Abstract
Background
The pharmacokinetic/pharmacodynamic (PK/PD) relationship for polymyxin B against Klebsiella pneumoniae infections is not known.
Methods
Dose-fractionation studies with subcutaneous polymyxin B were conducted in neutropenic mice in which infection with three strains of K. pneumoniae had been produced in thighs or lungs. Dosing (thigh infection 0.5–120 mg/kg/day; lung infection 5–120 mg/kg/day) commenced 2 h after inoculation, and bacterial burden was measured 24 h later. Plasma exposure measures for unbound polymyxin B were from population pharmacokinetic analysis of single doses and plasma protein binding by ultracentrifugation. The inhibitory sigmoid dose–effect model was employed to determine the relationship between exposure and efficacy. Antibacterial activities of polymyxin B and colistin against thigh infection were compared at equimolar doses generating exposures resulting in maximal antibacterial activity.
Results
The pharmacokinetics of polymyxin B were well described by a model comprising parallel linear and saturable pathways for absorption and elimination. Plasma binding of polymyxin B was constant (P > 0.05) over the range ∼0.9–37 mg/L; average (±SD) percentage bound was 91.4 ± 1.65. In thigh infection, antibacterial effect was well correlated with fAUC/MIC (R2 = 0.89). Target values of fAUC/MIC for stasis and 1 log10 kill were 1.22–13.5 and 3.72–28.0, respectively; 2 log10 kill was not achieved for any strain, even at the highest tolerated dose. There was no difference (P > 0.05) in antibacterial activity between polymyxin B and colistin with equimolar doses. It was not possible to achieve stasis in lung infection, even at the highest dose tolerated by mice.
Conclusions
The results will assist in the design of optimized dosage regimens of polymyxin B.
Introduction
Polymyxin B and colistin are now widely used for the treatment of infections caused by MDR Gram-negative pathogens such as Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. The two polymyxins have very similar chemical structures, and their MICs are within one 2-fold dilution for more than ∼98% of isolates.1,2 However, greater awareness that the administration of colistin in the form of an inactive prodrug (colistimethate) compared with direct administration of polymyxin B may lead to potentially important differences in clinical pharmacological profiles has led to increased interest in use of polymyxin B.3
Studies over the last few years have increased understanding of the pharmacokinetic/pharmacodynamic (PK/PD) driver of activity of the polymyxins against some important Gram-negative pathogens. Dose-fractionation studies of colistin against P. aeruginosa in a dynamic in vitro infection model4 and against P. aeruginosa and A. baumannii following subcutaneous administration in neutropenic mouse thigh and lung infection models5 indicated that antibacterial activity against these species correlated most closely with the ratio of the area under the unbound concentration–time curve to the MIC (fAUC/MIC). This PK/PD index has also been identified as the best predictor of antibacterial effect against P. aeruginosa following intraperitoneal6 or intratracheal7 administration of colistin in a neutropenic murine lung infection model. An in silico PK/PD model for colistin against P. aeruginosa in static in vitro time–kill studies when used for in silico replication of a dose-fractionation study in mice indicated fAUC/MIC as the PK/PD index most predictive of antibacterial activity.8 This PK/PD index also appeared relevant for polymyxin B against P. aeruginosa based on the time–kill profiles that were observed with three fractionated dosing regimens in a hollow-fibre infection model.9 Clearly, almost all the PK/PD information available relates to colistin against P. aeruginosa and A. baumannii. There is a dearth of information from dynamic PK/PD systems for polymyxin B against K. pneumoniae. Here, we describe such studies conducted in neutropenic mouse thigh and lung infection models.
Materials and methods
Chemicals and bacterial strains
Polymyxin B sulphate (Lot BCBF8382V; ≥6000 units/mg) and colistin sulphate (lot SLBD8306V; ≥15000 units/mg), both from Sigma–Aldrich (St Louis, MO, USA) were used to prepare aqueous solutions for administration to mice. Solutions were freshly prepared before each experiment, sterilized by passage through a 0.2 μm pore-size syringe filter and stored at 4 °C prior to use. Three bacterial strains were utilized: K. pneumoniae reference strain ATCC BAA-2146 from the American Type Culture Collection (Manassas, VA, USA) and two clinical isolates (FADDI-KP042 and FADDI-KP032). Polymyxin B MICs, determined by broth microdilution in untreated polystyrene trays in the absence of polysorbate 80 in accordance with the joint EUCAST and CLSI recommendations,10 were 0.5 mg/L for all strains. The colistin MIC was 0.25 mg/L for ATCC BAA-2146, 0.5 mg/L for FADDI-KP042 and 1 mg/L for FADDI-KP032. Prior to each experiment, strains were subcultured onto nutrient agar (Media Preparation Unit, The University of Melbourne, Parkville, Australia) and incubated overnight at 37 °C. A colony was then selected and grown overnight in 20 mL of cation-adjusted Mueller–Hinton broth (Oxoid, Hampshire, UK). Early logarithmic-phase growth was subsequently prepared.
Neutropenic murine thigh and lung infection models
Animal experiments were approved by the institutional animal ethics committee (approval numbers VCPA.2008.09 and MIPS.2012.47) and animals were maintained in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Eight-week-old, specific-pathogen-free female Swiss mice (24–30 g) were obtained from Monash Animal Services (Clayton, Victoria, Australia) and were fed, housed and rendered neutropenic by administration of cyclophosphamide, as described previously.5 Infections in thighs were initiated by intramuscular injection (50 μL) of the inoculum and in lungs by intratracheal aerosolization (25 μL) of the inoculum sprayed directly into the trachea above the carina using a MicroSprayer® Aerosolizer (model IA-1C; Penn-Century, Philadelphia, PA, USA).5 The inoculum was ∼105 and 106 cfu for the thigh and lung infection model, respectively.
Pharmacokinetics of polymyxin B in neutropenic infected mice
The PK of polymyxin B was determined following single-dose administration of 2, 4, 8, 16 and 32 mg/kg of polymyxin B to neutropenic thigh-infected mice. Plasma samples were collected from three or four mice at each of multiple times up to 12 h following administration; the sampling schedule varied somewhat across the five doses to maximize the yield of information. The protein binding of polymyxin B in pooled plasma collected from infected neutropenic mice was determined by ultracentrifugation using previously described conditions and procedures to minimize adsorption of polymyxin B in protein-free supernatant samples.5 Binding was determined in drug-free plasma that had been spiked to achieve 10 polymyxin B concentrations across the range ∼0.9–37 mg/L, encompassing the relevant range of total plasma concentrations in the single-dose PK studies (described above) and the dose fractionation PK/PD studies (described below). Concentrations of polymyxin B in samples of supernatant obtained by ultracentrifugation and whole plasma were quantified as before.5,11
Pharmacokinetics/pharmacodynamics of polymyxin B in thigh and lung infection models
Treatment with subcutaneously administered polymyxin B sulphate (daily dose range 0.5–120 mg/kg in the thigh infection model and 5–120 mg/kg in the lung infection model) commenced 2 h following inoculation. The maximum dose tolerated by mice was 120 mg/kg per day. The following dose-fractionated regimens were used against strain FADDI-KP032 in the thigh infection model: once-daily administration of 0.5, 10, 20, 30 and 45 mg/kg; 12 hourly administration of 5, 10, 15, 22.5, 30 and 45 mg/kg; 8 hourly administration of 0.83, 1.67, 5, 3.33, 6.67, 7.5, 10, 15, 20, 25, 30 and 40 mg/kg; and 4 hourly administration of 1.67, 3.33, 5, 7.5, 10, and 15 mg/kg. In the remaining PK/PD studies the daily doses were divided equally and administered at 8 hourly intervals. Bacterial burdens in lungs or thighs (dependent on the model) were determined at 2 h after inoculation (untreated controls) and 24 h later (untreated controls and polymyxin B-treated mice), as described previously.5
For strains FADDI-KP032 and ATCC BAA-2146, the antibacterial effects of polymyxin B and colistin were compared in the thigh infection model. For FADDI-KP032, equimolar daily doses of each polymyxin were studied at four dose levels (polymyxin B sulphate at 22.5, 45, 90 and 120 mg/kg/24 h, corresponding to colistin sulphate at 21.9, 43.9, 87.7 and 117.1 mg/kg/24 h; n = 4 for each treatment). For ATCC BAA-2146, the comparison was conducted with the first three equimolar dose levels. For each polymyxin, the daily dose was divided equally and administered 8 hourly.
Pharmacokinetic/pharmacodynamic analyses
The plasma polymyxin B concentration versus time data from the single-dose studies (spanning a 16-fold range in doses) were subjected to population PK analysis using S-ADAPT (version 1.57) with importance sampling (pmethod = 4) and SADAPT-TRAN.12–14 Briefly, models with one, two and three disposition compartments, and linear, saturable and parallel linear and saturable elimination were evaluated. Models with linear and saturable absorption, with and without absorption lag compartments, and with parallel and sequential absorption processes were tested. The inter-individual variability of the PK parameters was assumed to be log-normally distributed. Proportional and combined additive and proportional error models were explored. Standard goodness-of-fit plots, visual predictive checks and the normalized prediction distribution error were utilized to evaluate model performance.
The parameters determined in the population PK analysis were used together with the fraction of polymyxin B unbound in plasma and the MIC of the bacterial strains to determine values of each of the PK/PD indices for unbound drug (i.e. fAUC/MIC, fCmax/MIC and fT>MIC) corresponding to each of the regimens in the dose-ranging PK/PD studies. This was performed by using the developed population PK model to simulate the unbound polymyxin B concentration–time profiles for each of the regimens in the dose-ranging PK/PD studies. The simulations and calculation of the values of the PK/PD indices were conducted using Berkeley Madonna (version 8.3.23.0). Subsequently, the value of these indices for each dosage regimen and the corresponding bacterial burden in mouse tissue at 24 h after initiation of polymyxin B treatment in the mice were subjected to PK/PD analysis by use of the inhibitory sigmoid dose–effect model estimated in NONMEM (version 7).5
Results and discussion
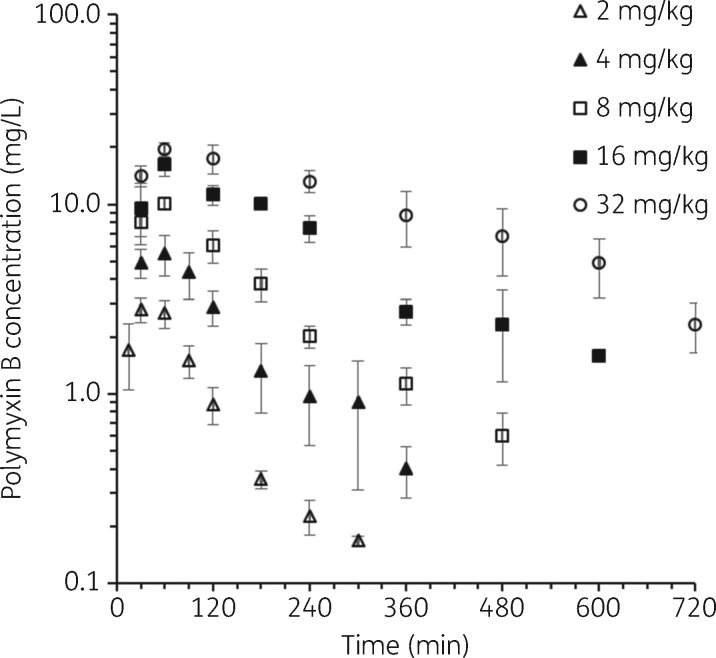
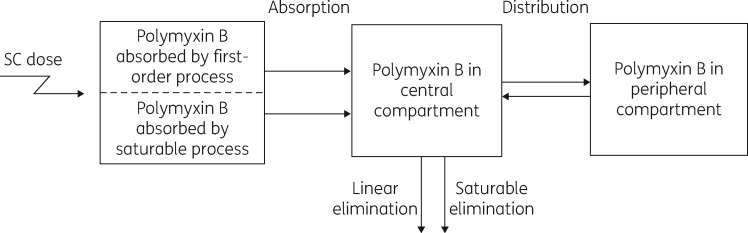
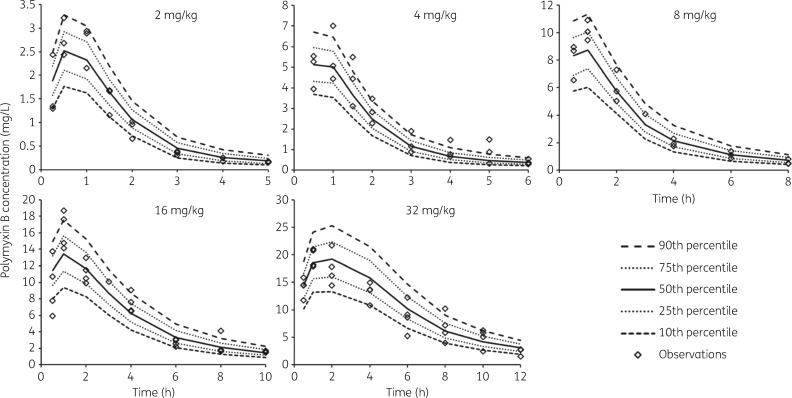
There has been only a small amount of information available on the PK of polymyxin B in mice. He et al.15 reported data from a study involving a single intravenous dose of 3 mg/kg, and Bowers et al.16 used a single 10 mg/kg intraperitoneal dose; plasma samples were collected for 6 and 4 h, respectively. To minimize animal numbers we have conducted studies in thigh-infected mice and assumed the PK of polymyxin B does not differ between thigh- and lung-infected animals. Previous studies have shown that the PK of ceftazidime and avibactam in thigh- and lung-infected mice did not differ between the two infection sites.17 The total plasma polymyxin B concentration versus time profiles from the single-dose PK studies in neutropenic infected mice in the present study are shown in Figure 1, and the PK parameter estimates from the population PK modelling are in Table 1. Close examination of the profiles indicates some degree of non-linearity in the PK across the 2–32 mg/kg range of polymyxin B subcutaneous doses. Non-linearity has also been observed for colistin following single subcutaneous doses spanning a more limited range (10–40 mg/kg) in neutropenic infected mice.5 In the current study with polymyxin B, various approaches to accommodate non-linearity were investigated in the population PK analysis. The superior model incorporated parallel linear and saturable pathways for both absorption from the subcutaneous injection site and elimination from the body (Figure 2). All parameters in the final model were required to describe the PK profiles. The visual predictive checks demonstrate excellent predictive performance of the model across the studied dose range (Figure 3).
Figure 1.
Total plasma polymyxin B concentration versus time after administration of single subcutaneous doses of 2, 4, 8, 16 or 32 mg/kg polymyxin B in neutropenic infected mice. Each symbol represents the mean ± SD (n = 3 or 4 mice).
Table 1.
Parameter estimates from the population PK analysis of the plasma polymyxin B concentration versus time data following subcutaneous administration of a single dose of 2, 4, 8, 16 or 32 mg/kg polymyxin B in neutropenic infected mice
| Parameter (unit) | Population PK estimate (CV%a) |
|---|---|
| First-order absorption rate constant (h−1) | 1.83 (9.6) |
| Maximum rate for saturable absorption (mg/h/kg) | 11.3 (4.4) |
| Amount in subcutaneous absorption compartment at half-maximum of saturable absorption (mg/kg)b | 11.5 (11) |
| Dose amount absorbed by first-order process (mg/kg) | 4.8 (8.4) |
| Linear elimination clearance (L/h/kg) | 0.178 (11) |
| Maximum rate for saturable elimination (mg/h/kg) | 0.255 (18) |
| Plasma concentration at half-maximum of saturable elimination (mg/L) | 0.823 (22) |
| Central volume of distribution (L/kg) | 0.325 (3.7) |
| Peripheral volume of distribution (L/kg) | 0.808 (42) |
| Inter-compartmental clearance (L/h/kg) | 0.198 (5.6) |
| Proportional residual error (%) | 22.5 |
| Additive residual error (mg/L) | 0.010 |
The bioavailability following subcutaneous administration was assumed to be 100%.
CV% represents the interindividual variability between the mice.
Maximum rate constant for saturable absorption component 0.98 h−1.
Figure 2.
Diagram of the final population pharmacokinetic model. SC, subcutaneous.
Figure 3.
Visual predictive checks of the model fits to the plasma polymyxin B concentration versus time data, stratified by dose. The diamonds represent the observed concentrations. The lines represent the model-predicted percentiles. Note the different axis scales.
The protein binding of polymyxin B in plasma of neutropenic infected mice was concentration independent over the concentration range of ∼0.9–37 mg/L (P > 0.05). For the 10 concentrations spanning this range, the mean (±SD) percentage of polymyxin B bound was 91.4 ± 1.65. Thus, the average unbound fraction of polymyxin B in plasma was 0.086, and this was the value used in determining the values of fAUC/MIC, fCmax/MIC and fT>MIC for each of the dosing regimens in the PK/PD studies. The unbound fraction of polymyxin B in the plasma of neutropenic infected mice (0.086) was almost identical to that reported previously for colistin (0.084).5 It is evident that the unbound fractions of polymyxin B,18 colistin5,19 and some polymyxin analogues20 are substantially lower in plasma of mice compared with those in humans. This is most likely the result of inter-species qualitative and/or quantitative differences in the plasma proteins involved in the binding of the polymyxins or in the concentrations of endogenous compounds that modify the binding. When translating PK/PD data to the clinical setting, it is important to recognize that the unbound fractions of polymyxin B and colistin in the plasma of neutropenic infected mice are substantially lower than the corresponding values for plasma of critically ill patients.5,18,19
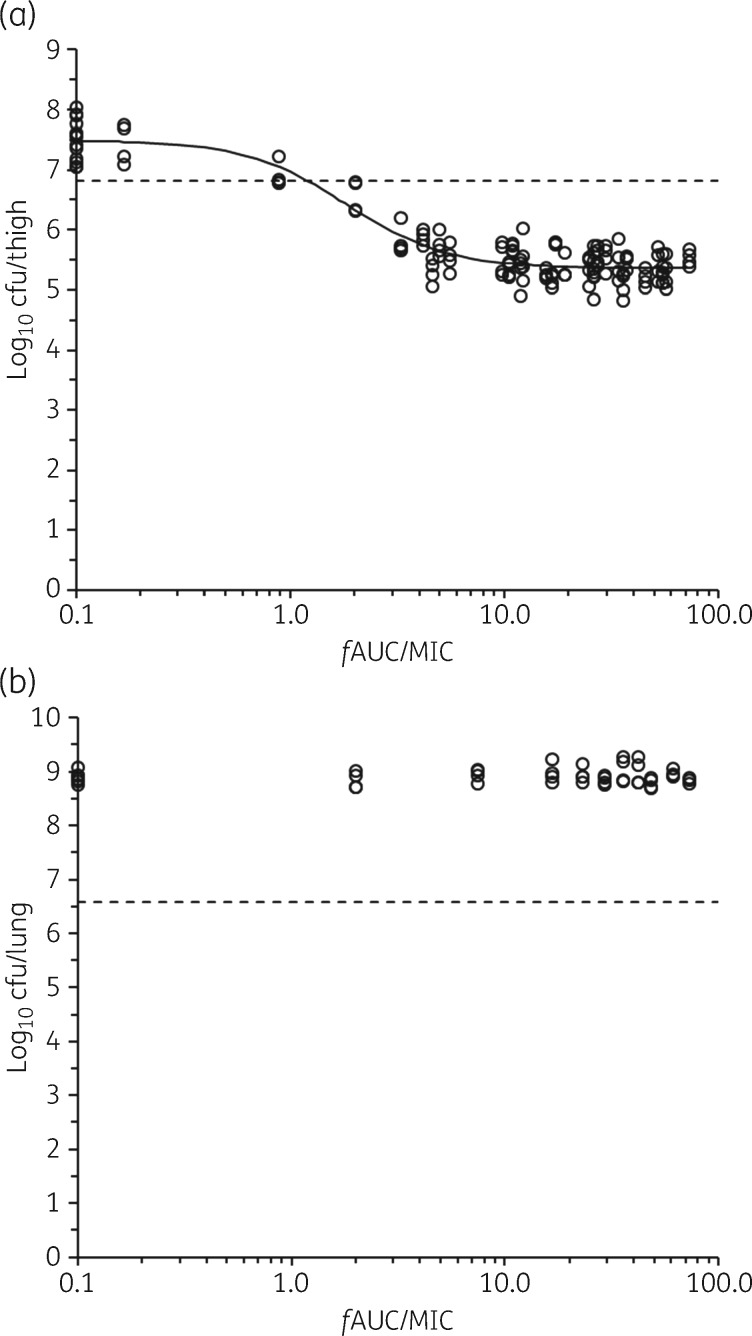
In the thigh infection model, the bacterial burdens at initiation of the polymyxin B regimens for strains ATCC BAA-2146, FADDI-KP032 and FADDI-KP042 were 6.30 ± 0.67 (n = 8), 6.81 ± 0.13 (n = 12) and 6.92 ± 0.13 (n = 8) log10 cfu/thigh, respectively. The relationship between the antibacterial effect of polymyxin B and fAUC/MIC in the dose-fractionation study for FADDI-KP032 in the thigh infection model is shown in Figure 4(a). The R2 value of 0.89 for the fit of the inhibitory sigmoid dose–effect model for the fAUC/MIC index was marginally higher than the corresponding value for fCmax/MIC (R2 = 0.88) and substantially higher than that for fT>MIC (R2 = 0.50); the data for the last two indices are shown in Figure S1 (available as Supplementary data at JAC Online). This is in keeping with the results of studies with colistin and polymyxin B against P. aeruginosa and A. baumannii that indicate that fAUC/MIC is the PK/PD index that best describes the antibacterial effect of the polymyxins, although in some reports fCmax/MIC was only modestly inferior to fAUC/MIC, as in the current study.4–9 The daily doses of polymyxin B needed to generate the fAUC/MIC values at the upper end of the exposure–response relationship (Figure 4a) were the maximum that were tolerated by the mice. The PK/PD model parameters for the fAUC/MIC index of polymyxin B against all three strains of K. pneumoniae in the thigh infection model were estimated with good precision (Table 2). The value of fAUC/MIC required to achieve 50% of the maximal drug effect (Emax) was similar to that reported for colistin against P. aeruginosa and A. baumannii in the murine thigh infection model.5 However, it is notable that the Emax for polymyxin B (2.13–2.90 log10 cfu/thigh) was substantially smaller than was observed for colistin against three strains each of P. aeruginosa (4.97–6.84 log10 cfu/thigh) and A. baumannii (3.78–4.61 log10 cfu/thigh) in the same model.5 In keeping with the smaller Emax for polymyxin B in the present study, it was not possible to determine a target value of fAUC/MIC for 2 log10 kill (Table 2); the target values for stasis and 1 log10 kill for polymyxin B against K. pneumoniae are in generally good agreement with the corresponding values for colistin against P. aeruginosa and A. baumannii in the murine thigh infection model.5 There was a relatively wide range of target values for stasis and 1 log10 kill for polymyxin B against the three strains of K. pneumoniae (Table 2). Similar or larger variability across strains has been observed in murine infection studies with non-polymyxin antibiotics against other bacterial species.21–23 As more information emerges on the clinical population PK of polymyxin B and the relationship between its plasma exposure and nephrotoxicity in patients, the PK/PD target values reported in Table 2, together with values from future studies on additional strains, can be translated to assist in development of optimized dosing regimens, as is occurring with colistin.19,24
Figure 4.
Relationships for K. pneumoniae FADDI-KP032 between log10 cfu per thigh at 24 h and fAUC/MIC (a) and log10 cfu per lung at 24 h and fAUC/MIC (b). Each symbol represents the value from a single thigh or lung in the respective infection models. The data points on the y-axis are for untreated (control) mice at 24 h after commencement of therapy in the other animals. The solid line in panel (a) is the fit of the inhibitory sigmoid dose–effect model (R2 = 0.89). The dotted line represents the average bacterial burden in the thighs or lungs at the start of polymyxin B treatment (i.e. the stasis value).
Table 2.
PK/PD model-fitted parameter estimates for the fAUC/MIC index of polymyxin B against all three strains of K. pneumoniae in the thigh infection model and corresponding target values of fAUC/MIC for stasis and 1 and 2 log10 kill
| PK/PD model-fitted parameter estimates of polymyxin B fAUC/MIC and coefficient of determinationa |
Target value of polymyxin B fAUC/MIC for various magnitudes of effect |
|||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Emax (log10 cfu/thigh) | E0 (log10 cfu/thigh) | EC50 | γ | R2 | stasis | 1 log10 kill | 2 log10 kill |
| ATCC BAA-2146 | 2.90 (0.47)b | 8.17 (0.22) | 13.9 (0.34) | 6.12 (0.77) | 0.75 | 13.5 | 17.4 | c |
| FADDI-KP032 | 2.13 (1.33) | 7.49 (1.24) | 1.83 (2.59) | 1.86 (2.99) | 0.89 | 1.22 | 3.72 | c |
| FADDI-KP042 | 2.84 (1.03) | 8.62 (1.02) | 4.24 (2.86) | 1.57 (1.49) | 0.96 | 5.47 | 28.0 | c |
Emax is maximal drug effect, E0 is the effect in the absence of drug, EC50 is the value of the PK/PD index required to achieve 50% of Emax, γ is the Hill coefficient, and R2 is the coefficient of determination.
Value in parentheses is the percentage relative standard error of the estimate.
Could not be determined because the highest tolerated dosage regimens of polymyxin B were not able to achieve 2 log10 kill.
To probe the possible reason(s) for the lower Emax for polymyxin B against K. pneumoniae, a comparison of the relative antibacterial effects of colistin and polymyxin B was conducted against two of the strains (FADDI-KP032 and ATCC BAA-2146). These strains were chosen because for polymyxin B in the thigh infection model they had the lowest and the highest respective EC50 values and fAUC/MIC target values for stasis (Table 2). Equimolar daily doses of each polymyxin were studied at three or four dose levels. The doses were chosen as they generated exposures that led to near-maximal reduction in bacterial burden with polymyxin B in the thigh infection model (Figure 4a). The results of the comparative study are presented in Table 3. The magnitude of the killing of FADDI-KP032, relative to the bacterial burden at the initiation of treatment with each of the polymyxins, was very similar to that shown for polymyxin B in Figure 4(a). There was no difference in the magnitude of killing between polymyxin B and colistin (P > 0.05) at any of the four dose levels against FADDI-KP032 or at two of the three dose levels for ATCC BAA-2146 (Table 3). For the latter strain, there was a small but marginally significant difference (P = 0.044) in the bacterial burden at the end of treatment for the second dose level. As colistin caused a substantially greater extent of killing of P. aeruginosa and A. baumannii in the murine thigh infection model,5 the smaller response for polymyxin B and colistin against K. pneumoniae in the current study (Figure 4a, Tables 2 and 3) is most likely related to intrinsic differences across the bacterial species. The presence of a capsule in K. pneumoniae is a possible reason for the lower maximal effect. The anionic bacterial capsule polysaccharide may serve to decrease the number of possible interactions of cationic polymyxin molecules with the anionic lipopolysaccharide of the outer membrane, which is a critical first step in the bactericidal action.25,26 Given the smaller response, it may be particularly important when polymyxins are used against infections caused by K. pneumoniae to consider combination therapy, although the benefit–cost of combination therapy with polymyxins remains to be determined in well designed and adequately powered clinical studies.3,27 The potential clinical significance of the differing maximal responses across these Gram-negative species warrants further evaluation.27
Table 3.
Comparative effects of polymyxin B and colistin at various equimolar dose levels against two strains of K. pneumoniae. Data are presented as mean ± SD (n = 4)
| Log10 cfu/thigh at 24 ha |
||||
|---|---|---|---|---|
| FADDI-KP032b |
ATCC BAA-2146c |
|||
| Dose leveld | polymyxin B | colistin | polymyxin B | colistin |
| 1 | 5.30 ± 0.27 | 5.42 ± 0.22 | 7.62 ± 0.81 | 7.03 ± 1.34 |
| 2 | 5.41 ± 0.17 | 5.57 ± 0.07 | 5.29 ± 0.25e | 5.68 ± 0.14 |
| 3 | 5.31 ± 0.20 | 5.55 ± 0.07 | 5.67 ± 0.98 | 5.28 ± 0.16 |
| 4 | 5.52 ± 0.13 | 5.43 ± 0.11 | ND | ND |
ND, not determined.
24 h represents end time of treatment with polymyxin B or colistin.
bStasis value 6.80 ± 0.08 log10 cfu/thigh at 0 h. 0 h represents time of commencing treatment with polymyxin B or colistin; the value of log10 cfu/thigh at this time is the baseline (stasis) value.
Stasis value 6.78 ± 0.13 log10 cfu/thigh at 0 h. 0 h represents time of commencing treatment with polymyxin B or colistin; the value of log10 cfu/thigh at this time is the baseline (stasis) value.
Dose levels 1, 2, 3 and 4 for polymyxin B were 22.5, 45, 90 and 120 mg/kg/24 h, respectively; the corresponding dose levels for colistin were 21.9, 43.9, 87.7 and 117.1 mg/kg/24 h. The daily dose was administered in three equal doses at intervals of 8 h.
Different from the corresponding value for colistin for this strain (P = 0.044).
In the lung infection model, the bacterial burdens at initiation of the polymyxin B regimens for strains ATCC BAA-2146, FADDI-KP032 and FADDI-KP042 were 6.61 ± 0.05 (n = 4), 6.58 ± 0.06 (n = 4) and 6.60 ± 0.05 (n = 4) log10 cfu/lung, respectively. There was no relationship between the antibacterial effect of polymyxin B and fAUC/MIC. The data for strain FADDI-KP032 are shown in Figure 4(b), and the results for the other two strains were almost identical. There was no evidence for bacterial killing, even at fAUC/MIC exposures achieved with the highest doses that could be tolerated by the mice. Clearly, it was not possible to undertake PK/PD modelling of the data. It is evident that subcutaneous (i.e. systemic) administration of polymyxin B resulted in very much less bacterial killing in lungs in comparison with thighs. Bowers et al.16 also reported a lack of antibacterial effect of polymyxin B (10 mg/kg) following intraperitoneal administration in neutropenic mice with A. baumannii lung infections. The authors of that study attributed the lack of responsiveness of lung infections to achievement of relatively low concentrations of polymyxin B in epithelial lining fluid (ELF) of mice administered the drug systemically. Lower responsiveness of infections in lungs compared with that in thighs was reported for colistin against P. aeruginosa and A. baumannii, although bacterial killing in lungs was observed at high (but tolerated) fAUC/MIC exposures for all three examined strains of P. aeruginosa and one of three examined strains of A. baumannii.5 This implies that colistin was able to access ELF in concentrations sufficient to elicit at least some degree of bacterial killing against these species. As discussed above in regard to thigh infections, it appears that lung infections caused by K. pneumoniae may be even less responsive to systemically administered polymyxin than is the case for P. aeruginosa and A. baumannii. Experimental lung infection with P. aeruginosa in piglets was substantially less responsive to systemically administered colistin than to nebulized colistin.28 This is not unexpected because recent preclinical28–31 and clinical32–34 studies have revealed that colistin concentrations in lung tissue, ELF or sputum are substantially higher after direct administration to the lungs than the corresponding concentrations achieved after systemic administration. The increasing amount of preclinical data from the current and previous studies together with the results of recent meta-analyses of clinical studies reinforce the need to consider nebulized delivery of polymyxins as an adjunct in the treatment of pulmonary infections.35,36
In conclusion, to the best of our knowledge, this is the first study to report the PK/PD of either polymyxin B or colistin against K. pneumoniae in a dynamic infection model. The study revealed that in the neutropenic murine thigh infection model fAUC/MIC is the PK/PD index that best correlates with bacterial killing. The fAUC/MIC target values for stasis and 1 log10 kill were in the same range as those reported for colistin against P. aeruginosa and A. baumannii in the same dynamic infection model. However, in a comparative component of the current study the maximal killing was substantially lower for both polymyxin B and colistin against K. pneumoniae, suggesting a difference in the responsiveness of this pathogen to the polymyxins. Murine lung infection with K. pneumoniae was even less responsive to systemically administered polymyxin B than were P. aeruginosa and A. baumannii lung infections treated systemically with colistin. As more is learned about the population PK of polymyxin B and the relationship between its plasma exposure and risk of nephrotoxicity in critically ill patients, the results of the current study will assist in the design of optimized dosing strategies for polymyxin B.
Supplementary Material
Acknowledgements
The authors are very grateful to Jie Lu for technical assistance.
Funding
This study was supported by internal funding, and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health provided support to B. T. T., J. L. and R. L. N. via award number R01AI111990 and to K. S. K., J. L. and R. L. N. via award number R01AI119446. C. B. L. is a recipient of an Australian National Health and Medical Research Council (NHMRC) Career Development Fellowship (APP1062509) and J. L. is a NHMRC Senior Research Fellow.
Transparency declarations
None to declare.
Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary data
Figure S1 appears as Supplementary data at JAC Online.
References
- 1. Nation RL, Velkov T, Li J.. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014; 59: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sader HS, Rhomberg PR, Farrell DJ. et al. Differences in potency and categorical agreement between colistin and polymyxin B when testing 15,377 clinical strains collected worldwide. Diagn Microbiol Infect Dis 2015; 83: 379–81. [DOI] [PubMed] [Google Scholar]
- 3. Nation RL, Li J, Cars O. et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2015; 15: 225–34. [DOI] [PubMed] [Google Scholar]
- 4. Bergen PJ, Bulitta JB, Forrest A. et al. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother 2010; 54: 3783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheah SE, Wang J, Nguyen VT. et al. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 2015; 70: 3291–7. [DOI] [PubMed] [Google Scholar]
- 6. Hengzhuang W, Wu H, Ciofu O. et al. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother 2012; 56: 2683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin YW, Zhou QT, Cheah SE. et al. Pharmacokinetics/pharmacodynamics of pulmonary delivery of colistin against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother 2017; 61: doi:10.1128/AAC.02025-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan DD, Friberg LE, Nielsen EI.. A pharmacokinetic/pharmacodynamic (PK/PD) model based on in vitro time-kill data predicts the in vivo PK/PD index of colistin. J Antimicrob Chemother 2016; 71: 1881–4. [DOI] [PubMed] [Google Scholar]
- 9. Tam VH, Schilling AN, Vo G. et al. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49: 3624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Recommendations for MIC Determination of Colistin (Polymyxin E): As Recommended by the Joint CLSI-EUCAST Polymyxin Breakpoints Working Group 2016. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf.
- 11. Cheah SE, Bulitta JB, Li J. et al. Development and validation of a liquid chromatography-mass spectrometry assay for polymyxin B in bacterial growth media. J Pharm Biomed Anal 2014; 92: 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauer RJ, Guzy S, Ng C.. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J 2007; 9: E60–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bulitta JB, Landersdorfer CB.. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J 2011; 13: 212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulitta JB, Bingolbali A, Shin BS. et al. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J 2011; 13: 201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He J, Gao S, Hu M. et al. A validated ultra-performance liquid chromatography-tandem mass spectrometry method for the quantification of polymyxin B in mouse serum and epithelial lining fluid: application to pharmacokinetic studies. J Antimicrob Chemother 2013; 68: 1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowers DR, Cao H, Zhou J. et al. Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59: 2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berkhout J, Melchers MJ, van Mil AC. et al. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 2015; 59: 2299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandri AM, Landersdorfer CB, Jacob J. et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57: 524–31. [DOI] [PubMed] [Google Scholar]
- 19. Nation RL, Garonzik SM, Li J. et al. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62: 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Utley L, Coleman S.. In-vitro ADME Properties of SPR741 Support Progression into Clinical Development ASM Microbe 2017, New Orleans http://www.abstractsonline.com/pp8/#!/4358/presentation/7135. American Society for Microbiology, Washington, DC, USA, 2017.
- 21. Lepak AJ, Andes DR.. In vivo pharmacodynamic target assessment of delafloxacin against Staphylococcus aureus, Streptococcus pneumoniae, and Klebsiella pneumoniae in a murine lung infection model. Antimicrob Agents Chemother 2016; 60: 4764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lepak AJ, Zhao M, Marchillo K. et al. In vivo pharmacodynamic evaluation of omadacycline (PTK 0796) against Streptococcus pneumoniae in the murine pneumonia model. Antimicrob Agents Chemother 2017; 61: pii=e02368-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulik CC, Okusanya OO, Lakota EA. et al. Pharmacokinetic-pharmacodynamic evaluation of gepotidacin against Gram-positive organisms using data from murine infection models. Antimicrob Agents Chemother 2017; 61: pii=e00115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nation RL, Garonzik SM, Thamlikitkul V. et al. Dosing guidance for intravenous colistin in critically ill patients. Clin Infect Dis 2017; 64: 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Llobet E, Tomas JM, Bengoechea JA.. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology 2008; 154: 3877–86. [DOI] [PubMed] [Google Scholar]
- 26. Velkov T, Deris ZZ, Huang JX. et al. Surface changes and polymyxin interactions with a resistant strain of Klebsiella pneumoniae. Innate Immun 2014; 20: 350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaye KS, Pogue JM, Tran TB. et al. Agents of last resort: polymyxin resistance. Infect Dis Clin North Am 2016; 30: 391–414. [DOI] [PubMed] [Google Scholar]
- 28. Lu Q, Girardi C, Zhang M. et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 2010; 36: 1147–55. [DOI] [PubMed] [Google Scholar]
- 29. Yapa SWS, Li J, Porter CJ. et al. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother 2013; 57: 5087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchand S, Gobin P, Brillault J. et al. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother 2010; 54: 3702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gontijo AV, Gregoire N, Lamarche I. et al. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob Agents Chemother 2014; 58: 3950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Athanassa ZE, Markantonis SL, Fousteri MZ. et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 2012; 38: 1779–86. [DOI] [PubMed] [Google Scholar]
- 33. Yapa SWS, Li J, Patel K. et al. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 2014; 58: 2570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boisson M, Jacobs M, Gregoire N. et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 2014; 58: 7331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu WJ, Wang F, Tang L. et al. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int J Antimicrob Agents 2014; 44: 477–85. [DOI] [PubMed] [Google Scholar]
- 36. Valachis A, Samonis G, Kofteridis DP.. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia a systematic review and metaanalysis . Crit Care Med 2015; 43: 527–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.