Abstract
Background: Improved antibiotic stewardship (AS) and reduced prescribing in primary care, with a parallel increase in personal internet use, could lead citizens to obtain antibiotics from alternative sources online.
Objectives: A cross-sectional analysis was performed to: (i) determine the quality and legality of online pharmacies selling antibiotics to the UK public; (ii) describe processes for obtaining antibiotics online from within the UK; and (iii) identify resulting AS and patient safety issues.
Methods: Searches were conducted for ‘buy antibiotics online’ using Google and Yahoo. For each search engine, data from the first 10 web sites with unique URL addresses were reviewed. Analysis was conducted on evidence of appropriate pharmacy registration, prescription requirement, whether antibiotic choice was ‘prescriber-driven’ or ‘consumer-driven’, and whether specific information was required (allergies, comorbidities, pregnancy) or given (adverse effects) prior to purchase.
Results: Twenty unique URL addresses were analysed in detail. Online pharmacies evidencing their location in the UK (n = 5; 25%) required a prescription before antibiotic purchase, and were appropriately registered. Online pharmacies unclear about the location they were operating from (n = 10; 50%) had variable prescription requirements, and no evidence of appropriate registration. Nine (45%) online pharmacies did not require a prescription prior to purchase. For 16 (80%) online pharmacies, decisions were initially consumer-driven for antibiotic choice, dose and quantity.
Conclusions: Wide variation exists among online pharmacies in relation to antibiotic practices, highlighting considerable patient safety and AS issues. Improved education, legislation, regulation and new best practice stewardship guidelines are urgently needed for online antibiotic suppliers.
Introduction
Antimicrobial stewardship (AMS) is recognized as the organizational or healthcare-system-wide approach to promoting and monitoring the judicious use of antimicrobials,1 such as antibiotics. Co-ordinated interventions within antibiotic stewardship (AS) programmes are designed to achieve optimal clinical outcomes whilst minimizing adverse events and antibiotic resistance.2 AS is a key priority within the UK3 and globally,4 as antimicrobial resistance (AMR) poses a profound threat to health security, healthcare quality and patient safety. The WHO global action plan for tackling AMR has specific objectives for international AS. These objectives include strengthening international regulations on the distribution, quality and use of antibiotics, with emphasis placed on those obtained through internet sales.4 Within the UK National Health Service (NHS), local antibiotic guidelines, a variety of hospital-based restrictive and persuasive interventions,5 community-based social norm feedback6 and national stewardship guidelines1,7 encourage judicious antibiotic prescribing. However, antibiotics may be acquired in much of the world without a prescription, despite this being illegal in many of the countries concerned.8–15 Within the UK, patient safety and current AS strategies may be threatened due to antibiotics being available to purchase online, without a prescription, from a variety of vendors globally.16 In 2013 a European survey reported that the use of the internet to resolve healthcare needs within the UK was modest.17 However, it is expected that the use of the internet within the UK for both consumer and healthcare needs will continue to rise based on the current trajectory.
Prescribing by healthcare professionals, all practices conducted within registered pharmacies and any advertisements for medicinal products are closely monitored and regulated within the UK. The General Medical Council (GMC) advises on remote and electronic prescribing decisions,18 and dentists, nurses, pharmacists, optometrists and midwives, who may also issue antibiotic prescriptions, have similar regulatory bodies. In Great Britain (GB), the General Pharmaceutical Council (GPhC) registers practising pharmacists as well as pharmacy premises and online pharmacies. Guidance for providing services online is also distributed by the Royal Pharmaceutical Society (RPS) in GB19 and by the Pharmaceutical Society of Northern Ireland (PSNI). The UK Medicines and Healthcare products Regulatory Agency (MHRA) also provides registration for online pharmacies, investigates web sites that are suspected of operating illegally and considers advertisements for prescription-only medicines (POMs), which are acceptable only on web sites whose content is directed at healthcare professionals.20 Formal MHRA registration for all online vendors selling medicines to UK consumers was mandated in 2015, with every web page legally required to display the EU common logo containing a hyperlink directing users to a list of registered online pharmacies.21 In contrast to the EU common logo, the GPhC logo is a voluntary scheme applicable only to pharmacies registered in GB.22
Currently, patients may obtain antibiotics online through legal registered pharmacy platforms, or through illegal web sites, which expose them to a variety of potential risks. These risks may include: no verbal or physical review prior to antibiotic supply; inappropriate choice, dose or duration; poor-quality medication; pressured antibiotic advertising; or payment information fraud. In November 2015 the Review on Antimicrobial Resistance, commissioned by the UK government, highlighted the risks of online antibiotic sales and emphasized the need for a safe, secure and controlled antibiotic supply chain.23 However, the extent of the associated problems is largely unknown.23
We report here an exploratory cross-sectional analysis of a representative sample of online pharmacies with the overarching objective being to improve understanding of the current state of online antibiotic sales in the UK. The specific aims of this cross-sectional analysis were: (i) to assess the quality and legality of online pharmacies identified (using registration status as a proxy indicator for quality and legality); (ii) to analyse the processes (whether prescriber-driven or consumer-driven) for purchasing an antibiotic online; and (iii) to identify any resulting AS or patient safety issues.
Methods
A multidisciplinary working group (A. H. H., S. E. B., M. G., L. S. P. M., C. C.), which included both healthcare professionals and academics with expertise in AS, agreed a study protocol and data collection tool by Delphi consensus. One researcher (S. E. B.) completed data collection based on the pre-agreed protocol using a computer for which the cached search history was cleared prior to the study.
Choice of search engine
The popularity of specific internet search engines will vary depending on the preference and geographical location of searchers. Google and Yahoo, widely recognized as two of the most popular search engines in the world, play a major role in how people address medical needs24 and were both used to reduce bias in the way that individual search engines may retrieve and rank results.25 Owing to varying degrees of overlap in the way these search engines present results,26 web sites that were duplicated were only included once. The Google search was completed first.
Choice of search term
Simple queries and keyword searches dominate when purchasing products online with searchers viewing fewer result pages.27 Consumer time-pressure and cognitive-resource limitations have been hypothesized to account for this.28 Search engine queries were therefore conducted with the search term ‘buy antibiotics online.’
Choice of sample size
In their default setting the search engines selected typically respond to queries with a ranked list of 10 web sites on the first page, with searchers being heavily influenced by the order in which they are presented.29 The first position in an internet search contributes to more traffic than the second and subsequent positions,29,30 with products or web sites at the top of a list being more likely to become part of a consumer’s consideration set.31 The first page of a Google search generates ∼92% of traffic from an average search; traffic drops by 95% when moving from the first to second page and by 78% and 58% for subsequent pages.30 When presented with options, consumers typically undergo a two-stage process by screening products or web sites, and subsequently reviewing a more relevant subset in detail before making a purchase decision.32 A sample size of 20, to include the first 10 unique web pages identified from each search engine that met the inclusion and exclusion criteria, was subsequently pre-determined.
Inclusion and exclusion criteria
Web sites were included if they were English-language vendors selling antibiotics online, for human use, to consumers within the UK. Web sites were excluded if they were advertisement links, primarily for veterinary medicine, did not ship to the UK, or were inactive when attempts were made to proceed to checkout. In some cases different uniform resource locator (URL) addresses were linked to a common stem vendor (CSV) selling antibiotics. Each CSV was included only once. The first 10 web sites from each search engine with a unique URL address, that fitted the criteria specified, were analysed in detail. Data were collected to meet the objectives, and the process for purchasing an antibiotic was followed until the point of payment. Purchasing an antibiotic was defined as a payment transaction.
The first objective was to assess the quality and legality of online pharmacies identified. Registration with the MHRA, evidenced by the presence of the mandatory EU common logo, was used as a proxy indicator of the quality and legality of the pharmacy. Evidence of accreditation and registration with the GPhC (or PSNI) was also recorded. All web sites displaying accreditation logos were cross-referenced with the relevant online register (MHRA and GPhC/PSNI) to ensure the validity of the logo displayed. Each web site was further studied to identify the location from where it was operating.
The second objective was to analyse the processes for purchasing an antibiotic online. Data were collected on prescription requirements and whether information for safe prescribing (allergies, comorbidities, pregnancy) was required prior to the purchase of an antibiotic. Web sites were thoroughly reviewed to identify statements on prescription requirements. All web pages specifying the sale of antibiotics were analysed in detail and the process for obtaining an antibiotic was followed up to the point of inputting payment information for each web site. In addition, the term ‘prescription’ was searched for and the ‘frequently asked questions’ section, or equivalent, was reviewed in detail for each online pharmacy included. Initial decisions regarding the choice of antibiotic were defined as being ‘prescriber-driven’ or ‘consumer-driven’. A prescriber-driven process was when the consumer was first directed through an online consultation after clicking on a specific ailment, and if appropriate, a prescription for an antibiotic was subsequently selected by the prescriber. A consumer-driven process was when the consumer initiated the antibiotic purchase by first selecting an antibiotic of their choice for placement in their ‘shopping basket’. Data were also collected on whether any safety information on adverse effects was provided to patients during the online process, whether oral or intravenous (iv) antibiotics were available for purchase, the standard delivery time to the UK, and whether an express delivery option was available. Each web site was explored in detail and data were collected on the name of all antibiotics that appeared available for purchase online.
The third objective was to identify any resulting AS and patient safety issues; this was met through integration of the above findings.
After completion of data collection, all vendors identified as illegally selling antibiotics to patients within the UK were reported to the MHRA. Ethics approval was not required for this study of open-source data.
Results
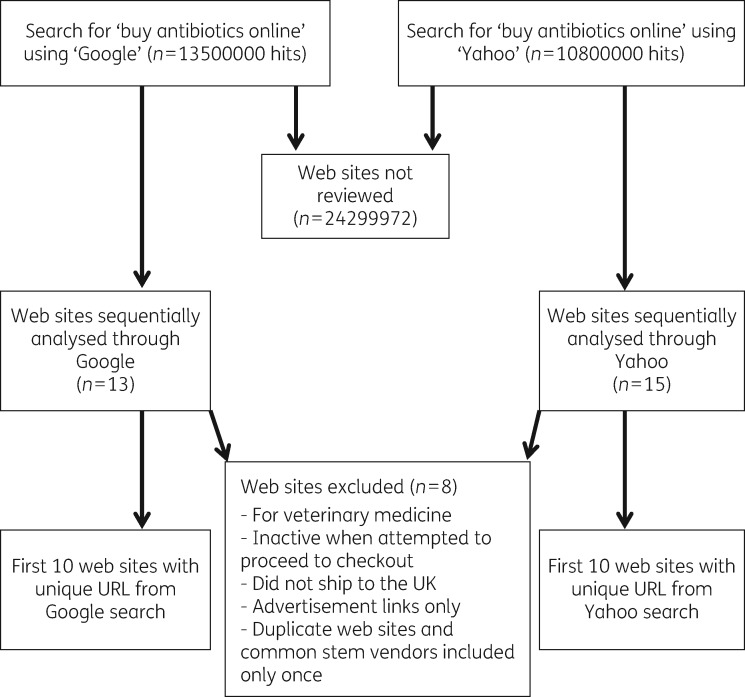
Results of the searches performed on 28 February 2016 are shown in Figure 1. Twenty-eight web sites were screened. Of the web sites analysed in detail (n = 20), five (25%) showed evidence of operating from within GB. All five displayed appropriate evidence of registration with both the MHRA and the GPhC. Table 1 shows the locations and registration status of the 15 other web sites analysed.
Figure 1.
Flow diagram displaying results from a search performed on 28 February 2016.
Table 1.
Online pharmacies selling antibiotics to consumers within the UK
| Characteristic | Number of online pharmacies (n = 20) |
|---|---|
| Registered with MHRA and GPhC | |
| yes | 5 (25%) |
| no | 15 (75%) |
| Location operating from | |
| Great Britain | 5 (25%)a |
| unclear | 10 (50%) |
| India | 3 (15%) |
| Cyprus | 2 (10%) |
All those operating from within Great Britain were registered with both the MHRA and GPhC.
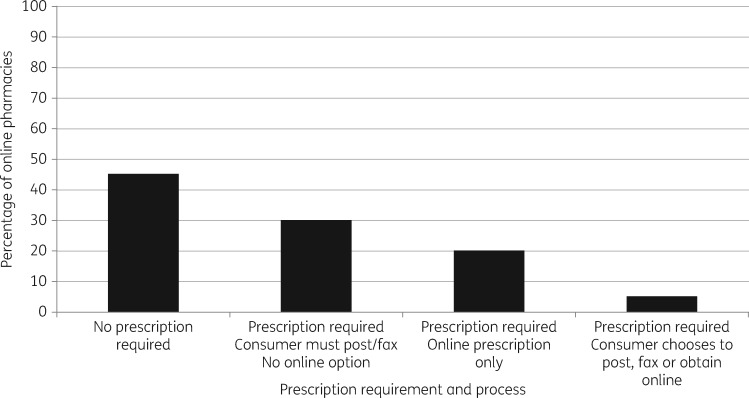
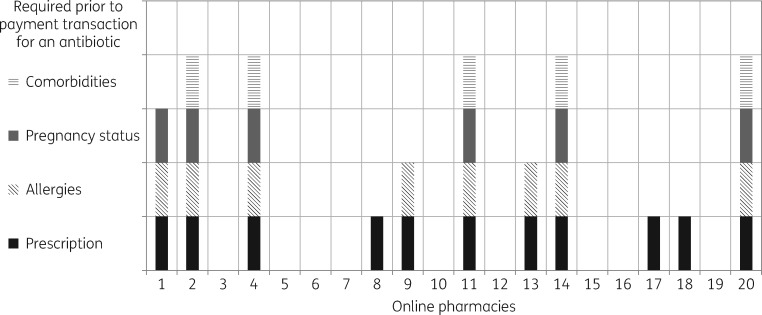
Figure 2 summarizes the prescription requirements and different processes for providing a prescription to the vendor prior to online purchase. All five GB-based online pharmacies required a prescription before an antibiotic would be delivered. For 16 (80%) web sites, decisions regarding antibiotic choice, dose and duration were initially consumer driven, with only 4 (20%) online pharmacies utilizing a prescriber-driven pathway (Table 2). All four of these were based in GB and registered with both the MHRA and GPhC. A further GB-based pharmacy, registered with both the MHRA and GPhC, permitted a consumer-driven process prior to the point of payment, through which consumers were directed to an antibiotic choice depending on the syndrome they clicked on the web page. However, despite initially adopting a consumer-driven approach, this pharmacy described a pathway whereby a health questionnaire would be made available after payment was received to allow a doctor to assess an individual’s suitability for an antibiotic. Six web sites (30%) did not issue online prescriptions and instead required that a prescription be faxed or posted before an antibiotic would be delivered. One of these web sites did not specify the location from where they were operating and it was not clear whether an address would have been provided to allow a consumer to post the prescription after a payment transaction. Figure 3 correlates the requirement for prescription through each individual online pharmacy with the information that was requested, prior to antibiotic purchase. All pharmacies offered oral antibiotics; one non-EU based web site also advertised iv antibiotics for sale. The cumulative frequency of all types of antibiotic available from the 20 pharmacies is presented in Table 3. Standard delivery time to the UK varied from 1 to 14 days (mean 10.5, median 14, IQR 6.75–14 days). Thirteen web sites (65%) had a standard delivery time of 14 days. An express option was available on request for all 20 web sites.
Figure 2.
Prescription requirements and processes for obtaining an antibiotic among sampled online pharmacies (n = 20).
Table 2.
Processes for obtaining an antibiotic online from within the UK
| Characteristic | Number of online pharmacies (n = 20) |
|---|---|
| Consumer-driven versus prescriber- driven antibiotic choice | |
| consumer-driven choice of drug, dose and quantity | 16 (80%) |
| prescriber-driven choice of drug, dose and quantity | 4 (20%) |
| Use of an online health questionnaire during purchasing | |
| yes | 6 (30%) |
| no | 14 (70%) |
| Safety information provided on contraindications or side effects prior to purchasing | |
| yes | 14 (70%) |
| no | 6 (30%) |
Figure 3.
Prescription and information requirements for obtaining an antibiotic among the top 20 online pharmacies analysed.
Table 3.
Cumulative frequency of antibiotics available from online pharmacies analysed (n = 20)
| Antibiotic class and agent | Number of online pharmacies that made clear on web site they were able to supply (n = 20) |
|---|---|
| Penicillins | |
| penicillin | 3 |
| amoxicillin | 17 |
| ampicillin | 14 |
| flucloxacillin | 3 |
| co-amoxiclav | 16 |
| Tetracyclines | |
| doxycycline | 19 |
| lymecycline | 1 |
| oxytetracycline | 8 |
| minocycline | 14 |
| tetracycline | 13 |
| Macrolides | |
| clarithromycin | 12 |
| erythromycin | 15 |
| azithromycin | 19 |
| roxithromycin | 9 |
| Cephalosporins | |
| cefalexin | 13 |
| cefuroxime | 10 |
| cefadroxil | 13 |
| cefixime | 16 |
| cefpodoxime | 13 |
| cefaclor | 9 |
| cefdinir | 10 |
| cefepime | 3 |
| cefprozil | 2 |
| Carbapenems | |
| faropenem | 2 |
| Quinolones | |
| ciprofloxacin | 15 |
| ofloxacin | 17 |
| levofloxacin | 9 |
| moxifloxacin | 9 |
| norfloxacin | 11 |
| sparfloxacin | 4 |
| nalidixic acid | 3 |
| Sulphonamides and trimethoprim | |
| co-trimoxazole | 10 |
| trimethoprim | 8 |
| Lincosamides | |
| clindamycin | 12 |
| lincomycin | 7 |
| Others | |
| nitrofurantoin | 14 |
| chloramphenicol | 11 |
| linezolid | 12 |
| metronidazole | 14 |
| rifaximin | 4 |
| rifampicin | 1 |
| cycloserine | 4 |
| ethambutol | 4 |
| ethionamide | 5 |
| pyrazinamide | 2 |
Discussion
This study raises several important issues regarding AS and patient safety with online pharmacies. Concerning heterogeneity was observed in the legality and quality of online pharmacies, the processes for obtaining an antibiotic, and in other safety procedures prior to the point of payment.
Assessing the quality and legality of online pharmacies
A similar study, carried out by Mainous et al.16 in the USA, found that 36.2% of 138 online pharmacies sold antibiotics without prescription, a figure slightly below the 45% identified in our sample. The relative paucity of published literature around selling antibiotics via the internet contrasts with numerous studies relating to other classes of medication. A systematic review published in 2011 assessed 193 relevant studies and aimed to determine the characteristics and quality of online pharmacies.33 The authors reported a wide variety of prescription-only medicines available with inconsistent prescription requirements and that the presence of at least one quality certification ranged from between 12% and 28% depending on the study in question.33 Among the 20 online pharmacies analysed in the present study, those that were operating from within the UK (25%) evidenced registration with both the MHRA and the GPhC. Confirming the registration status was facilitated by a user-friendly hyperlink, enabling potential consumers to check the legitimacy of a web site. However, this mechanism to reassure the public on quality and safety relies on consumers understanding what the logos represent. A concerning number of pharmacies within our sample (75%) lacked evidence of the registration that is required by current UK and European legislation. This may be because some of the identified pharmacies were operating outside of Europe, with three based in India. There was no information provided on where 10 (50%) of the pharmacies were operating from. Regardless of where they are based, vendors providing antibiotics to patients within the UK are subject to UK legislation. While non-prescription antibiotics are recognized as an important means for access in resource-poor settings,34 this is unlikely to be a concern within the UK, where healthcare is free at the point of need. This study raises concerns on the effectiveness of current legislation, licensing and regulation for platforms selling antibiotics via the internet to UK consumers.
The processes for obtaining antibiotics online from within the UK
We have identified a variety of processes for obtaining antibiotics online, including heterogeneity in the safety assessments made to determine whether antibiotics were required, and if so, the most appropriate and safe antibiotic choice, dose and duration. Overall, 16 (80%) of the pharmacies reviewed required that consumers directly select an antibiotic before proceeding to checkout. Health questionnaires were utilized in only six (30%) online pharmacies. These lacked consistency and often came subsequent to a consumer-driven choice on requirement and type of antibiotic. A systematic review of online pharmacies reported use of an online questionnaire during the purchasing process to be between 10% and 81%, depending on the study in question.33 We observed variation in the information sought via health questionnaires, and the methods used to collect this information. Some questionnaires comprised drop-down boxes, some free-text boxes and others a mixture of both. Additionally, it was not clear whether there would be feedback from the prescriber/dispenser if a mismatch was subsequently identified between the consumer-selected choice and the most appropriate course of action, taking into account the information in the questionnaire.
Opinion is mixed regarding whether antibiotics should be available without prescription.35,36 However, in line with current UK legal requirements37 and National Institute for Health and Care Excellence (NICE) guidance for AS,1 decision processes should be shared and crucially underpinned by prescriber-driven rationale. In addition, a uniform, consistent and thorough health questionnaire should be mandatory. This tool should be developed through collaboration with key UK stakeholders to ensure that online patient safety and antibiotic stewardship are consistent with national best practice. Key stakeholders may include representatives from PHE, the GMC, GPhC, RPS, PSNI, MHRA, Royal College of General Practitioners, NICE, the Department of Health Advisory Committee for Antimicrobial Resistance and Healthcare-Associated Infection, patient representatives and the public.
We identified a median delivery time of 14 days, representing a potential risk to patients acquiring antibiotics to treat an acute infection. Mainous et al.16 also analysed shipping times for antibiotic delivery. These authors suggested, based on similar results to our findings, that the prolonged ‘interval between ordering and receiving the medication suggest that these transactions will likely be used by individuals storing the drugs for future self-diagnosis and treatment or for sale’.
Consumers accessing health web sites have relatively high levels of digital health literacy,17 but there remains a need for a formal assessment of web sites to ensure uniform standards for user-friendly platforms and readability, and for important health messages to be conveyed. If antibiotics are to be sold online, advice to see a healthcare provider promptly if an adverse reaction occurs or if presenting symptoms do not improve must be at the forefront of the antibiotic purchasing process.
Additional concerns for antimicrobial stewardship and patient safety
Antibiotics were advertised directly to patients on several web sites, and although direct-to-consumer marketing may be permitted in other healthcare settings, this practice is not congruent with current MHRA regulations.20 The prevalence of antibiotic advertising was not a primary outcome measure in this study, but is raised as a concern on both ethical and safety grounds. Given the significant volume of funding and effort to develop effective strategies for antibiotic stewardship in the UK, further research should be conducted to determine the frequency with which this advertising occurs, the effect it has on patients’ expectation for antibiotics, and subsequent antibiotic-seeking behaviours. Recognition that inappropriate antibiotic prescribing is correlated with public expectation has been the focus of several educational campaigns led by the UK Department of Health and PHE.7,38 Technical solutions that prevent advertisement links should be implemented, with consideration of financial penalties for web sites who are in breach of MHRA regulations or who are supplying antibiotics outwith national stewardship guidelines, which are ‘Start Smart Then Focus’, ‘TARGET’ and the NICE Antimicrobial Stewardship guideline, within England.1,39,40 Responsibility also exists for individual prescribers to ensure they conform to nationally accepted best practice recommendations for antimicrobial stewardship, given the emphasis placed on this in recent NICE guidance.1
This research raises a question on the potential unintended consequences of stewardship initiatives that improve and reduce antibiotic prescribing through traditional routes.5 If the risks of inappropriate antibiotic use are not conveyed to patients there is concern that, as consumers, they may seek to obtain antibiotics from an alternative source. At present there is no way to estimate the acquisition of antibiotics through legal or illegal online pharmacies. Education and public awareness campaigns should encourage prescribers to identify patients’ ideas, concerns and expectations, whilst fully explaining why they do not need an antibiotic. Although the gains of this strategy have been modest to date, the prospect that a patient may seek to obtain an antibiotic from an alternative source, such as online, reinforces its importance. Practitioners should seek to address the issues surrounding obtaining medicines online with those felt most likely to engage in this behaviour, although further research is urgently required to understand who they may be. It seems likely that they represent a group that is hard to reach through traditional healthcare, given their preference to seek healthcare through non-traditional routes. A snowball approach that actively seeks to engage online healthcare communities may prove useful to identify these consumers. Facilitated small group or one-to-one sessions using formal qualitative behavioural research methods, aiming to understand how to engage their desire for self-management in a safe manner, is required. In addition to these strategies, the issues surrounding obtaining a variety of medicines online, including antibiotics, should be integrated into the curricula for all prescribers in order to raise awareness.
Strengths and limitations
This is the first analysis looking specifically at issues pertaining to the availability of antibiotics online to patients within the UK. Web sites were identified using a method felt to be widely representative of how consumers search for and buy products online. By using two popular search engines we identified a broad range of relevant web sites.
This study had limitations inherent to the constantly evolving online consumer domain. A Google or Yahoo search is not identical when different browsers are used for the same search, or when the same search is performed at different times. Different consumers may be faced with different purchasing options. However, it is widely accepted that the most popular sites will be placed higher on the result list for all searchers. Illegal vendors may also masquerade, and change their domain name frequently in order to remain operational. There is a possibility that if this occurred, the same vendor may have been included twice, although this is unlikely given the cross-sectional nature of the study. In addition, one researcher analysed all web sites and would have most likely noticed any striking similarities among them.
When antibiotics are dispensed in person, an opportunity to ensure patient safety exists when handing over a prescription. Actually purchasing antibiotics was beyond the scope of our analysis, and in not proceeding to payment we may have missed any patient safety prompts that occur only after a monetary transaction. Statements on web sites were sought to determine whether antibiotic prescriptions were required. However, by not proceeding through a payment transaction we cannot be certain whether web sites that made no statement on prescription requirement would subsequently refuse to process an order without a valid prescription, or whether web sites that had statements on prescription requirement would subsequently dispense antibiotics without a valid prescription. We did not explore whether or not information was sought on concomitant medications that may affect antibiotic suitability; collecting this additional data would be a valuable focus for future research.
Finally, the URL pages we identified may no longer be operational. All vendors identified as illegally selling antibiotics to patients within the UK were reported directly to the MHRA,41 who promptly responded by e-mail stating that all concerns had been passed to the Enforcement Team.
Conclusions
The way patients interact with healthcare is constantly evolving and shifts in consumer behaviour over the past decade mean increasing numbers are now opting to purchase products online. The availability of antibiotics online, or products being sold as such, poses a serious threat to patient safety and national antibiotic stewardship initiatives.
We make several key recommendations for stakeholders in the UK. GMC and RPS guidance for prescribers should be updated to reflect changes in healthcare-seeking behaviour, the increasing demand for remote and online prescribing, and the importance of antibiotic stewardship in this environment. Display of the GPhC/PSNI logo should be made mandatory in line with the EU common logo. A best practice toolkit based on current NICE guidelines for antibiotic stewardship with a standardized health questionnaire developed by key stakeholders is recommended if the sale of antibiotics online is to continue in the UK. Emphasis should be placed on prescriber responsibility for follow-up to ensure infective symptoms improve and to monitor antibiotic-associated adverse events in line with current NICE guidance. We also evidence the urgent need to improve the surveillance of online antibiotic sales. Antibiotic distribution through online channels should be mandatory to report, in line with antibiotic consumption data for the UK NHS. Engaging collaboration between international policy makers, governmental law enforcement agencies, pharmaceutical companies, individual prescribers and consumers will be a priority. In order to promote patient safety and preserve antibiotic therapy, an efficient and operational multidisciplinary taskforce is needed to address the issues we have identified.
Acknowledgements
The authors are all affiliated with the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE). The authors also acknowledge the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre, the NIHR Imperial Patient Safety Translational Research Centre and Imperial College NHS Trust.
Funding
This research was partially funded by the National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England and Imperial College Healthcare NHS Trust. This work was also supported by funding from the Imperial National Institute for Health Research Biomedical Research Centre. S. E. B. is a National Institute for Health Research Academic Clinical Fellow. E. C.-S. has received an Early Career Research Fellowship from the Antimicrobial Research Collaborative at Imperial College London, and acknowledges the support of the Florence Nightingale Foundation.
Transparency declarations
All authors have completed the ICMJE uniform disclosure form and declare: A. H. H. has consulted for bioMérieux in 2013 and 2014. L. S. P. M. has consulted for bioMérieux in 2014, and DNA electronics in 2015. M. G. reports attending advisory boards for Pfizer and MSD, in addition to receiving educational travel and speaker grants from Astellas Pharmaceuticals and Sanofi. The remaining authors have no conflicts of interest to declare.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England. The authors also acknowledge the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre, the NIHR Imperial Patient Safety Translational Research Centre and Imperial College NHS Trust. Sara Boyd is an NIHR Academic Clinical Fellow.
References
- 1. The National Institute for Health and Care Excellence (NICE). Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use. Guideline NG15 London: NICE, 2015. https://www.nice.org.uk/guidance/ng15/chapter/1-Recommendations.
- 2. Fishman N. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33: 322–7. [DOI] [PubMed] [Google Scholar]
- 3. Department of Health, Department for Environment Food and Rural Affairs. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf.
- 4. WHO. Global Action Plan on Antimicrobial Resistance. Geneva: WHO, 2015. http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua=1. [Google Scholar]
- 5. Davey P, Brown E, Fenelon L. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; issue 4: CD003543. [DOI] [PubMed] [Google Scholar]
- 6. Hallsworth M, Chadborn T, Sallis A. et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016; 387: 1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNulty CAM. European Antibiotic Awareness Day 2012: general practitioners encouraged to TARGET antibiotics through guidance, education and tools. J Antimicrob Chemother 2012; 67: 2543–6. [DOI] [PubMed] [Google Scholar]
- 8. Esimone CO, Nworu CS, Udeogaranya OP.. Utilization of antimicrobial agents with and without prescription by out-patients in selected pharmacies in South-eastern Nigeria. Pharm World Sci 2007; 29: 655–60. [DOI] [PubMed] [Google Scholar]
- 9. Sturm AW, van der Pol R, Smits AJ. et al. Over-the-counter availability of antimicrobial agents, self-medication and patterns of resistance in Karachi, Pakistan. J Antimicrob Chemother 1997; 39: 543–7. [DOI] [PubMed] [Google Scholar]
- 10. Grigoryan L, Haaijer-Ruskamp FM, Burgerhof JGM. et al. Self-medication with antimicrobial drugs in Europe. Emerg Infect Dis 2006; 12: 452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llor C, Cots JM.. The sale of antibiotics without prescription in pharmacies in Catalonia, Spain. Clin Infect Dis 2009; 48: 1345–9. [DOI] [PubMed] [Google Scholar]
- 12. Rathnakar UP, Sharma NK, Garg R. et al. A study on the sale of antimicrobial agents without prescriptions in pharmacies in an urban area in south India. J Clin Diagnostic Res 2012; 6: 951–4. [Google Scholar]
- 13. Nga DTT, Chuc NTK, Hoa NP. et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol 2014; 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zapata-Cachafeiro M, González-González C, Váquez-Lago JM. et al. Determinants of antibiotic dispensing without a medical prescription: a cross-sectional study in the north of Spain. J Antimicrob Chemother 2014; 69: 3156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mainous AG, Cheng AY, Garr RC. et al. Nonprescribed antimicrobial drugs in Latino community, South Carolina. Emerg Infect Dis 2005; 11: 883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mainous AG, Everett CJ, Post RE. et al. Availability of antibiotics for purchase without a prescription on the internet. Ann Fam Med 2009; 7: 431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abadie F, Lluch M, Lupiañez F. et al. Citizens and ICT for Health in 14 European Countries: Results from an Online Panel Luxembourg: Joint Research Centre of the European Commission, 2013. http://ftp.jrc.es/EURdoc/JRC71142.pdf.
- 18. General Medical Council (GMC). Prescribing Guidance: Remote Prescribing via Telephone, Video-link or Online London: General Medical Council, 2013. http://www.gmc-uk.org/mobile/14326.
- 19. Royal Pharmaceutical Society. Medicines, Ethics and Practice. The Professional Guide for Pharmacists. 39th edn London, 2015. [Google Scholar]
- 20. Medicines and Healthcare products Regulatory Agency (MHRA). The Blue Guide: Advertising and Promotion of Medicines in the UK .3rd edn London, 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/376398/Blue_Guide.pdf. [Google Scholar]
- 21. Medicines and Healthcare products Regulatory Agency (MHRA). New Mandatory Logo for Selling Medicines Online—Press Release https://www.gov.uk/government/news/new-mandatory-logo-for-selling-medicines-online.
- 22. General Pharmaceutical Council. Internet Pharmacy. http://www.pharmacyregulation.org/registration/internet-pharmacy.
- 23. O’Neill J. Safe, Secure and Controlled: Managing the Supply Chain of Antimicrobials . The Review on Antimicrobial Resistance. London, 2015. http://amr-review.org/sites/default/files/SafeSecureandControlledShortPaper.pdf. [Google Scholar]
- 24. Wang L, Wang J, Wang M. et al. Using Internet search engines to obtain medical information: a comparative study. J Med Internet Res 2012; 14: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mowshowitz A, Kawaguchi A.. Measuring search engine bias. Inf Process Manag 2005; 41: 1193–205. [Google Scholar]
- 26. Spink A, Jansen BJ, Blakely C. et al. A study of results overlap and uniqueness among major Web search engines. Inf Process Manag 2006; 42: 1379–91. [Google Scholar]
- 27. Jansen BJ, Spink A.. How are we searching the World Wide Web? A comparison of nine search engine transaction logs. Inf Process Manag 2006; 42: 248–63. [Google Scholar]
- 28. Diehl K. When two rights make a wrong: searching too much in ordered environments. J Mark Res 2005; 42: 313–22. [Google Scholar]
- 29. Pan B, Hembrooke H, Joachims T. et al. In Google we trust: users’ decisions on rank, position, and relevance. J Comput Commun 2007; 12: 801–23. [Google Scholar]
- 30.Chitika. Chitika Insights: The Value of Google Result Positioning 2013. http://info.chitika.com/uploads/4/9/2/1/49215843/chitikainsights-valueofgoogleresultspositioning.pdf.
- 31. Kleinmuntz DN, Schkade DA.. Information displays and decision processes. Psychol Sci 1993; 4: 221–7. [Google Scholar]
- 32. Häubl G, Trifts V.. Consumer decision making in online shopping environments: the effects of interactive decision aids. Mark Sci 2000; 19: 4–21. [Google Scholar]
- 33. Orizio G, Merla A, Schulz PJ. et al. Quality of online pharmacies and websites selling prescription drugs: a systematic review. J Med Internet Res 2011; 13: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendelson M, Røttingen J-A, Gopinathan U. et al. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2015; 387: 188–98. [DOI] [PubMed] [Google Scholar]
- 35. Knox K. Women should be able to get antibiotics for urinary tract infection without a prescription. BMJ 2015; 351: h3441.. [DOI] [PubMed] [Google Scholar]
- 36. Llor C. Antibiotics without prescription: more cons than pros. BMJ 2015; 351: h4202.. [DOI] [PubMed] [Google Scholar]
- 37.The Government of the United Kingdom. The Medicines Act. London, 1968. http://www.legislation.gov.uk/ukpga/1968/67/pdfs/ukpga_19680067_en.pdf. [Google Scholar]
- 38. Stockley JM. European Antibiotic Awareness Day 2012: getting smart about antibiotics, a public-professional partnership. J Infect 2012; 65: 377–9. [DOI] [PubMed] [Google Scholar]
- 39.Public Health England. Start Smart—Then Focus Antimicrobial Stewardship Toolkit for English Hospitals London, 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/417032/Start_Smart_Then_Focus_FINAL.PDF.
- 40. Royal College of General Practitioners. TARGET Antibiotics Toolkit London, 2012. http://www.rcgp.org.uk/clinical-and-research/toolkits/target-antibiotics-toolkit.aspx.
- 41.Medicines and Healthcare Products Regulatory Agency. Register of Authorised Online Sellers of Medicines.http://medicine-seller-register.mhra.gov.uk/.