Abstract
Background. Bloodstream infections (BSIs) due to ESBL-producing Enterobacteriaceae (ESBL-E) are frequent yet outcome prediction rules for clinical use have not been developed. The objective was to define and validate a predictive risk score for 30 day mortality.
Methods. A multinational retrospective cohort study including consecutive episodes of BSI due to ESBL-E was performed; cases were randomly assigned to a derivation cohort (DC) or a validation cohort (VC). The main outcome variable was all-cause 30 day mortality. A predictive score was developed using logistic regression coefficients for the DC, then tested in the VC.
Results. The DC and VC included 622 and 328 episodes, respectively. The final multivariate logistic regression model for mortality in the DC included age >50 years (OR = 2.63; 95% CI: 1.18–5.85; 3 points), infection due to Klebsiella spp. (OR = 2.08; 95% CI: 1.21–3.58; 2 points), source other than urinary tract (OR = 3.6; 95% CI: 2.02–6.44; 3 points), fatal underlying disease (OR = 3.91; 95% CI: 2.24–6.80; 4 points), Pitt score >3 (OR = 3.04; 95 CI: 1.69–5.47; 3 points), severe sepsis or septic shock at presentation (OR = 4.8; 95% CI: 2.72–8.46; 4 points) and inappropriate early targeted therapy (OR = 2.47; 95% CI: 1.58–4.63; 2 points). The score showed an area under the receiver operating curve (AUROC) of 0.85 in the DC and 0.82 in the VC. Mortality rates for patients with scores of < 11 and ≥11 were 5.6% and 45.9%, respectively, in the DC, and 5.4% and 34.8% in the VC.
Conclusions. We developed and validated an easy-to-collect predictive scoring model for all-cause 30 day mortality useful for identifying patients at high and low risk of mortality.
Introduction
ESBLs confer resistance to penicillins, cephalosporins and aztreonam. These enzymes have spread successfully among Enterobacteriaceae in recent decades and are now a significant worldwide problem. The mean prevalence of third-generation cephalosporin resistance among invasive isolates in Europe (most of which are due to ESBL production) has been increasing and is now 12% in Escherichia coli and 28% in Klebsiella pneumoniae, reaching 40% and 75%, respectively, in some countries.1 ESBLs frequently coexist with other mechanisms of resistance and so ESBL producers may also be resistant to other agents, such as fluoroquinolones, co-trimoxazole or the aminoglycosides, which significantly limits the available therapeutic options against these isolates.2
Crude mortality among patients suffering bacteraemic infections due to ESBL-producing Enterobacteriaceae (ESBL-E) varies across studies, ranging from 13% to 83%, with a pooled mean of 31%.3 Two meta-analyses have shown that ESBL production is associated with increased mortality when compared to non-ESBL producers, mainly due to the effect of the increased delay in administering active therapy.3,4 Other factors, such as the severity of underlying chronic and acute conditions or source of infection, are also associated with increased risk of death.2
Predicting risk of mortality using a scoring system can be useful for stratifying patients according to risk and as a reference for investigating the efficacy of therapeutic alternatives; however, the generalizability of a predictive score may be problematic if the data on which it is based have been obtained from only one centre or area, since epidemiology and overall clinical management can vary from area to area.5 To the best of our knowledge, risk-scoring systems related to ESBL-E have so far been developed only to detect patients who harboured these organisms upon hospital admission,6,7 but not to predict mortality. A previous study developed a predictive score for patients with bacteraemia due to Gram-negative bacteria, but only for those receiving appropriate therapy;8 the score was later validated in patients with bloodstream infection (BSI) due to E. coli or Pseudomonas aeruginosa.9 Because inappropriate empirical therapy and species other than E. coli are frequent among BSIs due to ESBL-E,3 a specific predictive score is needed. The aim of this study was to develop a scoring model to estimate the risk of 30 day all-cause mortality for patients with BSIs due to ESBL-E on the day susceptibility data are available, using data from a large multinational cohort. We were also interested in including early targeted therapy as a potential predictor because this is a variable amenable to intervention on the same day.
Methods
Study design, sites and participants
This analysis is part of the INCREMENT project, a multicentre, international retrospective cohort study including consecutive episodes of BSI due to ESBLs or carbapenemase-producing Enterobacteriaceae diagnosed at participating centres from January 2004 through December 2013 (ClinicalTrials.gov identifier: NCT01764490). The participating centres were selected because they had previous experience of characterizing the targeted bacteria and of collecting data from patients with BSIs. In the case of ESBL-E, a maximum of 50 consecutive cases per participating centre were included in the INCREMENT database. Subsequent episodes in a patient caused by the same microorganism were included only if the interval between their isolation dates was >3 months.
For this analysis, all episodes of ESBL-E included in the INCREMENT database were eligible. These came from 37 tertiary hospitals in 11 different countries (Spain, Germany, Italy, Greece, Israel, Turkey, South Africa, Canada, USA, Argentina and Taiwan). Exclusion criteria included polymicrobial BSIs, non-clinically significant episodes and unavailability of key data. Also, because the predictive variables are to be used when susceptibility data are available (typically, 48 h after blood cultures are taken), patients who died less than 48 h after the blood cultures were obtained were also excluded. All patients were followed for 30 days after the blood cultures were obtained. The patients included were randomly assigned to a derivation cohort (DC; two-thirds of the total number) and the rest to a validation cohort (VC; one-third) before the exclusion criteria were applied.
ESBL production was studied at each centre using standard phenotypic methods;10 susceptibility was studied using automated systems or disc diffusion at each local laboratory and interpreted using 2012 CLSI breakpoints;10 the genes coding for ESBLs were characterized by PCR and sequencing of selected isolates at each centre.
STROBE recommendations were followed in order to strengthen the reporting of the study (Table S1, available as Supplementary data at JAC Online).
Variables and definitions
The outcome variable was all-cause 30 day mortality. Day 0 was the day when the blood cultures were taken. The independent variables were assessed at day 0 except where specified, and included: demographics, chronic underlying conditions and their severity according to the McCabe classification (non-fatal underlying condition: death is not expected to occur as a consequence of the underlying condition in the next 5 years; ultimately fatal: death is expected to occur in the next 5 years; and rapidly fatal: death is expected to occur in the next 3 months)11 and the Charlson comorbidity index,12 severity of acute underlying condition according to the Pitt score (measured retrospectively the day before BSI),13 type of acquisition, type of admission ward (medical, surgical, emergency department or ICU), source of BSI according to CDC definitions,14 severity of systemic inflammatory response syndrome at presentation (sepsis, severe sepsis, septic shock),15 microorganism, and antibiotic therapy (see below).
Acquisition was considered nosocomial if signs or symptoms of infection started >48 h after hospital admission or <48 h after hospital discharge. Otherwise, the case was considered community onset. Antibiotic therapy was considered as empirical if administered before susceptibility data were available, and targeted thereafter. Antibiotics were considered appropriate if administered early enough (<1 day from the day blood cultures were taken for empirical drugs, and <4 days for targeted antibiotics) and if susceptible or intermediate in vitro according to 2012 CLSI recommendations;10 for isolates obtained before 2012, the susceptibility category was reviewed and assigned according to the MIC or inhibition size; these were not available in 15 isolates (1.2%) for which the breakpoint of the drug used for the treatment had changed, and for these the susceptibility was considered as reported by the local laboratory. The intravenous route was not a requirement for appropriate therapy, but active antibiotics were administered intravenously in all patients. In this analysis, because the prediction rule was to be used on day 3, only early targeted drugs (i.e. those administered on that day, which is the day when susceptibility results are typically available) were considered; any later change in therapy was not analysed.
Statistical analysis
The predictive score was calculated using the DC. Categorical variables were compared by χ2 test or Fisher’s exact test, and continuous variables by the Student’s t-test or Mann–Whitney U-test, as appropriate. Odds ratios (ORs) and 95% CIs were calculated. Continuous and polychotomous variables were dichotomized using classification and regression tree (CART) analysis according to their association with the outcome. The participating centres were classified as high or low mortality risk after considering all other variables using TreeNet (Salford Systems) (see Table S2, and Figures S1–S6). Variables with a P value of ≤0.2 in univariate analysis in the DC and those considered to be of clinical interest were included in a logistic regression model and selected manually in a stepwise manner. The variance inflation factor value (VIF) for every variable was calculated to control for the influence of multicollinearity. The ‘centre’ variable (dichotomized) was included as a covariate in the multivariate analysis to control for the influence of unmeasured variables related to the site effect. Interactions were explored. A predictive score was developed by dividing each regression coefficient by half of the smallest and rounding to the nearest unit. The discriminatory power of the models was evaluated by calculating the area under the receiver operating characteristic curve (AUROC), and goodness-of-fit was evaluated with the Hosmer–Lemeshow test. Sensitivity (SE), specificity (SP), positive and negative predictive values (PPV, NPV), accuracy (AC), and positive and negative likelihood ratios16 (PLR, NLR) were calculated. The score was then applied to the VC for validation.
Statistical analysis was performed using SPSS version 21 software (IBM Statistics for Windows, version 21.0; IBM Corp, Armonk, NY, USA), CART software 7.0 (Salford Systems) and TreeNet (Salford Systems).
Ethics
The Institutional Review Board of the Hospital Universitario Virgen Macarena approved the study (reference number 1921). Approval was also obtained at each participating centre according to local requirements; the need for informed consent was waived because of the observational nature of the study.
Results
The study included 1004 episodes of BSIs due to ESBL-E. One patient was excluded because of important missing data and 53 died within 48 h and were also excluded; therefore, 950 cases were finally included. The ESBL was characterized in 330 (34.7%), of which 260 (78.8%) were CTX-M-type, 58 (17.7%) SHV-type and 51 (15.5%) TEM type (27 isolates had≥1 ESBL). Of these 950 cases, 622 were allocated to the DC, and 328 to the VC (see Figure S7). The epidemiological features and predisposing factors of patients in both cohorts are shown in Table 1.
Table 1.
Epidemiological features and predisposing factors of patients with bacteraemia due to ESBL-E in the derivation and validation cohort
| Variable | Derivation cohort (n = 622) | Validation cohort (n = 328) | P value |
|---|---|---|---|
| Median age in years (IQR) | 69 (56–79) | 68 (58–79) | 0.95 |
| Male sex | 353 (57) | 182 (55) | 0.70 |
| Median previous hospital stay in days (IQR)a | 2 (0–13) | 1 (0–10) | 0.19 |
| Nosocomial acquisition | 301 (49) | 155 (47) | 0.73 |
| Source | 0.56 | ||
| urinary tract | 274 (44) | 135 (41) | |
| unknown | 108 (17) | 50 (15.1) | |
| biliary tract | 62 (10) | 43 (13) | |
| intra-abdominal | 59 (9) | 43 (13) | |
| vascular | 45 (7) | 20 (6.1) | |
| respiratory tract | 44 (7) | 20 (6.1) | |
| CNS | 1 (0.3) | 0 (0) | |
| osteoarticular | 4 (0.7) | 1 (0.3) | |
| skin and soft tissue | 16 (3) | 11 (3.4) | |
| other | 9 (2) | 5 (2) | |
| Ward admission | 0.83 | ||
| emergency department | 295 (47) | 147 (45) | |
| medical | 165 (27) | 89 (27) | |
| ICU | 91 (15) | 49 (15) | |
| surgical | 71 (11) | 43 (13) | |
| Microorganisms | 0.59 | ||
| E. coli | 435 (70) | 218 (66) | |
| K. pneumoniae | 133 (21) | 80 (24) | |
| Other Klebsiella spp. | 3 (0.5) | 4 (1.2) | |
| Morganella morganii | 1 (0.2) | 0 (0) | |
| Citrobacter freundii | 3 (0.5) | 2 (0.9) | |
| Enterobacter cloacae | 37 (6) | 16 (5) | |
| Enterobacter aerogenes | 2 (0.4) | 3 (1) | |
| Proteus mirabilis | 5 (0.8) | 2 (0.9) | |
| Serratia marcescens | 2 (0.4) | 3 (1) | |
| Other Enterobacter spp. | 1 (0.2) | 0 (0) | |
| Median Charlson Index (IQR) | 2 (1–4) | 2 (1–4) | 0.33 |
| Median Pitt score (IQR) | 1 (0–3) | 1 (0–2) | 0.16 |
| Ultimately or rapidly fatal underlying condition | 306 (49) | 173 (52.7) | 0.88 |
| Severe sepsis/septic shock | 224 (36) | 104 (32) | 0.18 |
| Appropriate empirical therapy | 339 (54) | 174 (53) | 0.66 |
| Appropriate targeted therapy | 505 (81) | 265 (81) | 0.88 |
| 30 day crude mortality | 115 (18) | 50 (15) | 0.2 |
Data are expressed as numbers (percentage) except where specified.
Time from admission to positive blood culture.
The following variables were dichotomized according to CART as follows: age, >50 versus ≤50 years; Charlson index, ≥2 versus <2; McCabe, ultimately and rapidly fatal underlying condition versus non-fatal; Pitt score, >3 versus ≤3, severity of inflammatory response, severe sepsis and septic shock versus sepsis (Figures S2–S6). The variable centre was dichotomized according to TreeNet as high-risk versus low-risk sites (Figure S1). There was no trend towards differences in mortality according to study year and this variable was not therefore considered.
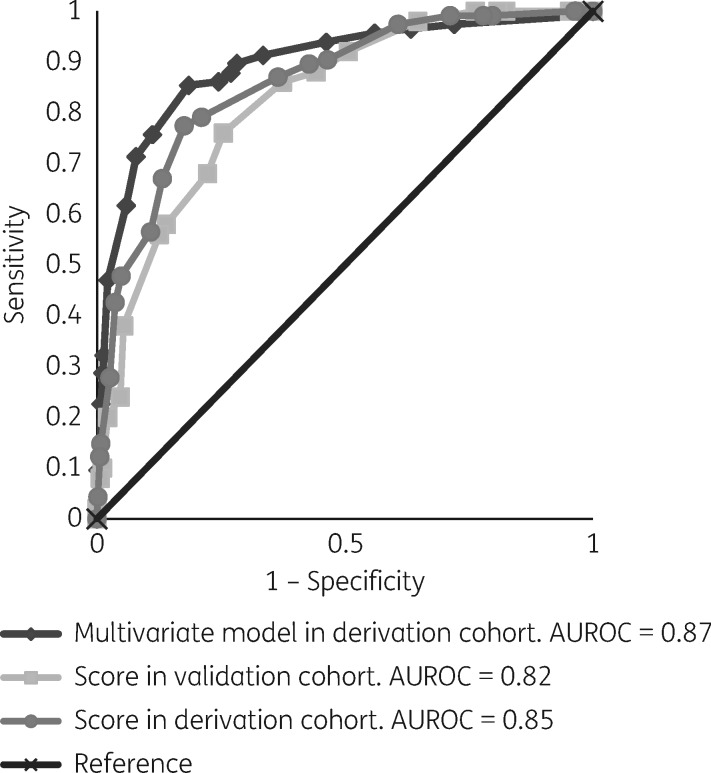
The univariate analysis of variables associated with 30 day mortality in the DC showed significantly increased risk for age >50 years, infection due to Klebsiella spp, nosocomial acquisition, Charlson index ≥2, Pitt score >3, ultimately or rapidly fatal underlying disease, source other than urinary tract, severe sepsis/septic shock at presentation, and inappropriate early targeted therapy; while infection due to E. coli was protective (Table 2). Variables in the final multivariate analysis model independently associated with an increased risk of 30 day mortality were: age >50 years, infection due to Klebsiella spp., ultimately or rapidly fatal underlying disease, Pitt score >3, source other than the urinary tract, severe sepsis/septic shock at presentation of symptoms, and inappropriate early targeted treatment. When early appropriate targeted therapy was subdivided into carbapenems and non-carbapenems, both showed a similar protective effect (data not shown). The Charlson comorbidity index was also significantly associated with higher mortality in the univariate model but was not included in the final multivariate model because it was collinear with the McCabe classification. The data are shown in Table 2. The point values assigned to each independent risk factor for mortality are also shown in Table 2. The sum of the scores applied to each individual patient ranged from 0 to 21 points. The AUROC of the final model was 0.87 (95% CI: 0.83–0.90), and the P value for the Hosmer–Lemeshow test was 0.93, indicating good discrimination and calibration (Figure 1). When the scoring model was applied to the DC, it showed an AUROC of 0.85 (95% CI: 0.82–0.89) (Figure 1), and the Hosmer–Lemeshow test also demonstrated a good fit (P = 0.44). The SE, SP, PPV, NPV and AC for different cut-offs are shown in Table 3; a cut-off of ≥7 showed 97% SE and 98% NPV, but, in contrast, a moderate SP (39%) and low PPV (27%). On the other hand, a cut-off of ≥14 showed high SP (89%) and moderate PPV (54%) but a high NPV (90%).
Table 2.
Univariate and multivariate analysis of risk factors associated to all-cause 30 day mortality in the derivation cohort with calculated scores
| Crude analysis |
Adjusted analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | No. deceased (%) n = 115 | No. alive (%) n = 507 | OR (95% CI) | P value | β coefficient | OR (95% CI) | P value | Score |
| Age >50 years | 103 (89) | 416 (82) | 1.87 (0.99–3.55) | 0.05 | 0.97 | 2.63 (1.18–5.85) | 0.01 | 3 |
| Male sex | 64 (55) | 289 (57) | 0.94 (0.63–1.42) | 0.79 | ||||
| Enterobacteriaceae | ||||||||
| E. coli | 58 (50) | 377 (74) | 0.35 (0.23–0.53) | <0.001 | ||||
| Klebsiella spp. | 44 (38) | 92 (18) | 2.79 (1.8–4.33) | <0.001 | 0.73 | 2.08 (1.21–3.58) | 0.008 | 2 |
| others | 13 (11) | 38 (7.4) | 1.57 (0.8–3.05) | 0.17 | ||||
| Nosocomial acquisition | 72 (62) | 229 (45) | 2.03 (1.34–3.08) | 0.001 | ||||
| Source other than UTI | 91 (79) | 257 (50) | 3.68 (2.27–5.97) | <0.001 | 1.28 | 3.60 (2.02–6.44) | <0.001 | 3 |
| ICU admission | 25 (21) | 46 (9) | 2.78 (1.62–4.76) | <0.001 | ||||
| Charlson Index ≥2 | 99 (86) | 310 (61.1) | 3.93 (2.25–6.86) | <0.001 | ||||
| McCabe (UF and RF) | 87 (75) | 219 (43) | 4.08 (2.57–6.47) | <0.001 | 1.36 | 3.91 (2.24–6.80) | <0.001 | 4 |
| Pitt score >3 | 55 (47) | 57 (11) | 7.23 (4.57–11.44) | <0.001 | 1.11 | 3.04 (1.69–5.47) | <0.001 | 3 |
| Severe sepsis/septic shock | 85 (73) | 139 (27) | 7.50 (4.73–11.87) | <0.001 | 1.56 | 4.80 (2.72–8.46) | <0.001 | 4 |
| Inappropriate empirical therapy | 64 (55) | 275 (54) | 1.05 (0.70–1.59) | 0.78 | ||||
| Inappropriate early targeted therapy | 74 (64) | 80 (15) | 3.22 (2.0–5.0) | <0.001 | 0.90 | 2.47 (1.58–4.63) | 0.002 | 2 |
UTI, urinary tract infection; UF, ultimately fatal; RF, rapidly fatal.
Figure 1.
Receiver operating curves for the multivariate model and scoring system in the DC and VC.
Table 3.
Risk score performance in the derivation cohort (data shown are percentages)
| Score | Proportion of patients | SE | SP | PPV | NPV | AC | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| ≥4 | 83 | 99 | 20 | 22 | 99 | 35 | 1.23 | 0.05 |
| ≥5 | 82 | 99 | 22 | 22 | 99 | 36 | 1.26 | 0.04 |
| ≥6 | 76 | 99 | 29 | 24 | 99 | 42 | 1.39 | 0.03 |
| ≥7 | 67 | 97 | 39 | 27 | 98 | 50 | 1.59 | 0.07 |
| ≥8 | 55 | 90 | 54 | 31 | 96 | 60 | 1.95 | 0.18 |
| ≥9 | 51 | 89 | 58 | 32 | 96 | 63 | 2.11 | 0.18 |
| ≥10 | 46 | 87 | 63 | 35 | 95 | 68 | 2.35 | 0.20 |
| ≥11 | 32 | 79 | 78 | 46 | 94 | 79 | 3.59 | 0.26 |
| ≥12 | 29 | 77 | 83 | 50 | 94 | 81 | 4.52 | 0.27 |
| ≥13 | 23 | 67 | 86 | 53 | 92 | 83 | 4.78 | 0.38 |
| ≥14 | 19 | 56 | 89 | 54 | 90 | 83 | 5.09 | 0.49 |
| ≥15 | 13 | 47 | 95 | 69 | 88 | 86 | 9.4 | 0.55 |
| ≥16 | 11 | 43 | 96 | 72 | 88 | 86 | 10.75 | 0.59 |
| ≥17 | 7 | 28 | 97 | 71 | 85 | 85 | 9.33 | 0.74 |
| ≥18 | 3 | 15 | 99 | 81 | 83 | 84 | 15.00 | 0.85 |
| ≥19 | 3 | 12 | 99 | 82 | 83 | 83 | 12.00 | 0.90 |
| ≥20 | 1 | 4 | 100 | 83 | 82 | 82 | – | 0.96 |
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; AC, accuracy; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
When the scoring model was applied to the VC, the AUROC of the model was 0.82 (95% CI: 0.76–0.88) (Figure 1) and the P value for the Hosmer–Lemeshow test was 0.96. The prediction rules derived from applying the scores to the VC are shown in Table 4. For a score of ≥7, the scoring system showed 98% SE and 99% NPV. When a cut-off of ≥14 was considered, SP was 87% and NPV 92%.
Table 4.
Risk score performance in the validation cohort. Data are shown in percentages
| Score | Proportion of patients | SE | SP | PPV | NPV | AC | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| ≥4 | 85 | 100 | 17 | 17 | 100 | 30 | 1.20 | 0 |
| ≥5 | 84 | 100 | 19 | 18 | 100 | 31 | 1.23 | 0 |
| ≥6 | 80 | 100 | 24 | 19 | 100 | 35 | 1.31 | 0 |
| ≥7 | 70 | 98 | 36 | 21 | 99 | 45 | 1.53 | 0.05 |
| ≥8 | 57 | 92 | 50 | 25 | 97 | 56 | 1.84 | 0.16 |
| ≥9 | 51 | 88 | 56 | 26 | 96 | 61 | 2.00 | 0.21 |
| ≥10 | 45 | 86 | 62 | 29 | 96 | 66 | 2.26 | 0.22 |
| ≥11 | 33 | 76 | 74 | 35 | 94 | 75 | 2.92 | 0.32 |
| ≥12 | 30 | 68 | 78 | 35 | 93 | 76 | 3.09 | 0.41 |
| ≥13 | 21 | 58 | 86 | 43 | 92 | 82 | 4.14 | 0.48 |
| ≥14 | 19 | 56 | 87 | 44 | 92 | 83 | 4.30 | 0.50 |
| ≥15 | 10 | 38 | 95 | 56 | 89 | 86 | 7.60 | 0.65 |
| ≥16 | 8 | 24 | 95 | 48 | 87 | 84 | 4.80 | 0.80 |
| ≥17 | 5 | 20 | 98 | 62 | 87 | 86 | 10.00 | 0.81 |
| ≥18 | 2 | 10 | 99 | 62 | 86 | 85 | 10.00 | 0.90 |
| ≥19 | 2 | 8 | 99 | 67 | 86 | 85 | 8.00 | 0.92 |
| ≥20 | 0.3 | 2 | 100 | 100 | 85 | 85 | – | 0.98 |
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value; AC, accuracy; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Two groups of risk categories were identified for risk of mortality in both the DC and the VC: a low-risk (<11) and a high-risk (≥11) category. Mortality rates for patients with scores of <11 and ≥11 were 5.6% and 45.9% in the DC; and 5.4% and 34.8% in the VC, respectively.
Discussion
We describe a predictive scoring model (the INCREMENT-ESBL score) for all-cause 30 day mortality in patients with BSIs due to ESBL-E that can be calculated when susceptibility data are available. To our knowledge, this is the first prognostic score developed for this condition. Relevant aspects of the study are that the score was obtained from a multinational study with a large number of patients and that it was also validated.
A previous study identified a predictive score for patients with bacteraemia due to Gram-negative bacteria who had received appropriate empirical therapy;8 the predictive factors in that study were malignancy (3 points), liver cirrhosis (4), source other than urinary tract or catheter (4), and Pitt score 2–3 (2) or ≥4 (5). Because appropriate empirical therapy was not frequent (∼54% of patients in our cohort), that score would not be applicable to many patients with BSIs due to ESBL-E. When that score was calculated for our patients, considering only those with appropriate empirical therapy, its discriminative ability was only moderate (AUROC: 0.74; 95% CI: 0.68–0.79). This is probably due to the specific features of patients with BSIs due to ESBL-E. Additionally, we were interested in the impact of appropriate early targeted therapy administered on the same day that the score was calculated.
The predictive score developed showed good (but not excellent) predictive ability in both the DC and VC. However, in terms of implementation, the scoring system readily made it possible to identify patients at both a low and high risk of mortality. Overall, two-thirds of patients had a score of <11 and low mortality (≤5.6%). In contrast, 32% of patients had a ≥34.8% risk of mortality. The score is expected to be useful primarily to calculate the expected rate of mortality in future non-comparative studies when old or new drugs are used, and also in comparative studies to check if the mortality found in the standard-of-care arm is as expected. As regards its potential utility for clinical management beyond risk-stratification, this should be investigated in specific studies, i.e. it is necessary to test whether changes in management according to the score-based stratification of the patients (e.g. use of a more aggressive approach in higher-risk patients) are associated with any benefit. Despite including data that must be usually collected when evaluating patients with bacteraemia, the score is not as simple as we would have liked, which is surely a limitation for its clinical use.
The study identified the key risk factors associated with 30 day mortality among patients with BSIs due to ESBL-E. These include: age >50 years, infection due to Klebsiella spp. rather than other species, a source of infection other than urinary tract infections (UTIs), a fatal underlying disease according to the McCabe classification (i.e. death is expected to occur in <5 years as a consequence of the underlying condition), a condition of acute severity as measured by a Pitt score of >3, presentation of BSI with severe sepsis or septic shock, and inappropriateness of early targeted antibiotics (administered up until day 3). Most of these have been found in previous studies performed in specific countries and are now confirmed and measured here.17–19 Both the McCabe classification and the Charlson index have been validated as mortality predictors in many bloodstream studies; they measure the same concept and, in fact, correlated well in this study. While the McCabe index may be more subjective, the dichotomization used (non-fatal versus ultimately or rapidly fatal underlying condition) somehow limits its subjectivity; we decided to keep it in the score because it was more predictive than the Charlson index. As regards the Pitt score, it has also been repeatedly validated as a predictor of mortality.8,9,13 Different thresholds have been used in previous studies; by using CART analysis, we selected >3 points as our mortality predictor. The fact that Klebsiella spp. was associated with increased mortality should be noted; this might be due to increased intrinsic virulence or to the fact that Klebsiella may be a surrogate for baseline severity not adequately controlled for by other variables; in any case, data from studies including only E. coli isolates should not be extrapolated to all ESBL-E.
An important finding is the fact that inappropriate early targeted therapy was associated with increased mortality. We could not investigate the reasons for the delay in administering active targeted therapy, but lack of early and active reporting of the susceptibility data is a probable cause. This means that there is still an opportunity to improve the mortality rate of patients who receive inappropriate empirical therapy by reviewing them early, as soon as the susceptibility results are known, and providing advice for an active drug. This can be done by implementing active bacteraemia services.20–22 The fact that inappropriate empirical therapy was not shown to be independently associated with mortality, as was shown in previous studies with all-causes of bacteraemia,23 has also been found in other studies in ESBL-producers.19 Anyway, the exclusion of patients who died within 48 h according to the objectives in this study might have underestimated the importance of empirical therapy.
Our group recently published a predictive score for 14 day mortality in patients with BSIs due to carbapenemase-producing Enterobacteriaceae (CPE).24 Despite the fact that mortality was much higher in that cohort (45%), the score was similar to the one found in this study; the main difference was that Klebsiella spp. was not associated with outcome among patients with BSIs due to CPE, which is probably due to the fact that these organisms caused 85% of the episodes.
This study has several limitations. First, the data were obtained retrospectively, and some aspects of management beyond antimicrobial therapy were not collected. Second, despite the fact that the cases should be consecutive, we could not assess that and therefore the possibility of selection bias cannot be eliminated. Anyway, the features of the patients are very similar to those in other smaller cohorts. Third, residual confounding is possible. Fourth, despite the fact that the participating centres were expert in investigating patients with bacteraemia, the quality of the data may not have been equal across all sites. Fifth, validation was performed on the same cohort, not a new, prospective one. Finally, the ESBL were characterized only in a subgroup of patients.
In summary, a predictive score with good discriminative ability for the risk of all-cause 30 day mortality in adult patients with BSIs due to ESBL-E was developed and validated. The INCREMENT-ESBL score provides a baseline risk for future controlled and uncontrolled studies and may help physicians to identify patients at high and low risk of mortality.
Supplementary Material
Acknowledgements
We would like to thank the European Study Group of Bloodstream Infections and Sepsis (ESGBIS) from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) for endorsing the INCREMENT project. We thank Virginia Palomo for her contribution in reviewing the database, Alejandro González for his work with the online database programming and Luisa Baena for her help with the graphs.
Other members of the REIPI/ESGBIS/INCREMENT project Group
J. Gálvez (Hospital Universitario Virgen Macarena, Seville, Spain); M. Falcone, A. Russo (Policlinico Umberto I, Rome, Italy); G. Daikos (Laikon General Hospital, Athens, Greece); E. M. Trecarichi and A. R. Losito (Catholic University of the Sacred Heart, Rome, Italy); J. Gómez (Hospital Universitario Virgen de la Arrixaca, Murcia, Spain); E. Iosifidis and E. Roilides (Hippokration Hospital of Thessaloniki, Thessaloniki, Greece); I. Karaiskos (Hygeia General hospital, Athens, Greece); Y. Doi (University of Pittsburgh, Pittsburgh, USA); F. F. Tuon (Hospital da Universidade Federal do Paraná, Paraná, Brazil); F. Navarro and B. Mirelis (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain); R. San Juan and M. Fernández-Ruiz (Hospital 12 de Octubre, Madrid, Spain); N. Larrosa and M. Puig (Hospital Universitario Vall d’Hebrón, Barcelona, Spain); J. Molina and V. González (Hospitales Universitarios Virgen del Rocío y Virgen Macarena, Seville, Spain); V. Rucci (Hospital Español, Rosario, Argentina); E. Ruiz de Gopegui and C. I. Marinescu (Hospital Universitario Son Espases, Palma de Mallorca, Spain); M. C. Fariñas, M. E. Cano and M. Gozalo (Hospital Universitario Marqués de Valdecilla-IDIVAL, Santander, Spain); J. R. Paño-Pardo and Marta Mora-Rillo (Hospital Universitario La Paz-IDIPAZ, Madrid, Spain); S. Gómez-Zorrilla and F. Tubau (Hospital de Bellvitge, Barcelona, Spain); S. Pournaras, A. Tsakris and O. Zarkotou (University of Athens, Athens, Greece); Ö. K. Azap (Baskent University, Ankara, Turkey); M. Souli, A. Antoniadou and G. Poulakou (University General Hospital Attikon, Chiadiri, Greece); D. Virmani (University of Calgary, Calgary, Canada); Á. Cano and J. Guzmán-Puche (Hospital Universitario Reina Sofía-IMIBIC, Córdoba, Spain); Ö. Helvaci and A. O. Sahin (Hacettepe University, Ankara, Turkey); R. Cantón and P. Ruiz-Garbajosa (Hospital Ramón y Cajal, Madrid, Spain); M. Bartoletti and M. Giannella (Teaching Hospital Policlinico S. Orsola Malpighi, Bologna, Italy); S. Peter (Tübingen University Hospital, Tübingen, Germany); C. Badia and M. Xercavins (Hospital Universitario Mútua de Terrassa, Terrassa, Spain); D. Fontanals and E. Jové (Hospital Parc Taulí, Sabadell, Spain).
Funding
This study was funded by: the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III co-financed by the European Development Regional Fund ‘A way to achieve Europe’ ERDF, the Spanish Network for Research in Infectious Diseases (REIPI RD12/0015), FIS grant PI10/02021 and FIS grant PI14/01832. B. G. G., J. R. B., A. P. and Y. C. also received funds from the COMBACTE-CARE project, Innovative Medicines Initiative (IMI), the European Union’s Seventh Framework Programme (FP7/2007-2013) and in-kind contributions from EFPIA companies. R. A. B. was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program and the Geriatric Research Education and Clinical Center VISN 10 (VISN 10 GRECC), and the NIAID of the NIH under award numbers R01AI072219 and R01AI063517.
Transparency declarations
J. R. B. has been an advisor for AstraZeneca, Merck, InfectoPharm, Achaogen and Basilea, and a speaker at educational courses for AstraZeneca and Merck. R. A. B. received research grants from the NIH, Veteran Affairs, AstraZeneca, Merck, Melinta and Steris. D. L. P. has received honoraria for advisory board participation from Merck, AstraZeneca, Cubist, Pfizer and Novartis. Y. C. received grants, honoraria, travel support, consulting fees, and other forms of financial support from Achaogen, Allecra Therapeutics, AstraZeneca, Basilea Pharmaceutica LTD, Biomérieux, Cepheid, DaVolterra, Durata Therapeutics, Intercell AG, Merck, PPD, Proteologics, Rempex Pharmaceuticals, Rib-X Pharmaceuticals, Syntezza Bioscience Ltd and Takeda Pharmaceutical Company. B. A. has been scientific advisor for AstraZeneca, Merck, Pfizer, Novartis, Astellas and Gilead, and a speaker for AstraZeneca, Merck, Pfizer, Astellas, Gileead and Novartis. A. P. has been a speaker for Merck and B Braun; he has been scientific advisor for Merck and has received unrestricted research grants from B Braun and AstraZeneca. All other authors declare no conflicts of interest.
Author contributions
Z. R. P. B., B. G. G and J. R. B. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conception or design: B. G. G., R. A. B., Y. C., D. L. P., A. P. and J. R. B. Acquisition, analysis or interpretation of data: Z. R. P. B., B. G. G., M. dC., P. V., M. V., A. H. T., A. O., L. M. M., E. C., V. P., O. G., B. A., J. A. L., J. P., M. A., C. P. M., M. J. S., M. T., E. T., J. O., N. P., G. B., H. G., J. B., A. H., F. P., M. Almela, W. L., P. R. H., C. N. S. F., J. T. C., Y. C., R. A. B., D. L. P., A. P. and J. R. B. Drafting of the manuscript: Z. R. P. B., B. G. G., A. P. and J. R. B. Critical revision of the manuscript for important intellectual content: M. dC., P. V., M. V., A. H. T., A. O., L. M. M., E. C., V. P., O. G., B. A., J. A. L., J. P., M. A., C. P. M., M. J. S., M. T., E. T., J. O., N. P., G. B., H. G., J. B., A. H., F. P., M. Almela, W. L., P. R. H., C. N. S. F., J. T. C., Y. C., R. A. B and D. L. P. Statistical analysis: Z. R. P. B., B. G. G. and J. R. B. Obtaining funding: R. A. B., A. P. and J. R. B. Supervision: Y. C., R. A. B, D. L. P, A. P. and J. R. B. Administrative, technical or material support: B. G. G., M. dC., P. V, M. V, A. H. T, A. O., L. M. M., E. C., V. P., O. G., B. A., J. A. L., J. P., M. A., C. P. M., M. J. S., M. T., E. T., J. O., N. P., G. B., H. G., J. B., A. H., F. P., M. Almela, W. L., P. R. H., C. N. S. F. and J. T. C.
Supplementary data
Tables S1 and S2 and Figures S1–S7 are available as Supplementary data at JAC online (http://jac.oxfordjournals.org/).
References
- 1. European Centre for Disease Prevention and Control. Annual Epidemiological Report 2014. Antimicrobial Resistance and Healthcare-associated Infections Stockholm: ECDC; 2015. http://ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-annual-epidemiological- report.pdf.
- 2. Rodríguez-Baño J, Pascual A. Clinical significance of extended spectrum β-lactamases. Expert Rev Anti Infect Ther 2008; 6: 671–83. [DOI] [PubMed] [Google Scholar]
- 3. Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 2012; 67: 1311–20. [DOI] [PubMed] [Google Scholar]
- 4. Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum β-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007; 60: 913–20. [DOI] [PubMed] [Google Scholar]
- 5. Taconelli E, Cataldo MA, De Angelis G. et al. Risk scoring and bloodstream infections. Int J Antimicrob Agents 2007; 30: S88–92. [DOI] [PubMed] [Google Scholar]
- 6. Tumbarello M, Trecarichi EM, Bassetti M. et al. Identifying patients harbouring ESBL-producing Enterobacteriaceae on hospital admission. Antimicrob Agents Chemother 2011; 55: 3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson SW, Anderson DJ, Byron May D. et al. Utility of a clinical risk factor scoring model in predicting infection with extended-spectrum-β-lactamase-producing Enterobacteriaceae on hospital admission. Infect Control Hosp Epidemiol 2013; 34: 385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Hasan MN, Lahr BD, Eckel-Passow JE. et al. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin Microbiol Infect 2013; 19: 948–54. [DOI] [PubMed] [Google Scholar]
- 9. Al-Hasan MN, Juhn YJ, Bang DW. et al. External validation of bloodstream infection mortality risk score in a population-based cohort. Clin Microbiol Infect 2014; 20: 886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Second Informational Supplement M100-S22. CLSI, Wayne, PA, USA, 2012.
- 11. McCabe WR, Jackson GG. Gram-negative bacteremia: II. Clinical, laboratory, and therapeutic observations. Arch Intern Med 1962; 110: 856–64. [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic co-morbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 13. Hilf M, Yu V, Sharp J. et al. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 1989; 87: 540–6. [DOI] [PubMed] [Google Scholar]
- 14. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–32. [DOI] [PubMed] [Google Scholar]
- 15. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–74. [PubMed] [Google Scholar]
- 16. Bai AD, Showler A, Burry L. et al. Clinical prediction rules in Staphylococcus aureus bacteremia demonstrate the usefulness of reporting likelihood ratios in infectious diseases. Eur J Clin Microbiol Infect Dis 2016; 35: 1393–8. [DOI] [PubMed] [Google Scholar]
- 17. Kang CI, Kim SH, Park WB. et al. Bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother 2004; 48: 4574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tumbarello M, Sanguinetti M, Montuori E. et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51: 1987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frakking FN, Rottier WC, Dorigo-Zetsma JW. et al. Appropriateness of empirical treatment and outcome in bacteremia caused by extended-spectrum-β-lactamase-producing bacteria. Antimicrob Agents Chemother 2013; 57: 3092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodríguez-Baño J, Picón E, Gijón P. et al. Risk factors and prognosis of nosocomial bloodstream infections caused by extended-spectrum-β-lactamase-producing Escherichia coli. J Clin Microbiol 2010; 48: 1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minton J, Clayton J, Sandoe J. et al. Improving early management of bloodstream infection: a quality improvement project. BMJ 2008; 336: 440–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodríguez-Baño J, de Cueto M, Retamar P. et al. Current management of bloodstream infections. Expert Rev Anti Infect Ther 2010; 8: 815–29. [DOI] [PubMed] [Google Scholar]
- 23. Retamar P, Portillo M, López-Prieto M. et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 2012; 56: 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M. et al. A predictive model of mortality in patients with bloodstream infection due to carbapenemase-producing Enterobacteriaceae. Mayo Clin Proc 2016; 91: 1362–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.