Abstract
Background: Newborns of HIV-infected mothers are given daily doses of nevirapine to prevent HIV-1 acquisition. Infants born to mothers with TB should also receive TB preventive therapy. TB preventive regimens include isoniazid for 6 months or rifampicin plus isoniazid for 3 months (RH preventive therapy). The effect of concomitant RH preventive therapy on nevirapine concentrations in infants is unknown.
Patients and methods: Tshepiso was a prospective case–control cohort study of pregnant HIV-infected women with and without TB whose newborn infants received standard doses of nevirapine for HIV prophylaxis. Infants born to mothers with TB also received RH preventive therapy. Infant plasma nevirapine concentrations were measured at 1 and 6 weeks. The effects of RH preventive therapy on nevirapine disposition were investigated in a population pharmacokinetic model.
Results: Of 164 infants undergoing pharmacokinetic sampling, 46 received RH preventive therapy. After adjusting for weight using allometric scaling, the model estimated a 33% reduction in nevirapine trough concentrations with RH preventive therapy compared with TB-unexposed infants not receiving concomitant rifampicin and a 30% decline in trough concentrations in a typical infant between day 7 and 35 post-partum.
Conclusions: Rifampicin-based TB preventative treatment reduces nevirapine concentrations significantly in HIV-exposed infants. Although the nevirapine exposures required to prevent HIV acquisition in breastfeeding infants are undefined, given the potential risks associated with underdosing nevirapine in this setting, it is prudent to avoid rifampicin-based preventive therapy in HIV-exposed children receiving prophylactic nevirapine.
Introduction
Antiretrovirals for mothers and their infants provide the most effective interventions for the prevention of mother-to-child transmission (PMTCT) of HIV-1. In South Africa >90% of HIV-infected pregnant women receive such regimens and the transmission rates 6 weeks post-partum are <3%.1 In keeping with WHO guidelines, HIV-exposed newborns in South Africa receive daily nevirapine for at least 6 weeks.2,3 In high burden settings, TB is common among HIV-infected pregnant women.4–6 Isoniazid preventive therapy (IPT) for 6 months is recommended to prevent TB in newborn infants of pregnant women with TB disease; however, adherence to daily IPT for 6 months is challenging for infants and their caretakers. Daily rifampicin plus isoniazid for 3 months (RH preventive therapy) is an acceptable alternative that has the benefit of higher completion rates.7–9
Apart from dose and body size, several factors may affect nevirapine exposure in infants. Nevirapine is metabolized primarily by cytochrome P450 (CYP) 3A4 and 2B6. Maturation of these enzymes during the early weeks of life and autoinduction through activation of the constitutive androstane receptor reduce bioavailability and increase clearance over time. Genetic polymorphisms of CYP 2B6 have been shown to impact nevirapine concentrations significantly in pregnant women and infants.10–12 Conceivably, growth and maturation, and transplacental and breast milk transfer of nevirapine would further modulate the requirement for oral dosing of nevirapine.12,13
Drugs co-administered to breastfeeding mothers or infants may result in altered nevirapine concentrations. Rifampicin induces CYP3A4 potently and CYP2B6 to a lesser extent, while isoniazid inhibits several enzymes including CYP3A4. The net effect of TB treatment containing rifampicin and isoniazid is to reduce substantially the nevirapine concentrations in HIV–TB co-infected adults and children.14,15 However, there is little information about the impact of regimens containing rifampicin and isoniazid on nevirapine concentrations in newborns and infants receiving nevirapine for PMTCT.
We used a population modelling approach to describe the impact of concomitant maternal and/or infant treatment with rifampicin on the pharmacokinetics of daily nevirapine in newborns of HIV-infected women enrolled in a prospective study in South Africa.
Methods
Cohort study design
Tshepiso was a prospective cohort study evaluating the effects of TB on HIV-infected pregnant women and their infants in Soweto, South Africa. HIV-infected pregnant women with TB disease were enrolled as cases. For each case, two HIV-infected pregnant women with no evidence of TB were selected as controls. The recruitment and enrolment procedures have been reported previously.16,17 Briefly, women ≥18 years old and pregnant for at least 13 weeks were eligible. Cases had confirmed or probable TB and controls were matched on gestational age, maternal age and date of enrolment. Antiretrovirals for maternal treatment and PMTCT as well as TB treatment and prevention regimens were provided through routine public sector clinics, in line with national treatment guidelines.3 Women were followed antenatally and during delivery, thereafter mother–infant pairs were followed to 1 year post-partum.
Ethics
The study was approved by the institutional review boards of Johns Hopkins University, the University of the Witwatersrand and the University of Cape Town. Written informed consent was provided by each woman enrolled.
Drug dosing
Infants started daily doses of nevirapine at birth and continued nevirapine until at least 6 weeks of age (longer if mothers continued to breastfeed). Infants ≤2.0 kg at birth were given daily 2 mg/kg doses of oral suspension for 2 weeks, then 4 mg/kg until 6 weeks of age. Infants with a birth weight of 2.0–2.5 kg were given 10 mg once daily and infants weighing >2.5 kg at birth were given a once-daily 15 mg dose, until 6 weeks of age. The daily dose of nevirapine was increased to 20 mg for infants 6 weeks to 6 months old. Infants receiving RH preventive therapy were administered rifampicin and isoniazid doses for each drug of ∼10 mg/kg daily.
Pharmacokinetic and HIV laboratory assessments
As part of a pharmacokinetic substudy, infant nevirapine concentrations were measured in a single plasma sample taken ∼7 days after birth and repeated at the 6 week follow-up visit. Plasma nevirapine concentrations were also determined in cord blood if the mother had received nevirapine for PMTCT and a suitable sample could be obtained at delivery. Maternal HIV viral load and CD4+ lymphocyte count were measured at enrolment and infants were tested for HIV using DNA PCR testing in their first week and again at 6 weeks of age.
Plasma nevirapine concentrations were determined using liquid chromatography/tandem mass spectrometry in the Division of Clinical Pharmacology at the University of Cape Town. The assay was validated over the concentration range 0.078–20 mg/L and had accuracies of 99.4%, 100.1% and 102.1% at low, medium and high concentrations, respectively, during inter-day sample analysis. Coefficients of variation were <7% across the range of concentrations, confirming excellent precision.
Pharmacokinetic model development
Non-linear mixed-effects modelling in NONMEM 7.318 was used to describe the population pharmacokinetics of nevirapine in the infants. Model development was guided by decreases in objective function value (OFV), with a drop of 3.84 points assumed to be statistically significant at P < 0.05 for 1 additional degree of freedom, and inspection of goodness-of-fit plots and visual predictive checks.19 Several structural models were tested. These included models describing one- or two-compartment disposition kinetics with first-order elimination and first-order absorption, with or without absorption lag time or with a series of transit compartments.20 Between-subject (BSV) and -occasion variability (BOV) was included assuming a log-normal distribution for the individual parameters. A combined additive and proportional error model was used to describe residual unexplained variability. Allometric scaling was included to account for the effect of body size at an early stage of model development, as previously suggested.21 Nevirapine concentrations measured in cord blood were tested as initial concentrations in the neonate model. Further potential effects on clearance and bioavailability that were investigated in the model included post-menstrual age, treatment duration, infant RH preventive therapy and, in breastfed infants, maternal nevirapine or rifampicin ingestion. Continuous covariates were tested as linear, exponential and sigmoidal effects. Individual Bayesian post-hoc estimates of nevirapine trough concentrations (Cmin) were derived from the model.
Results
A total of 164 infants were started on daily nevirapine and underwent pharmacokinetic evaluation. Maternal and infant characteristics are shown in Table 1. RH preventive therapy was the only TB prevention regimen used and was started in 45 of 53 (85%) infants born to mothers with TB and one infant whose mother did not have TB. Amongst mothers with TB (cases), 44 were on combination ART (cART) at the time of delivery, 7 received single dose nevirapine for PMTCT, 1 was on zidovudine monotherapy and 1 did not receive antiretrovirals perinatally. Amongst 111 pregnant women without TB (controls), 74 were on cART, while 25 received single dose nevirapine and 12 zidovudine monotherapy for PMTCT.
Table 1.
Maternal and infant characteristics for cases and controls, respectively
| Maternal TB disease, n = 53 | No maternal TB, n = 111a | |
|---|---|---|
| Infant sex—male, n (%) | 29 (55) | 55 (49) |
| Birth weight (kg), median (IQR), min–max | 3.04 (2.64–3.35), 0.98–3.80 | 3.06 (2.75–3.30), 1.02–4.43 |
| Maternal age (years), median (IQR) | 30 (26–32) | 30 (26–32) |
| Maternal CD4+ lymphocyte count at enrolment (cells/mm3), median (IQR)b* | 289 (149–382) | 365 (261–449) |
| Maternal viral suppression at enrolment, n (%)b | 20 (31) | 54 (49) |
| Maternal viral load (viral load not suppressed) at enrolment (copies/mL), median (IQR), min–max | 4052 (127–21 105), 0–137 040 | 1023 (398–9418), 71–423 065 |
| Mothers on cART at delivery n (%)* | 44 (83) | 74 (67) |
| Maternal antiretrovirals at delivery, n (%) | ||
| cART | 44 (83)c | 74 (67)d |
| single-dose nevirapine | 7 (13) | 25 (22) |
| other | 2 (4) | 12 (11) |
| Maternal duration on ART at delivery (weeks), median (IQR)* | 14 (10–19) | 21 (12–88) |
| Initial feeding, n (%) | ||
| breast feeding | 13 (25) | 30 (27) |
| formula feeding | 37 (73) | 76 (69) |
| mixed feeding | 1 (2) | 4 (4) |
| Gestational age (weeks), median (IQR) | 38 (37–40) | 39 (38–40) |
| Infant nevirapine dose (mg/kg), by time of pharmacokinetic sampling visit, median (IQR), n | ||
| <28 dayse | 4.93 (4.35–5.10), 43 | 4.85 (4.23–5.30), 96 |
| ≥8 daysf | 3.30 (2.99–3.75), 28 | 3.23 (2.99–3.75), 74 |
| Infant TB chemoprophylaxis, n (%)g* | 45 (85) | 1 (0.9) |
| Infant TB diagnosed, n (%)* | 3 (5.7) | 0 (0) |
| Infants acquiring HIV, n (%) | 2 (3.8) | 2 (1.8) |
| Infant death, n (%) | 1 (1.9)h | 0 (0) |
Percentage values are calculated separately for the ‘Maternal TB disease’ and ‘No maternal TB’ groups.
One mother of twins (control) is counted twice.
Values closest to the date of delivery are presented.
At delivery, 40 mothers were on efavirenz-based ART, while 1 was receiving nevirapine-based ART and 3 were receiving PI-based ART.
At delivery, 59, 11 and 4 were on efavirenz-, nevirapine- and PI-based ART, respectively.
Median (IQR) = 7 (6–10) days after birth.
Median (IQR) = 45 (43–48) days after birth.
Combined rifampicin plus isoniazid daily for 3 months.
Died of gastroenteritis 366 days after birth (HIV status at time of death unconfirmed).
Difference between case and control groups is statistically different at P < 0.05 level.
A total of 296 infant nevirapine concentrations were measured. Of these, 55 concentrations obtained at the second pharmacokinetic visit from infants who had stopped taking nevirapine several days before sampling were below the limit of quantification of the assay. After exclusion of these values, the model was used to fit nevirapine concentrations from 155 infants, measured in 241 samples taken a median (IQR) 4.25 h (3.29, 14.9) after a nevirapine dose, 2–83 days after birth.
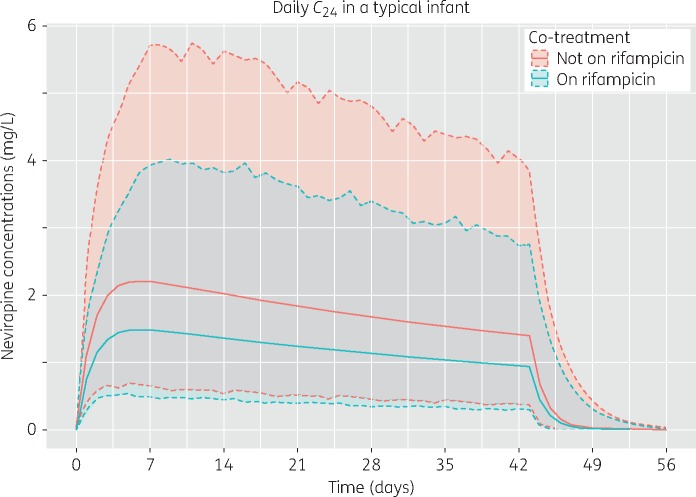
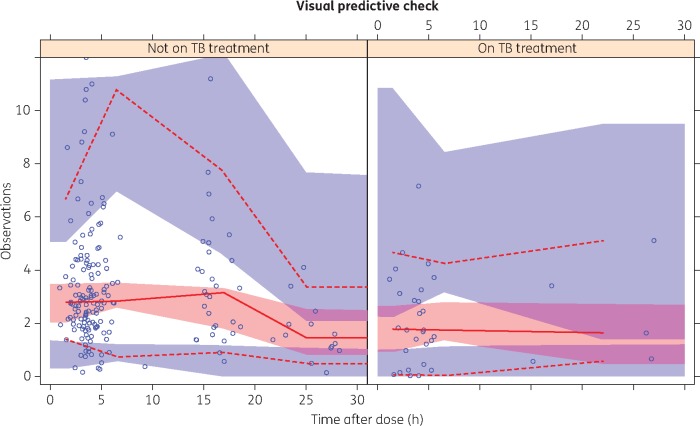
The sparse data supported a one-compartment model with first-order elimination. Absorption was described using a series of transit compartments20 (NN was fixed to 2 and no separate ka was estimated), which provided a fit similar to a simple first-order absorption model, but produced more stable and precise parameter estimates. Allometric scaling of clearance and volume using body weight improved the model fit (OFV reduction of 40 points). While the data did not support a sigmoidal maturation function, clearance was found to increase linearly with post-menstrual age (4% for each week; 7.6 point OFV drop, P < 0.01). Infants given RH preventive therapy had a 33% reduction in bioavailability (OFV drop of 9.4 points, P < 0.01), resulting in lower nevirapine exposures. The changes in nevirapine trough concentrations over time, for typical infants with and without RH preventive therapy, are illustrated in Figure 1. The median (IQR) estimated Cmin for samples taken early in treatment (within 28 days of birth; a median 7 days after birth) were 1.66 (1.32, 2.37) mg/L for infants receiving RH preventive therapy and 2.11 (1.48, 3.27) mg/L for infants not receiving RH preventive therapy. Later in treatment (a median 45 days after birth), the Cmin values were 0.89 (0.60, 1.57) mg/L with and 1.39 (1.01, 1.98) mg/L without concomitant RH preventive therapy. Use of cord blood measurements as the initial concentrations of nevirapine in the infants did not improve the model, possibly due to the small number of children with mothers on nevirapine that were sampled during the first days after birth. Neither maternal rifampicin intake nor maternal cART regimens impacted infant nevirapine pharmacokinetics. The final model parameter estimates and visual predictive checks are displayed in Table 2 and Figure 2, respectively. See Figure S1 (available as Supplementary data at JAC Online) for goodness-of-fit plots.
Figure 1.
Change in nevirapine trough concentrations with time, from birth to 6 weeks, with and without combined TB preventive therapy (rifampicin plus isoniazid). Solid lines are the median values, while shaded areas represent the 95% prediction intervals generated using between-subject and -occasion variability. Simulations (n = 1000) were performed with 15 mg daily dosing administered to a typical infant in our cohort: 39 weeks of gestation, birth weight of 3.13 kg and weight increase of 0.22 kg/day after birth. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 2.
Parameter estimates for the final population pharmacokinetic model describing nevirapine disposition in infants
| Parameter | Typical value (95% CI)b | Random variability (95% CI)b |
|---|---|---|
| Clearance, CL (L/h)a | 0.244 (0.198–0.288) | BSV: 44.5% (22.8%–59.8%) |
| Volume (L)a | 8.48 (5.02–11.1) | |
| Bioavailability, F | 1 fixed | BOV: 45.7% (29.8%–58.1%) |
| Absorption mean transit time (h) | 2.64 (0.469–4.98) | |
| Absorption transit compartments, NN | 2 fixed | |
| Effect of RH preventive therapy on F (%) | −32.6% (−49.8% to − 11.9%) | |
| Effect of age on CL (%/week) | +4.11% (+0.753% to + 6.69%) | |
| Proportional error (%) | 14.6% (5.19%–18.3%) | |
| Additive error (mg/L) | 0.438 (0.127–0.606) |
Clearance and volume have been allomerically scaled and clearance is affected by age; these typical values refer to a child with a weight of 3.5 kg and an age of 42 weeks after conception.
CIs are obtained from empirical percentiles of a non-parameteric bootstrap with replacement (n = 200). BSV, between subject variability; BOV, between occasion variability.
Figure 2.
Visual predictive check for nevirapine concentrations by time after dose, stratified for infants without and with rifampicin plus isoniazid TB preventive therapy, respectively. Open circles represent the observed concentration–time points. Lower and upper broken lines represent the 5th and 95th percentiles and the continuous line represents the 50th percentile of the observations. Shaded areas represent the model-predicted 95% CIs for the corresponding percentiles. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Three infants who received RH preventive therapy were started on treatment at 3–6 months of age for clinically suspected, microbiologically unconfirmed TB disease. HIV infection was confirmed in four infants. Two of the mothers of these infants had received efavirenz-based cART, one had received zidovudine only prior to delivery and one had received daily zidovudine with nevirapine perinatally. Two were born to mothers with TB. The nevirapine concentration in one infant was below the limit of quantification. The predicted Cmin values amongst the remaining infants acquiring HIV ranged from 1.35 to 2.00 mg/L and 0.49 to 0.65 mg/L at first and second sampling, respectively.
Discussion
We described highly variable trough concentrations of nevirapine in infants dosed according to the current guidelines for PMTCT and estimated an average reduction in nevirapine trough concentrations of 33% (95% CI 12%–50%) in infants receiving RH preventive therapy compared with infants not receiving TB preventive therapy. The recommended doses of rifampicin and isoniazid for children have been increased by 50% and 100%, respectively, since the Tshepiso study. The revised doses could conceivably induce or inhibit the metabolism of nevirapine to an even greater extent, resulting in a different net effect on nevirapine exposures.22 Early studies of nevirapine for PMTCT targeted trough concentrations >0.1 mg/L (10 times the in vitro IC50 of nevirapine),23 and 30-fold lower than the recommended trough concentration required to maintain viral suppression in patients infected with HIV.24 In our study the estimated nevirapine concentrations remained >0.1 mg/L, even with the full effects of both nevirapine and rifampicin induction on metabolizing enzymes and transporters. However, as no study has validated the nevirapine concentration threshold required to prevent HIV acquisition, the clinical implications of reduced concentrations of nevirapine in infants on RH preventive therapy are unclear. Defining such a threshold is complicated by multiple other factors contributing to HIV transmission risk, such as maternal ART, maternal viral load, mode of delivery, infant adherence to nevirapine, and infant feeding (breastfeeding, mixed feeding, formula), etc.
Although providing cART to all pregnant women (Option B+) significantly reduces the risk of transmission of HIV to infants, giving daily doses of nevirapine to newborn infants until at least 6 weeks after birth is recommended. The latter is particularly important among women who have a detectable HIV viral load at the time of delivery, either because the woman did not access ART or because ART was not given for a period sufficient to reduce viraemia to undetectable levels. WHO estimates that 77% of HIV-infected pregnant women in the African region Global Plan priority countries received antiretrovirals in 2014. In South Africa and six other countries in the region, maternal antiretrovirals were provided to >90% of HIV-infected pregnant women.25
Nevirapine dosing for infants was initially based on the HIVNET 012 study, in which a dose of 2 mg/kg given within 72 h of birth reduced HIV acquisition.26 Extension of the nevirapine dosing period was recommended as it became clear that the 6 weeks following delivery are of particularly high risk for HIV acquisition, especially among infants without safe alternatives to breast feeding.27 Studies supporting current guidelines for infant prophylaxis with daily nevirapine doses of 10 or 15 mg (depending on the infant’s weight at birth) demonstrated that the approach is highly effective and well tolerated.28,29 However, nevirapine has a low barrier to the selection of resistance and high proportions of viral resistance have been reported when there is regimen failure resulting in transmission.30,31 Our data illustrate the potential danger of treating infants receiving nevirapine with rifampicin and underscore the importance of using isoniazid for TB prophylaxis in this population.
Our study had several limitations including insufficient power to evaluate the risks of HIV acquisition by nevirapine exposure and lack of a control group of TB-exposed infants given IPT for 6 months, for comparison with RH preventive therapy for 3 months. Resistance mutations were not analysed in the four infants who acquired HIV. Pharmacokinetic sampling was sparse and data on the pharmacogenetic determinants of nevirapine exposure were not available to support the pharmacokinetic model estimates. While we did not find maternal rifampicin ingestion to impact nevirapine exposures, the study was not designed to separate this effect from that of infant RH preventive therapy. Similarly, while the linear effect describing a 4% increase in clearance for each week of post-menstrual age fitted the data best, a model including duration on nevirapine described a similar effect on clearance. Hence, the effects of enzyme maturation and autoinduction could not be separated.
Given the potentially grave consequences of underdosing nevirapine among HIV-exposed infants who are breastfeeding and thus remain at high risk for HIV acquisition, we suggest that the 3 month regimen of rifampicin plus isoniazid for TB prevention be avoided in infants born to mothers with HIV and TB disease. It would be prudent to avoid other preventive regimens that include a rifamycin in the absence of evidence to support their use in this population, as they too might impact nevirapine exposures in infants on nevirapine prophylaxis. Isoniazid prophylaxis requires a longer treatment duration (6 rather than 3 months), but risk–benefit considerations favour this approach among infants exposed to both HIV and TB.
Supplementary Material
Acknowledgements
We acknowledge the women and their children who participated in the Tshepiso cohort and Mr Yudesh Baliram who managed entering and cleaning and transmission of data.
Other Tshepiso Study Team members
Matebogu Letutu, Susan Raedani, Ziyaad Waja, Chris Hoffmann, Jenny Hull and Yudesh Baliram.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Allergy and Infectious Diseases and the National Institute of Mental Health of the National Institutes of Health (NIH; grant R01HD064354 to R. E. C.; grant K23AI080842 to K. E. D.; grants UM1 AI068634, UM1 AI068636 and UM1 AI106701, U01 AI068632 and AI068632 to the University of Cape Town), the Johns Hopkins Center for AIDS Research (NIH grant P30AI094189 to R. E. C.) and the National Research Foundation of South Africa (grant 90729 to H. M.).
Transparency declarations
R. E. C. is a consultant to Merck and his spouse owns Merck stock. All other authors: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of any funder.
Supplementary data
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
References
- 1. South African National AIDS Council. Enhanced Progress Report of the South African National Strategic Plan on HIV, STIs and TB: 2012-2016 2016. http://sanac.org.za/2015/05/25/progress-report-national-strategic-plan-on-hiv-stis-and-tb-2012-2016/.
- 2. WHO. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV infection in Infants. Recommendations for a Public Health Approach—2010 Version.2010. http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/. [PubMed]
- 3. Department of Health, Republic of South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. National Department of Health, South Africa, April 2015 http://www.sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf.
- 4. Deluca A, Chaisson RE, Martinson NA.. Intensified case finding for tuberculosis in prevention of mother-to-child transmission programs: a simple and potentially vital addition for maternal and child health. J Acquir Immune Defic Syndr 2009; 50: 196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gounder CR, Wada NI, Kensler C. et al. Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr 2011; 57: e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann CJ, Variava E, Rakgokong M. et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 2013; 8: e62211.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spyridis NP, Spyridis PG, Gelesme A. et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis 2007; 45: 715–22. [DOI] [PubMed] [Google Scholar]
- 8. Getahun H, Matteelli A, Abubakar I. et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015; 46: 1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathivha KT, Velaphi S.. Characteristics of infants exposed to maternal tuberculosis and chemoprophylaxis using 3 months of isoniazid and rifampicin. Paediatr Int Child Health 2017; 37: 129–34. [DOI] [PubMed] [Google Scholar]
- 10. Vardhanabhuti S, Acosta EP, Ribaudo HJ. et al. Clinical and genetic determinants of plasma nevirapine exposure following an intrapartum dose to prevent mother-to-child HIV transmission. J Infect Dis 2013; 208: 662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olagunju A, Khoo S, Owen A.. Pharmacogenetics of nevirapine excretion into breast milk and infants' exposure through breast milk versus postexposure prophylaxis. Pharmacogenomics 2016; 17: 891–906. [DOI] [PubMed] [Google Scholar]
- 12. Bienczak A, Cook A, Wiesner L. et al. Effect of diurnal variation, CYP2B6 genotype and age on the pharmacokinetics of nevirapine in African children. J Antimicrob Chemother 2017; 72: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kunz A, Frank M, Mugenyi K. et al. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J Antimicrob Chemother 2009; 63: 170–7. [DOI] [PubMed] [Google Scholar]
- 14. Cohen K, van Cutsem G, Boulle A. et al. Effect of rifampicin-based antitubercular therapy on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J Antimicrob Chemother 2008; 61: 389–93. [DOI] [PubMed] [Google Scholar]
- 15. Oudijk JM, McIlleron H, Mulenga V. et al. Pharmacokinetics of nevirapine in HIV-infected children under 3 years on rifampicin-based antituberculosis treatment. AIDS 2012; 26: 1523–8. [DOI] [PubMed] [Google Scholar]
- 16. Denti P, Martinson N, Cohn S. et al. Population pharmacokinetics of rifampin in pregnant women with tuberculosis and HIV coinfection in Soweto, South Africa. Antimicrob Agents Chemother 2015; 60: 1234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dooley KE, Denti P, Martinson N. et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis 2015; 211: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beal S, Sheiner L, Boeckmann A. et al. NONMEM User’s Guides (1989-2009). Ellicott City, MD: ICON Development Solutions, 2009. [Google Scholar]
- 19. Karlsson M, Holford N. A tutorial on visual predictive checks. In: Abstracts of the Annual Meeting of the Population Approach Group in Europe, Marseille, France, 2008 Abstract 1434. www.page-meeting.org/?abstract=1434.
- 20. Savic RM, Jonker DM, Kerbusch T. et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26. [DOI] [PubMed] [Google Scholar]
- 21. Anderson BJ, Holford NHG.. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32. [DOI] [PubMed] [Google Scholar]
- 22. WHO. Rapid Advice: Treatment of Tuberculosis in Children.2010. WHO/HTM/TB/2010.13. http://apps.who.int/iris/handle/10665/44444. [PubMed]
- 23. Mirochnick M, Dorenbaum A, Blanchard S. et al. Predose infant nevirapine concentration with the two-dose intrapartum neonatal nevirapine regimen: association with timing of maternal intrapartum nevirapine dose. J Acquir Immune Defic Syndr 2003; 33: 153–6. [DOI] [PubMed] [Google Scholar]
- 24. Department of Health and Human Services. Panel on Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Role of Therapeutic Drug Monitoring in Management of Pediatric HIV Infection. (Updated March 1 2016) https://aidsinfo.nih.gov/contentfiles/lvguidelines/PediatricGuidelines.pdf.
- 25. WHO. Global Health Sector Response to HIV, 2000-2015: Focus on Innovations in Africa: Progress Report 2015. http://www.who.int/hiv/pub/progressreports/2015-progress-report/en/.
- 26. Guay LA, Musoke P, Fleming T. et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999; 354: 795–802. [DOI] [PubMed] [Google Scholar]
- 27. Miotti PG, Taha TE, Kumwenda NI. et al. HIV transmission through breastfeeding: a study in Malawi. JAMA 1999; 282: 744–9. [DOI] [PubMed] [Google Scholar]
- 28. Hudgens MG, Taha TE, Omer SB. et al. Pooled individual data analysis of 5 randomized trials of infant nevirapine prophylaxis to prevent breast-milk HIV-1 transmission. Clin Infect Dis 2013; 56: 131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taha T, Flynn P, Shapiro D. et al. Maternal triple antiretrovirals (MART) and infant nevirapine (iNVP) prophylaxis for the prevention of mother-to-child transmission (MTCT) of HIV during breastfeeding (BF). Rev Antiviral Ther Infect Dis 2016; 7: 21–2 (Abstract O_18 from the Eighth International Workshop on HIV Pediatrics, Durban, South Africa). [Google Scholar]
- 30. Martinson NA, Morris L, Gray G. et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr 2007; 44: 148–53. [DOI] [PubMed] [Google Scholar]
- 31. Nelson JA, Fokar A, Hudgens MG. et al. Frequent nevirapine resistance in infants infected by HIV-1 via breastfeeding while on nevirapine prophylaxis. AIDS 2015; 29: 2131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.