Abstract
Background
Antimicrobial resistance threatens human health worldwide. Antimicrobial misuse is a major driver of resistance. Promoting appropriate antimicrobial use requires an understanding of how clinical microbiology services are utilized, particularly in resource-limited settings.
Objectives
To assess the appropriateness of antimicrobial prescribing and the factors affecting utilization of the established clinical microbiology service (CMS). The CMS comprises the microbiology laboratory, clinical microbiologists (infection doctors) and antimicrobial treatment guidelines.
Methods
This mixed-methods study was conducted at a non-governmental Cambodian paediatric hospital. Empirical and post-culture antimicrobial prescriptions were reviewed from medical records. The random sample included 10 outpatients per week in 2016 (retrospective) and 20 inpatients per week for 4 weeks in the medical, neonatal and intensive care wards (prospective). Post-culture prescriptions were assessed in patients with positive blood and cerebrospinal fluid cultures from 1 January 2014 to 31 December 2016. Focus group discussions and semi-structured interviews with clinicians explored barriers and facilitators to use of the CMS.
Results
Only 31% of outpatients were prescribed empirical antimicrobials. Post-culture prescriptions (394/443, 89%) were more likely to be appropriate than empirical prescriptions (447/535, 84%), based on treatment guidelines, microbiology advice and antimicrobial susceptibility test results (P = 0.015). Being comprehensive, accessible and trusted enabled CMS utilization. Clinical microbiologists provided a crucial human interface between the CMS and physicians. The main barriers were a strong clinical hierarchy and occasional communication difficulties.
Conclusions
Antimicrobial prescribing in this hospital was largely appropriate. A culturally appropriate human interface linking the laboratory and physicians is essential in providing effective microbiology services and ensuring appropriate antimicrobial prescribing in resource-limited settings.
Introduction
Antimicrobial resistance (AMR) is an increasingly dangerous threat to human health worldwide. Preserving the utility of existing antimicrobials is vital.1
Ensuring appropriate antimicrobial use can reduce AMR and improve outcomes, in both inpatient and community settings,2–4 and is advocated by the WHO.5,6 Dellit et al.7 describe the frequency of inappropriate antimicrobial use as a ‘surrogate marker for the avoidable impact on antimicrobial resistance’.
Addressing prescribing practices is a key feature of antimicrobial stewardship,8,9 with targeted interventions needed specifically for paediatrics and in resource-limited settings.10,11 Establishing a clinical microbiology service is a fundamental component of this.12,13 In contrast to reports evaluating technical microbiology laboratory processes, there are few reports evaluating clinical microbiology services,14 which is vital if the efficacy of services is to be optimized.15
Reasons for inappropriate antimicrobial prescribing include fear of treatment failure, lack of trust in laboratories and lack of knowledge.16–18 In a survey of Cambodian physicians working in government hospitals, Om et al.19 report that clinicians do not ‘fully utilize microbiology services’ based on the lack of knowledge of AMR rates and treatment of resistant organisms.
This study evaluated the existing clinical microbiology service at a non-governmental paediatric hospital in Cambodia, with regard to its effect on antimicrobial prescribing. This study explored the barriers to and facilitators of use of the service from the perspective of key stakeholders (physicians, microbiologists and hospital managers). In doing so, this study highlights the features of a clinical microbiology service that make it most useful, and which need to be prioritized. In an era of growing concern around AMR, it is essential that the resources dedicated to ensuring the appropriate use of antimicrobials are deployed effectively, particularly where those resources are limited.
Methods
Setting
This study was conducted at Angkor Hospital for Children (AHC), a non-profit paediatric referral hospital in Siem Reap, Cambodia. AHC is an 87 bed facility which in 2016 saw 127900 outpatients and 5596 inpatients.20
The clinical microbiology service (CMS) at AHC consists of a laboratory, two clinical microbiologists (one Cambodian and one expatriate) and treatment guidelines (in the format of the MicroGuide application from 2015). AHC has had a microbiology laboratory since 2006, with a comprehensive CMS since 2012. In 2016 the CMS processed 4518 blood and 272 cerebrospinal fluid cultures.
Study design
This mixed-methods study involved quantitative data collected from medical records, to evaluate current prescribing practice by determining the proportion of antimicrobial prescriptions that were appropriate to a recommendation. Focus group discussions (FGDs) and semi-structured interviews (SSIs) with hospital staff were conducted to determine the reasons for current prescribing practice, and facilitators and barriers to use of the CMS. Data collection occurred from February to June 2017. All data were anonymized.
Quantitative data
Empirical antimicrobial prescriptions were evaluated by randomly selecting 10 outpatients per week for the year 2016, and 20 inpatients per week for 4 weeks (prospectively) in each of the inpatient, neonatal and paediatric intensive care departments. A patient was included once per admission. Data collected included anonymized patient information, diagnosis, the empirical and guideline antimicrobial prescriptions, and documented reasons for discrepancy.
Positive cultures (excluding those deemed to be contaminated by skin flora) from blood and cerebrospinal fluid were identified from 1 January 2014 to 31 December 2016. Post-culture antimicrobial prescriptions were evaluated for these patients. The data collected additionally included the clinical microbiologist’s recommendations, and post-culture antimicrobials. Cultures from the same patient-episode (one illness episode as determined from the medical record) with the same result were recorded once.
Prescriptions were assessed to see whether the antimicrobial (name only) chosen matched the recommendation. English is used in clinical practice and for medical documentation at AHC.
For empirical prescriptions, the recommendation was the guideline antimicrobial for that diagnosis. Patient-episodes with no empirical antimicrobial prescriptions were assessed for the appropriateness of this according to the guideline for that diagnosis. For post-culture prescriptions the recommendation was either microbiology advice, antimicrobial susceptibility test (AST) results or the guideline, in that order of priority. For unclear prescriptions, expert opinion from the senior clinical microbiologist was sought. Documented reasons for discrepancy from the recommendation were deemed appropriate. Prescriptions not in accordance with any recommendation were categorized ‘not appropriate’.
Statistics
Data were analysed using the R statistical package (R Foundation for Statistical Computing, Vienna, Austria) and described as proportions. Comparisons between groups were done using the χ2 test (z-test).
Qualitative data
Prescribers at AHC (outpatient triage nurses and resident, middle-grade and senior doctors) from different departments were convenience sampled using sign-up sheets for potential participants to register their interest. Participants were invited to FGDs exploring how they utilize the CMS, and factors that facilitate or inhibit its use.
The two clinical microbiologists and two senior medical management staff were purposively sampled for individual face-to-face semi-structured interviews exploring participants’ opinions on antimicrobial prescribing at AHC.
With participants’ written informed consent FGDs and SSIs were audio-recorded. One author (S. F.-L.) facilitated and transcribed in English, with Khmer (national language) translation as needed by another author (S. P.). Concurrent data collection and analysis allowed iteration, and ceased when data saturation was reached.21 Transcripts and field notes were imported into the NVivo software package (QSR International Pty Ltd., version 11, 2015) to aid analysis. An inductive approach was taken to thematic content analysis.21
Ethics
Ethics approval was obtained from the Oxford University Tropical Research Ethics Committee (OxTREC 504-17) and the AHC Institutional Review Board (AHC-IRB 089/17). Written informed consent was obtained from all participants in the FGDs and SSIs. This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice, and adhered to the Research Governance policies of the University of Oxford.
Results
Appropriateness of antimicrobial prescribing
Overall, 84% of empirical prescriptions and 89% of post-culture prescriptions were appropriate to a recommended choice of antimicrobial.
In total, 1028 patient-episodes were included for analysis; 666 empirical patient-episodes and 362 post-culture patient-episodes (Figure S1, available as Supplementary data at JAC Online, summarizes the patient-episodes included). The most commonly prescribed classes were β-lactams, macrolides and aminoglycosides, with ceftriaxone the most frequently prescribed antibiotic.
Empirical antimicrobials were prescribed in 518 (50%) patient-episodes (Table 1). The outpatient department prescribed the lowest proportion of empirical antimicrobials (P < 0.0001). The most common diagnoses for which empirical antimicrobials were prescribed were pneumonia, typhoid and sepsis.
Table 1.
The number of empirical antimicrobial prescriptions per department
| Department | Total number of patient-episodes | Number of patient-episodes containing empirical antimicrobial prescriptions (%) |
|---|---|---|
| Outpatient | 567 | 175 (31) |
| Inpatient | 331 | 240 (73) |
| Intensive care | 130 | 103 (79) |
| Total | 1028 | 518 (50) |
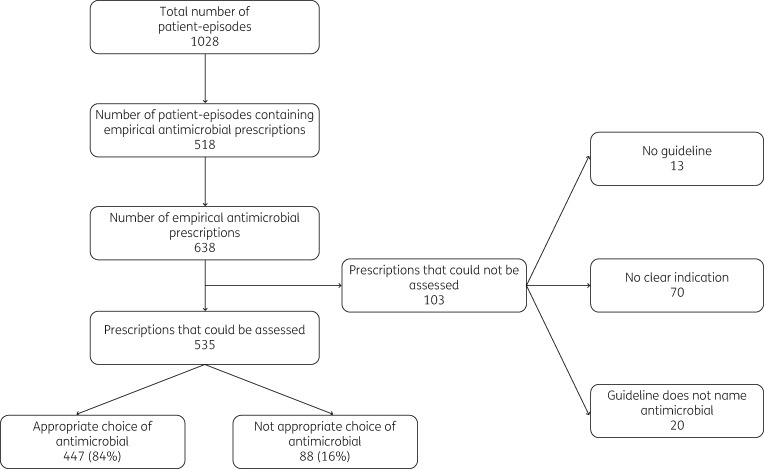
The 518 patient-episodes involved 638 empirical antimicrobial prescriptions (92 patient-episodes with two or more antimicrobials prescribed). Of these, 103 could not be assessed because there was no clear indication (70), no guideline (13) or the guideline stated ‘discuss with microbiology’ (20). Of the 535 prescriptions that could be assessed, 447 (84%) were appropriate (Figure 1).
Figure 1.
The number of empirical antimicrobial prescriptions that were appropriate.
There was no statistically significant difference in empirical antimicrobial appropriateness by department (P = 0.3207) or patient age group (P = 0.157) (Tables S1 and S2).
Among the 510 patient-episodes with no empirical antimicrobial prescriptions, the most common diagnoses were upper respiratory tract infection, gastroenteritis and bronchiolitis. The majority of these patient-episodes [428 (84%)] appropriately contained no empirical antimicrobial prescriptions.
The 362 post-culture patient-episodes (Figure S2 summarizes the patient-episodes included) involved 443 prescriptions. Microbiology advice was documented for 159 prescriptions, and was followed in 143 (90%). Overall, 394 (89%) prescriptions were appropriate (Table 2). Of the recommendation groups that these prescriptions were appropriate to, microbiology advice formed the largest group (32%).
Table 2.
Recommendation groups that post-culture antimicrobial prescriptions were appropriate to
| Recommendation group that prescriptions were appropriate to | Number of prescriptions (%), n = 443 |
|---|---|
| Microbiology advice | 143 (32) |
| AST result | 119 (27) |
| Guideline | 107 (24) |
| Other | 25 (6) |
| Total appropriate prescriptions | 394 (89) |
While overall prescribing appropriateness was high, post-culture prescriptions were significantly more likely to be appropriate than empirical prescriptions [89% versus 84% (P = 0.015)].
Facilitators of and barriers to CMS utilization
Five FGDs and four SSIs with 35 participants were conducted; Table 3 summarizes their characteristics.
Table 3.
Characteristics of focus group discussion (FGD) and semi-structured interview (SSI) participants
| Characteristic | FGD participants (n = 31), n (%) | SSI participants (n = 4), n (%) |
|---|---|---|
| Grade of cliniciana | ||
| triage nurse | 5 (16) | |
| resident doctor | 14 (45) | |
| middle-grade doctor | 9 (29) | |
| senior doctor | 3 (10) | 4 (100) |
| Male | 23 (74) | 4 (100) |
| Median age in years (range) | 29 (24–40) | 41.5 (37–47) |
| Years worked at AHC, median (range) | 3 (0.08–12) | 10.5 (4–18) |
| <1 year | 3 (10) | |
| 1 year | 8 (26) | |
| 2–4 years | 7 (23) | 1 (25) |
| 5–7 years | 6 (19) | 1 (25) |
| ≥8 years | 4 (13) | 2 (50) |
| question not answered | 3 (10) | |
| Department | ||
| outpatient | 8 (26) | |
| inpatient (medical) | 9 (29) | |
| neonatal unit | 7 (23) | |
| paediatric intensive care unit | 7 (23) | 1 (25) |
| microbiology | 2 (50) | |
| management | 1 (25) | |
| Training in microbiology previously | NA | |
| no | 29 (94) | |
| yes | 1 (3) | |
| question not answered | 1 (3) |
NA, not applicable.
Middle-grade doctors are often considered in the same bracket as senior doctors at AHC. Therefore, in this text resident doctors will be referred to as ‘junior doctors’ and the term ‘senior doctors’ refers to middle-grade and senior doctors.
As shown in Table 3, equal numbers of junior and senior doctors participated in FGDs. Fewer triage nurses participated, due to the fact that the majority of antimicrobial prescribing at AHC is done by doctors. Most participants were male, reflecting that the majority of staff at AHC are male. The SSI participants were older and had worked at AHC for longer, reflecting their more senior positions. Clinical departments were evenly represented in the FGDs.
Eight key themes emerged from the qualitative data regarding utilization of the CMS: facilitators (understanding the role of the CMS, comprehensive, accessibility and trust) and barriers (lack of clinical confidence, hierarchy, fixed beliefs and behaviours, and communication).
Facilitators
Understanding the role of the CMS
All groups unanimously agreed that the CMS was valuable and had improved antimicrobial use at AHC (quote 1.1, Table 4). Specific training was directed towards ensuring physicians understood the role of the CMS. Management staff said that this was a key reason why the CMS was used effectively at AHC (quote 1.2, Table 4).
Table 4.
Key quotes from the FGDs and SSIs by theme
| Theme | Quote number | Quote | Participant |
|---|---|---|---|
| Understanding the CMS | 1.1 | Before, when we worked without microbiologists we used a lot of antibiotics, abused [emphasized] antibiotics … And now we use them more correctly … And we can know the organism grown, and we know the resistance, so we can use narrow spectrum antibiotics and minimize the cost to AHC. | senior doctor |
| 1.2 | I think the training, education we provide to physicians is important, so they know the important role of the microbiology service, to make sure they understand what is really useful about it and how it can help their practice to take care of the patient. | management | |
| 1.3 | First of all about blood cultures, how to be sterile, otherwise maybe there will be mixed growth. And another thing is to make sure that we thoroughly check the condition of the patient before we give antibiotics. It will be much better to reduce resistance or unnecessary use of antibiotics, also spending money for nothing. | junior doctor | |
| Comprehensive | 2.1 | We’ve got the microbiology team, and we’ve got the rounds two times a week, and we’ve got an on-call service so we can call them anytime. And we’ve got the facility that can grow the organisms, a reliable lab. And I think the micro team has developed guidelines for us. | management |
| 2.2 | You need to have a person who understands the way clinicians work, and understands the way the microbiology laboratory works. And then that person is just like a bridge to bring them together. You need to build a person in house to do that activity, it’s very important. | clinical microbiologist | |
| 2.3 | They know things we don’t know [laughter]. Mostly about organisms, like how they survive, how they kill, how the antibiotics work on them, the side-effects of antibiotics. | junior doctor | |
| Accessibility | 3.1 | I got a phone call from [name of microbiologist] when the patient grew Gram-negative bacteria and he told me that it might be melioidosis. The patient was treated with ceftriaxone, and then he called me to change it. And ultimately it was melioidosis. I could change it very fast because when he got the result he called me. | senior doctor |
| Trust | 4.1 | I think we have open lines of communication basically, and mutual trust. And that’s come with time and generation of results that seem to be useful … Our doors are always open and we can have open dialogue about problems as they come up. | clinical microbiologist |
| 4.2 | I think that MicroGuide is great for us because it is based on Cambodian research, not global research. | junior doctor | |
| Lack of clinical confidence | 5.1 | I think that if we have no microbiology to determine the pathogen, maybe we would still treat with blind reason on the clinical features. We would not be confident about source of infection, or the pathogen. | junior doctor |
| 5.2 | Sometimes the clinical picture does not fit the microbiology result. Like, the blood culture is positive but the clinical features of the patient do not fit, it doesn’t fit together. So we have to think again, to revise again …We have to discuss. Microbiologists come and we have to discuss together, and focus on the benefit to the patient. | senior doctor | |
| Hierarchy | 6.1 | So it is very difficult. I accept the ideas of the microbiologists, but we have no choice. | junior doctor |
| 6.2 | Because unless I’m happy, because the decision is made, in the end, is made by the people who are taking care of the patient … if you think that the idea [from microbiology] is right, and you accept it, you do it. But if you disagree and you think that you’re doing the right thing and the patient’s getting better, just keep on with that. | senior doctor | |
| Fixed beliefs and behaviours | 7.1 | Sometimes they [doctors] believe in this antibiotic, so they don’t want to change their behaviour. It’s not the problem of communication, it’s not the problem of the facility, but they just personally, yeah. And I appreciate that the micro team works hard, they generally try very hard to talk with the physicians, even when they don’t accept their advice but they try hard to explain it to them. | management |
| 7.2 | Sometimes, like at the private hospital, they prescribe because they can charge. You understand? They charge money. And also they can get benefits from the pharmaceutical company … For patients, their culture when they come to the hospital is that they need medicine. Mostly if we don’t prescribe antibiotics they don’t feel confident, they are not happy … So this is the challenge, to change this culture. | management | |
| 7.3 | We continue, we try, we will not stop doing, and we will continue providing our service. And we encourage them to understand, and I hope that the challenge will be reduced … I am a person to bridge, to narrow the gap between the clinicians bit by bit, bit by bit. And with time it’s going to be closer together. And people come to understand each other. | clinical microbiologist | |
| Communication | 8.1 | Sometimes the microbiologist doesn’t know everything, doesn’t know all the guidelines. But I suppose we can learn from each other. Because we learn a lot from him, but can he learn from us? | senior doctor |
| 8.2 | Yeah, saving face, it’s a critical part of cultural interactions here. It’s very bad form to criticize somebody directly and make them feel or appear incorrect or wrong or less knowledgeable, than their status would predict. So that really limits the direct challenging of a doctor’s diagnosis or a treatment plan that can be done. | clinical microbiologist | |
| 8.3 | [Referring to differing opinions of microbiologists and physicians] We are different people, different concepts, different ideas and opinions. We don’t totally agree with each other, so we need to find one solution that is appropriate for most partners. | senior doctor |
Equally important was a clear understanding of the physician’s role in providing accurate clinical information, ensuring aseptic sample collection and using antimicrobials judiciously. Physicians partaking in this study understood the impact this has on AMR and optimizing the cost of healthcare (quote 1.3, Table 4).
All groups of participants reported that in their experience AHC had better antimicrobial prescribing practices than other healthcare facilities in Cambodia. Participants related this to the presence and nature of the CMS at AHC, specifically that it was comprehensive, accessible and trustworthy, as discussed below. Participants recognized that the effective use of the CMS was due to physicians understanding its value and remit, and also their role in achieving useful results (quote 1.3, Table 4).
Comprehensive
Participants appreciated the complete service offered by the CMS of laboratory tests, guidelines and clinical microbiologists. Physicians particularly spoke of how useful contact with clinical microbiologists was in aiding their decision-making (quote 2.1, Table 4).
Clinical microbiologists provided a human interface linking the laboratory and physicians, optimizing understanding between the teams (quote 2.2, Table 4). This interface reinforced when and how best to use the laboratory, helped the laboratory understand which tests to conduct, and by engaging directly with physicians enhanced their knowledge of microbiology. As such, this human interface provided a channel through which to bolster antimicrobial stewardship activities (quote 2.3, Table 4).
Accessibility
Physicians valued the accessibility of the CMS, particularly the ease of contact with clinical microbiologists. Clinical microbiologists would promptly relay significant laboratory results and their meaning to physicians. Participants acknowledged how useful this accessible human interface was in providing timely, high-quality patient care (quote 3.1, Table 4).
Trust
The efficacy of a comprehensive accessible CMS hinged on physicians acting on information provided. For this to occur, physicians must trust the information delivered by the CMS. Participants said that they trust the CMS because of its demonstrably high quality (e.g. participation in external quality assurance) and the generation of useful results (quote 4.1, Table 4). Open channels of communication allowed the development of ‘mutual trust’ between the CMS and physicians, enabled by the human interface of clinical microbiologists.
Generating locally relevant data was deemed very important by all participants, and enhanced trust in the stewardship messages delivered by the CMS (quote 4.2, Table 4).
Participants also spoke of barriers to effective utilization of the CMS: lack of clinical confidence, hierarchy, fixed beliefs and behaviours, and communication.
Barriers
Lack of clinical confidence
Physicians described how uncomfortable they were with ‘treating blindly’, feeling more confident with microbiological evidence to guide their decisions. Physicians could then feel sure that they were doing the right thing. Without microbiological evidence, they would fall back on clinical features to make decisions, but suggested that they were less comfortable with this (quote 5.1, Table 4).
Physicians said they did not feel confident in selecting antimicrobial treatment when the clinical features and microbiological result were discrepant. In these situations they discussed with the microbiologists, relying on this interface between them and the microbiological result (quote 5.2, Table 4).
Hierarchy
Occasionally microbiology advice was not followed. Senior doctors made the final treatment decisions. Participants described a strong clinical hierarchy, which made discussions around suitable treatment challenging.
Even when they would treat a patient based on microbiology advice, junior doctors were unable to if their senior held a different view, because of the clinical hierarchy. Junior doctors described discomfort with questioning their senior’s actions, and in practice their prescriptions could only be actioned after a countersign from their senior (quote 6.1, Table 4).
Participants mentioned that the advisory nature of clinical microbiology meant that overall responsibility for the patient lay with the clinical team. Senior doctors said that the microbiologist’s advice would be considered, but ultimately the treatment decision was theirs (quote 6.2, Table 4).
Microbiologists and management staff also felt that the established hierarchy provided the biggest challenge to antimicrobial stewardship.
Fixed beliefs and behaviours
Participants reported that (mainly senior) doctors harboured fixed beliefs about antimicrobial treatment. Diagnostic microbiology has advanced over the years, but participants reported that some senior doctors had not updated their practice accordingly (quote 7.1, Table 4).
Participants thought this may be because the senior doctors were reluctant to update their practice, or because they practised in an over-cautious way (as explained in ‘Lack of clinical confidence’). Competing interests, such as monetary gain and patient demands for antimicrobials, were also reported as reasons for inappropriate prescribing (quote 7.2, Table 4). Some physicians at AHC also worked at other fee-charging facilities, which may have influenced their prescribing practices.
Participant-suggested strategies to resolve these challenges were perseverance and maintaining the human interface (quotes 7.1 and 7.3, Table 4). Maintaining open channels between physicians and the laboratory, by way of the clinical microbiologists, was regarded as crucial by participants in affecting behavioural change over time.
Communication
Communication was the main barrier to CMS utilization for physicians. Discussion occasionally resulted in conflict between the clinical and microbiology teams. Senior doctors said this made them feel frustrated and undervalued (quote 8.1, Table 4). Both teams desired more mutual understanding at times.
Participants noted that the cultural context of these challenging discussions was important. In Cambodian society, the difficulties in challenging someone in a position of higher hierarchy were compounded by the concept of ‘saving face’. Directly challenging or questioning someone was taboo because they would ‘lose face’ (quote 8.2, Table 4). It also meant that someone may not openly say they disagree or do not understand.
All groups agreed that working together as a team was essential, as was keeping the discussion patient-centred, as ultimately all parties wanted the best possible outcome for the patient (quote 8.3, Table 4).
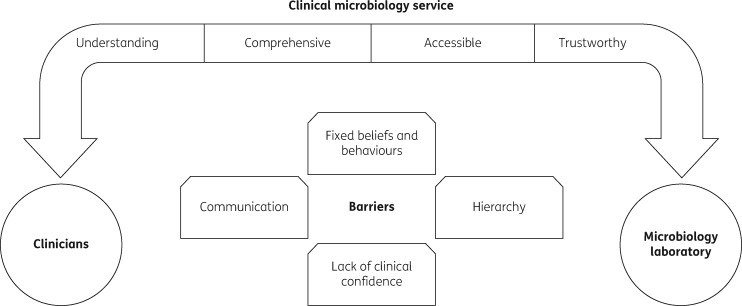
Overall, these findings showed that the CMS, by way of the clinical microbiologists, could bridge the microbiology and clinical teams, resulting in optimal patient care (illustrated in Figure 2). Factors that facilitated effective use of the CMS were understanding its role, and it being comprehensive, accessible and trustworthy. These factors helped overcome the barriers to its use: lack of clinical confidence, clinical hierarchy, fixed prescribing beliefs and behaviours, and communication.
Figure 2.
Facilitators of and barriers to utilization of the clinical microbiology service.
Discussion
There is growing global concern about AMR, and focus on solutions to tackle it. Several reviews call for the strengthening of microbiology laboratory facilities.12,13,16,22 This study finds that, in addition, in order to address antimicrobial prescribing behaviour the human interface provided by clinical microbiologists is essential: it would be remiss to neglect this. Furthermore the CMS must be delivered in a culturally appropriate manner, with training to help physicians understand its value.
Review articles report that roughly half of inpatients receive antimicrobial treatment.10,23 This study also found that 50% of patients were prescribed empirical antimicrobials. However, outpatient antimicrobial use was lower, at only 31% of outpatients.
Reviews also found that 30%–50% of prescriptions are inappropriate.2,23 A Cambodian study found that 85% of clinicians would inappropriately prescribe antimicrobials for common cold.19 Contrastingly, in this study prescribing practices were mainly appropriate: 84% of empirical and 89% of post-culture prescriptions contained the appropriate choice of antimicrobial. There were no differences in appropriateness between departments, which is expected since junior doctors rotate through departments.
Previous work found that most Cambodian physicians did not have access to microbiology services, and even when they did only 58% of physicians would act upon microbiology results.19 However, this study found that where microbiology advice was given, it was followed (90%). Physicians at AHC associate the current good practice with the presence of the CMS, and as this study shows, act upon the information from it.
Key facilitators to utilization of the CMS include accessibility, with the presence of onsite clinical microbiologists important in facilitating discussions. Clinical microbiologists were identified as an essential component to a comprehensive service. Developing this resource of clinical microbiologists or infection doctors will require particular attention in resource-limited settings, where postgraduate specialty training programmes are often lacking.19 When implementing a CMS, training dedicated to helping physicians understand its role will make it more likely that they engage with and use the service. Trust in a high-quality CMS means physicians believe and act upon the results.
Similar to previous reviews, this study found that lack of physician confidence, inappropriate prescribing behaviours, patient demands and economic pressures drive antimicrobial misuse.16,22 In addition, this study found that clinical hierarchy, occasional communication difficulties and the sociocultural phenomenon of ‘saving face’ are further barriers. These concepts are not unique to Cambodia; in any global setting local cultural practices will need to be addressed. By working alongside physicians and retaining Cambodian staff, the CMS endeavours to strike a delicate balance between culturally sensitive communication and effective antimicrobial stewardship.
To our knowledge, this is the first study to evaluate the effect of an existing clinical microbiology service on antimicrobial prescribing practices, using a mixed-methods approach to holistically understand current practice, as advocated by Radyowijati et al.16 Limitations of this study include that medical record review was the only way to gather much of the quantitative data. Thus, there may be cases of inaccuracy in the clinical documentation which could have affected the results. Qualitative data collection needed to rely on convenience sampling, in order to respect participants’ commitments to their clinical duties, and therefore some clinicians who may have wished to participate could have been excluded. This single-centre study conducted at a non-government hospital may not be representative of other institutions in resource-limited settings. However, the very nature of being conducted at one site that is non-government has allowed this study to examine in depth the ways in which a CMS can best be used to improve antimicrobial prescribing. The conclusions drawn from this study would potentially be applicable to a wide variety of settings where there is a need to establish or strengthen microbiology services.
This study highlights how a CMS can most effectively be implemented, making it globally applicable. In order to halt the growing problem of AMR, ensuring appropriate antimicrobial use is key. In order to change prescribing practices to enhance antimicrobial stewardship, establishing microbiology laboratory facilities, or providing data, is alone not sufficient. There must be a human interface of trained microbiologists or infection doctors to liaise between the laboratory and physicians. Implementing this in a culturally appropriate manner, with training for physicians to understand its use, will enable the most effective utilization of the clinical microbiology service.
Supplementary Material
Acknowledgements
The Cambodia Oxford Medical Research Unit/Angkor Hospital for Children microbiology laboratory is funded by grants from the Kadoorie Charitable Foundation, the Li Ka Shing University of Oxford Global Health Programme and by the Wellcome Trust as part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme.
The findings from this study were presented as a poster at the Singapore International Infectious Diseases Conference 2017 (Poster reference number: P50).
We thank the staff at Angkor Hospital for Children. In particular we thank Ms Sophea Srey and her team for their assistance in retrieving medical records, and Mr Sopheary Sun for his assistance in retrieving data from the hospital database.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
Author contributions
S. F.-L., N. P. J. D., P. T. and C. T. conceived and designed the study. S. F.-L. and T. M. collected quantitative data. S. F.-L. facilitated focus group discussions and semi-structured interviews; S. P. was note-taker for focus group discussions. S. F.-L. transcribed audio-recordings which were checked by S. P. for accuracy and translation. S. F.-L., S. P. and C. T. analysed the qualitative data. S. F.-L. wrote the manuscript with support from C. T. All authors edited the manuscript, and approved the final version for submission.
Supplementary data
Figures S1 and S2 and Tables S1 and S2 appear as Supplementary data at JAC Online.
References
- 1. World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1.
- 2. Davey P, Brown E, Charani E. et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2013; issue 4: CD003543. [DOI] [PubMed] [Google Scholar]
- 3. Bell BG, Schellevis F, Stobberingh E. et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costelloe C, Metcalfe C, Lovering A. et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: c2096.. [DOI] [PubMed] [Google Scholar]
- 5. Leung E, Weil DE, Raviglione M. et al. The WHO Policy Package to Combat Antimicrobial Resistance http://www.who.int/bulletin/volumes/89/5/11-088435/en/. [DOI] [PMC free article] [PubMed]
- 6. World Health Organization. Global Action Plan on Antimicrobial Resistance 2015. http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. [DOI] [PubMed]
- 7. Dellit TH, Owens RC, McGowan JE Jr. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 8. Okeke IN, Lamikanra A, Edelman R.. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis 1999; 5: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmes AH, Moore LSP, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 10. Le Doare K, Barker CI, Irwin A. et al. Improving antibiotic prescribing for children in the resource-poor setting. Br J Clin Pharmacol 2015; 79: 446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayukekbong JA, Ntemgwa M, Atabe AN.. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 2017; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Branda JA, Lewandrowski K.. Utilization management in microbiology. Clin Chim Acta 2014; 427: 173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laxminarayan R, Duse A, Wattal C. et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis 2013; 13: 1057–98. [DOI] [PubMed] [Google Scholar]
- 14. Wooster SL, Sandoe JA, Struthers JK. et al. Review of the clinical activity of medical microbiologists in a teaching hospital. J Clin Pathol 1999; 52: 773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newland JG, Hersh AL.. Purpose and design of antimicrobial stewardship programs in pediatrics. Pediatr Infect Dis J 2010; 29: 862–3. [DOI] [PubMed] [Google Scholar]
- 16. Radyowijati A, Haak H.. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc Sci Med 2003; 57: 733–44. [DOI] [PubMed] [Google Scholar]
- 17. Quet F, Vlieghe E, Leyer C. et al. Antibiotic prescription behaviours in Lao People's Democratic Republic: a knowledge, attitude and practice survey. Bull World Health Organ 2015; 93: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia C, Llamocca LP, Garcia K. et al. Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru. BMC Clin Pharmacol 2011; 11: 18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Om C, Vlieghe E, McLaughlin JC. et al. Antibiotic prescribing practices: a national survey of Cambodian physicians. Am J Infect Control 2016; 44: 1144–8. [DOI] [PubMed] [Google Scholar]
- 20.Angkor Hospital for Children. Angkor Hospital for Children Annual Report 2016. http://angkorhospital.org/wp-content/uploads/newsletters/2016-annual-report.pdf.
- 21. Chandler CIR, Reynolds J, Palmer JJ. et al. ACT Consortium Guidance: Qualitative Methods for International Health Intervention Research. London School of Hygiene & Tropical Medicine, 2013. http://www.actconsortium.org/data/files/resources/72/ACTc-Guidance.-Qualitative-methods-for-international-health-intervention-research_Dec2013.pdf. [Google Scholar]
- 22. Phuong NTK, Hoang TT, Van PH. et al. Encouraging rational antibiotic use in childhood pneumonia: a focus on Vietnam and the Western Pacific Region. Pneumonia (Nathan) 2017; 9: 7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spivak ES, Cosgrove SE, Srinivasan A.. Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 2016; 63: 1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.