the functional effects of this mutation on cellular gelsolin processing are yet to be fully elucidated. The findings presented here, however, open the doorway for future research. Additionally, further work should focus on potential therapeutic targets in an attempt to decrease disease burden and improve quality of life.
Acknowledgments
We would like to acknowledge assistance from Dr. Saied Mirshahidi PhD and Dr. Rosalia de Necochea-Campion, as well as the Loma Linda University Cancer Center Biospecimen Laboratory for the provision of technical advice, resources, and time in the laboratory.
References:
- 1.Vrana JA, Gamez JD, Madden BJ, et al. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114(24):4957–59. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 2.Meretoja J. Familial systemic paramyloidosis with lattice dystrophy of the cornea, progressive cranial neuropathy, skin changes and various internal symptoms. A previously unrecognized heritable syndrome. Ann Clin Res. 1969;1(4):314–24. [PubMed] [Google Scholar]
- 3.Meretoja J. Genetic aspects of familial amyloidosis with corneal lattice dystrophy and cranial neuropathy. Clin Genet. 1973;4(3):173–85. doi: 10.1111/j.1399-0004.1973.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 4.Nikoskinen T, Schmidt EK, Strbian D, et al. Natural course of Finnish gelsolin amyloidosis. Ann Med. 2015;47(6):506–11. doi: 10.3109/07853890.2015.1075063. [DOI] [PubMed] [Google Scholar]
- 5.Alabdali M, Barnett C, Abraham A, et al. Gelsolin familial amyloidosis peripheral neuropathy in Canada: A case report. Can J Neurol Sci. 2015;42(5):353–55. doi: 10.1017/cjn.2015.56. [DOI] [PubMed] [Google Scholar]
- 6.Sekijima Y. [Clinical diversity, diagnosis and treatment of hereditary amyloid neuropathy] Rinsho Shinkeigaku. 2014;54(12):953–56. doi: 10.5692/clinicalneurol.54.953. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 7.Solomon JP, Page LJ, Balch WE, Kelly JW. Gelsolin amyloidosis: Genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit Rev Biochem Mol Biol. 2012;47(3):282–96. doi: 10.3109/10409238.2012.661401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiuru S. Gelsolin-related familial amyloidosis, Finnish type (FAF), and its variants found worldwide. Amyloid. 1998;5(1):55–66. doi: 10.3109/13506129809007291. [DOI] [PubMed] [Google Scholar]
- 9.Bucki R, Levental I, Kulakowska A, Janmey PA. Plasma gelsolin: Function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci. 2008;9(6):541–51. doi: 10.2174/138920308786733912. [DOI] [PubMed] [Google Scholar]
- 10.Nag S, Ma Q, Wang H, et al. Ca2+ binding by domain 2 plays a critical role in the activation and stabilization of gelsolin. Proc Natl Acad Sci USA. 2009;106(33):13713–18. doi: 10.1073/pnas.0812374106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274(47):33179–82. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 12.Westberg JA, Zhang KZ, Andersson LC. Regulation of neural differentiation by normal and mutant (G654A, amyloidogenic) gelsolin. FASEB J. 1999;13(12):1621–26. doi: 10.1096/fasebj.13.12.1621. [DOI] [PubMed] [Google Scholar]
- 13.Kwiatkowski DJ, Mehl R, Izumo S, et al. Muscle is the major source of plasma gelsolin. J Biol Chem. 1988;263(17):8239–43. [PubMed] [Google Scholar]
- 14.Efebera YA, Sturm A, Baack EC, et al. Novel gelsolin variant as the cause of nephrotic syndrome and renal amyloidosis in a large kindred. Amyloid. 2014;21(2):110–12. doi: 10.3109/13506129.2014.891502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Overbeke W, Verhelle A, Everaert I, et al. Chaperone nanobodies protect gelsolin against MT1-MMP degradation and alleviate amyloid burden in the gelsolin amyloidosis mouse model. Mol Ther. 2014;22(10):1768–78. doi: 10.1038/mt.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Overbeke W, Wongsantichon J, Everaert I, et al. An ER-directed gelsolin nanobody targets the first step in amyloid formation in a gelsolin amyloidosis mouse model. Hum Mol Genet. 2015;24(9):2492–507. doi: 10.1093/hmg/ddv010. [DOI] [PubMed] [Google Scholar]
- 17.Gelderman AH, Levine RA, Arndt KA. Dermatomyositis complicated by generalized amyloidosis. Report of a case. N Engl J Med. 1962;267:858–61. doi: 10.1056/NEJM196210252671704. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–62. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 19.Koskelainen S, Pihlamaa T, Suominen S, et al. Gelsolin amyloid angiopathy causes severe disruption of the arterial wall. APMIS. 2016;124(8):639–48. doi: 10.1111/apm.12554. [DOI] [PubMed] [Google Scholar]