Abstract
Background
Delafloxacin is an investigational anionic fluoroquinolone in development for oral or intravenous administration for the treatment of infections caused by Gram-positive (including MRSA), Gram-negative, atypical and anaerobic organisms.
Objectives
To establish the non-inferiority of delafloxacin compared with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections and to compare the safety of the two antimicrobials.
Patients and methods
A Phase 3, multicentre, randomized, double-blind, active-controlled study with 660 patients compared delafloxacin 300 mg or vancomycin 15 mg/kg plus aztreonam 2 g each administered twice daily intravenously for 5–14 days. Non-inferiority was evaluated by objective response (≥20% erythema reduction) at 48–72 h after initiation of study drug, investigator subjective assessment of outcome and microbiological responses. Clinical Trials Registration: NCT01811732. EudraCT number: 2012-001767-71.
Results
In the ITT analysis set, the objective response was 78.2% in the delafloxacin arm and 80.9% in the vancomycin/aztreonam arm (mean treatment difference, −2.6%; 95% CI, −8.78% to 3.57%). Investigator-assessed cure was similar between the two groups at follow-up (52.0% versus 50.5%) and late follow-up (70.4% versus 66.6%). Bacterial eradication of MRSA was 100% and 98.5% in the delafloxacin group and the vancomycin/aztreonam group, respectively. Frequency of treatment-emergent adverse events in the delafloxacin and vancomycin/aztreonam groups was similar. Treatment-emergent adverse events leading to study drug discontinuation were higher in the vancomycin/aztreonam group compared with the delafloxacin group (4.3% versus 0.9%).
Conclusions
Delafloxacin, an anionic fluoroquinolone, was statistically non-inferior to vancomycin/aztreonam at 48–72 h following the start of therapy and was well tolerated as monotherapy in the treatment of acute bacterial skin and skin structure infections.
Introduction
Acute bacterial skin and skin structure infections (ABSSSIs) have diverse aetiologies due, in part, to varying epidemiological settings, with many different microbes identified as potential causes.1,2 MRSA and other MDR Gram-positive and Gram-negative bacteria are of particular concern due to significant patient morbidity coupled with high utilization of healthcare resources and costs.3–6 Many antimicrobials are generally effective for ABSSSIs, although their use is limited by resistance7,8 and adverse effects.9 These challenges have stimulated efforts to develop new antimicrobial agents with a favourable safety profile and clinical efficacy with appropriate pathogen coverage aligning with antimicrobial stewardship principles.5
Delafloxacin is an anionic fluoroquinolone for oral or intravenous administration recently approved by the FDA for the treatment of ABSSSI, caused by designated susceptible bacteria including Gram-positive organisms (including MRSA) and Gram-negative organisms. Delafloxacin is chemically distinct from other quinolones in its size, shape and charge profile. These properties result in a highly active agent, particularly against Gram-positive pathogens.10,11 Delafloxacin is more active in vitro than levofloxacin against most Gram-positive pathogens, including levofloxacin non-susceptible isolates, and is 32-fold more active than levofloxacin against MRSA isolates.12 The spectrum of activity13–15 of delafloxacin suggests a potential for use in infections with Gram-positive and Gram-negative pathogens and mixed pathogens.
Previous Phase 216,17 ABSSSI studies demonstrated that delafloxacin is well tolerated and has favourable clinical efficacy compared with tigecycline,16 linezolid and vancomycin.17 Statistically better outcomes in obese patients with delafloxacin compared with vancomycin in one Phase 2 study led to pre-specified secondary endpoints in obese patients in the study reported here.17
The primary objective of this study was to evaluate the clinical efficacy and safety of delafloxacin monotherapy compared with vancomycin plus aztreonam for the treatment of ABSSSIs at 48–72 h following treatment initiation in accordance with FDA18 guidelines and at the follow-up (FU) visit (Day 14 ± 1) per EMA19 guidelines.
Patients and methods
Study design
This Phase 3, multicentre, multinational, stratified, randomized, double-blind trial assessed the efficacy and safety of intravenous delafloxacin compared with vancomycin/aztreonam for the treatment of adults with ABSSSIs. Clinical Trials Registration: NCT01811732. EudraCT number: 2012-001767-71.
Ethics
This study was conducted in compliance with the principles of ICH E6(R1) and the principles of the World Medical Association Declaration of Helsinki. A written informed consent in compliance with US Title 21 Code of Federal Regulations Part 50, ICH E6(R1) and other applicable regulatory requirements was obtained from each patient or legally authorized representative before entering the study or performing any unusual or non-routine procedure that involved risk to the patient. A list of institutional review boards used for this study is available.
Patients
Eligibility criteria were age ≥18 years and a diagnosis of ABSSSI classified as cellulitis/erysipelas, wound infection, major cutaneous abscess or burn infection with ≥75 cm2 of erythema and ≥2 signs of systemic infection. Exclusion criteria were consistent with current guidance and included receipt of systemic antibiotic therapy in the 14 days before enrolment unless one of the following was documented: the patient received at least 48 h of antibiotic therapy for ABSSSI and clinical progression of ABSSSI was documented; the patient completed a treatment course within 7 days for an infection other than ABSSSI with an antibacterial drug not having activity against bacterial pathogens that cause ABSSSI; and patients who received one dose of a single, potentially effective, short-acting antimicrobial drug for the treatment of the ABSSSI under study in the 14 days before study entry. Prior single dose antibiotic use was limited to no more than 25% of the total randomly assigned patients. Further details regarding the eligibility and exclusion criteria are available in Table S1 (available as Supplementary data at JAC Online).
Patients were enrolled at 34 study centres in seven countries between April 2013 and June 2014 and randomized (1:1) to delafloxacin or vancomycin/aztreonam using an interactive web response system. Vancomycin was chosen as the comparator in accordance with FDA and EMA guidelines.18,19 Randomization was stratified by infection type, ensuring that ≤25% and ≤35% of patients had a major cutaneous abscess or wound infection, respectively.
Interventions
Patients received intravenous delafloxacin 300 mg or vancomycin 15 mg/kg (actual body weight) plus aztreonam 2 g every 12 h on an inpatient or outpatient basis. Vancomycin levels were monitored on Day 2 (+1) and Day 6 (± 1), with dose adjustments based on a target trough of 15–20 μg/mL.20 Patients received aztreonam for Gram-negative coverage, which was discontinued once baseline cultures did not reveal Gram-negative organisms. Both groups were treated for at least 5 days and no more than 14 days, with the duration of therapy based on the investigators’ assessment of signs and symptoms of ABSSSI.
Study visits took place at screening, daily during treatment, FU (Day 14 ± 1) and late FU (LFU; Days 21–28). Telephone FU was conducted for all patients 30 days after the last dose of study drug to obtain 28 day all-cause mortality rates, adverse events (AEs) and use of post-treatment medications.
Efficacy assessments
The FDA-defined primary efficacy endpoint was the objective response at 48–72 h (± 2) following treatment initiation and defined as ≥20% reduction in erythema of the ABSSSI lesion determined by digital planimetry of the leading edge without evidence of clinical failure. Clinical failure at this timepoint was defined as any of the following: (i) <20% reduction in erythema area determined by digital planimetry; (ii) administration of rescue antibacterial drug therapy or non-study antibacterial drug therapy before the primary efficacy endpoint assessment; (iii) unplanned surgical intervention except for limited bedside debridement and standard wound care before the primary efficacy endpoint assessment; or (iv) death within 74 h after treatment initiation. Patients were classified as clinical failures in the ITT analysis if digital planimetry was not available within the 48–72 h (± 2) window.
The EMA-defined primary efficacy measure was the investigator assessment of clinical cure (no remaining signs or symptoms) at the FU visit in the ITT population. An additional secondary endpoint was investigator-assessed success (cure or improved and no further antibiotic needed) at the FU visit.
Clinical response at FU and LFU was based on investigator assessment of ABSSSI signs and symptoms and categorized as cure (complete resolution of symptoms); improved (near resolution with some remaining symptoms not requiring antibiotic therapy); failure (additional non-study antibiotics or unplanned major surgical intervention required); or indeterminate. Patients lost to FU (or with indeterminate outcomes) and those categorized as improved were considered failures in the primary analysis. Other antibiotic studies in skin infections have defined a successful outcome as resolution or near resolution of signs and symptoms that no longer require antibiotic therapy. This definition aligns with the definition of success in this study.
Because a previous Phase 2 study showed statistically better outcomes in obese patients, it was pre-specified that data sets would be analysed by patient baseline BMI for patients with baseline BMI <30 kg/m2 and patients with baseline BMI ≥30 kg/m2.17
Patients’ subjective assessment of pain was recorded on a numerical rating scale ranging from 0 (no pain) to 10 (worst imaginable pain) at screening, during treatment, end of treatment (EOT), FU and LFU.
Microbiological assessments
Microbiological response for patients in the microbiological ITT (MITT) and microbiologically evaluable (ME) analysis sets was based on results of the baseline and post-baseline cultures through the FU and LFU visits, susceptibility testing and the clinical response assigned by the investigator. ABSSSI samples were collected before initiation of any rescue therapy and from all clinical failures that had material available for culture. Microbiological response was categorized as documented eradicated (baseline pathogen absent in FU cultures); presumed eradicated (no FU material available for culture with a clinical response of success); documented persisted (baseline pathogen present in FU cultures); or presumed persisted (no FU material available for culture with an investigator-assessed response of failure).
Safety and tolerability assessments
Safety assessments included all AEs, physical examinations, vital signs, 12-lead ECGs at baseline and if clinically indicated thereafter, and clinical laboratory tests. AEs were summarized by treatment group and overall for the safety analysis set. Treatment-emergent AEs (TEAEs) were those that occurred or worsened in severity after administration of the first dose of the study drug through the telephone FU with patients 30 days after the last dose. The Medical Dictionary for Regulatory Activities Version 16.1 was used to code AEs.
Statistical analysis
Separate statistical analysis plans were prospectively developed prior to database lock and unblinding for the FDA and the EMA endpoints. All clinical efficacy outcomes were analysed for the ITT population. The safety analysis set included all enrolled patients who received ≥1 dose of the study drug.
Analysis of microbiological outcomes was based on the MITT population. There were multiple ME and clinically evaluable (CE) analysis sets based on the timing of assessments [48–72 h (± 2), EOT, FU and LFU].
Continuous variables were described with descriptive statistics such as mean, standard deviation, median, minimum and maximum while counts and percentages were calculated for categorical data. The rate of the primary FDA-defined efficacy endpoint was the sample responder rate defined as [responder/(responder + non-responder)]. The rate of the primary EMA-defined efficacy endpoint was the sample cure rate defined as [cure/(cure + failure)].
A two-sided 95% CI was calculated based on differences between delafloxacin and vancomycin/aztreonam in responder rates at 48–72 h (± 2) after initiation of treatment (FDA-defined endpoint) and investigator-assessed response rates (EMA-defined endpoint) using a non-stratified method proposed by Miettinen and Nurminen.21 Non-inferiority was concluded if the lower limit of this 95% CI was > −10%, with mean differences between treatments expressed as delafloxacin minus vancomycin/aztreonam. The FDA-defined primary efficacy analysis was performed on the ITT analysis set while the EMA-defined primary efficacy analysis included the ITT and CE at FU analysis sets.
The same analysis method was applied to the comparison of microbiological response rates between treatment groups.
All analyses and summaries were produced using SAS® software (SAS Institute, Inc., Cary, NC, USA) Version 9.3 (or higher).
Results
Patient disposition and analysis sets
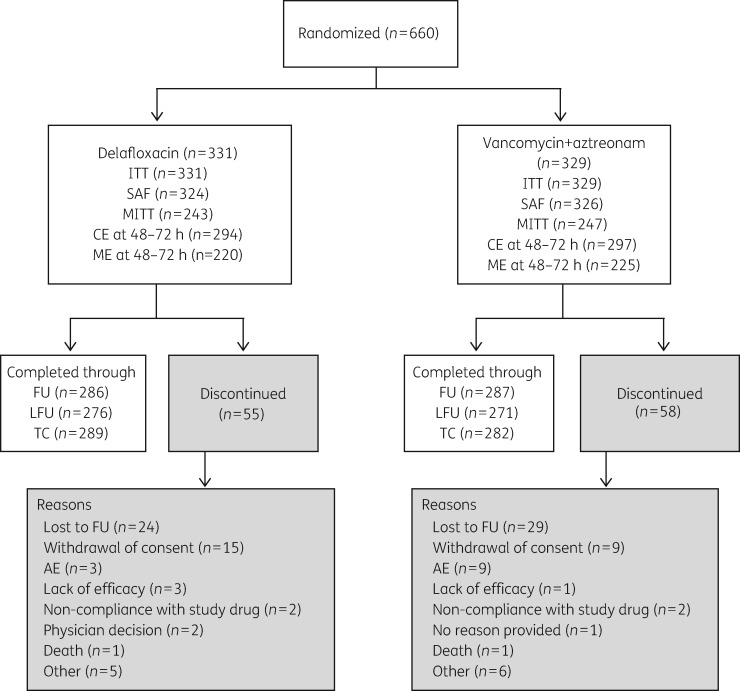
Six-hundred and sixty patients were randomized to delafloxacin or vancomycin/aztreonam and comprised the ITT analysis population (Figure 1). Among patients enrolled, 547 (82.9%) completed the study through the LFU visit. The safety analysis set included 650 patients who received study drug. The median duration of treatment with delafloxacin was 5 and 5.5 days for the vancomycin/aztreonam group. The median duration of aztreonam/aztreonam placebo use was 2 days for either group.
Figure 1.
CONSORT diagram of patient disposition. ITT analysis set included all patients who were randomly assigned to treatment. CE analysis set included all patients in the ITT population who: (i) received ≥80% of the total expected doses of the assigned study drug or were clinical failures and received ≥4 doses of study drug; (ii) did not receive any concomitant, systemic antibacterial therapy with activity against the identified pathogen; and (iii) had no major protocol deviations. MITT analysis set consisted of all patients in the ITT analysis set that had bacterial pathogens known to cause ABSSSI at baseline. ME analysis set included all patients in the MITT population who met the criteria established for the CE analysis set. SAF, safety; TC, telephone call.
Patient demographic and clinical characteristics
Baseline characteristics were similar between treatment groups (Table 1). The majority of patients were men (62.9%) and Caucasian (91.1%), with a mean (SD) age of 45.8 (± 14.2) years and a mean (SD) BMI of 28.1 (6.4) kg/m2; 32.4% of patients had BMI ≥30 kg/m2. Less than 20% of patients received antibacterial therapy prior to randomization (15.7% in the delafloxacin group and 21.6% in the vancomycin/aztreonam group).
Table 1.
Patient demographics and baseline characteristics (ITT population)
| Characteristic | Delafloxacin, N = 331 | Vancomycin + aztreonam, N = 329 |
|---|---|---|
| Age (years), mean ± SD (range) | 46.3 ±13.91 (18–94) | 45.3 ±14.4 (19–90) |
| Age category (years), n (%) | ||
| ≤65 | 309 (93.4) | 309 (93.9) |
| >65 | 22 (6.6) | 20 (6.1) |
| >75 | 7 (2.1) | 10 (3.0) |
| Men, n (%) | 206 (62.2) | 209 (63.5) |
| Race, n (%) | ||
| white | 297 (89.7) | 304 (92.4) |
| black or African American | 27 (8.2) | 19 (5.8) |
| American Indian or Alaska native | 5 (1.5) | 2 (0.6) |
| Asian | 1 (0.3) | 1 (0.3) |
| native Hawaiian or other Pacific Islander | 1 (0.3) | 2 (0.6) |
| other | 0 | 1 (0.3) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 101 (30.5) | 103 (31.3) |
| Region, n (%) | ||
| Europe | 63 (19.0) | 55 (16.7) |
| North America | 268 (81.0) | 274 (83.3) |
| BMI (kg/m2), mean ± SD | 28.4 ± 6.42 | 27.9 ±6.36 |
| BMI ≥30 kg/m2, n (%) | 120 (36.3) | 94 (28.6) |
| Diabetes, n (%) | 30 (9.1) | 27 (8.2) |
| Prior antibiotic use, n (%) | 52 (15.7) | 71 (21.6) |
| Baseline pain score, mean ± SD | 7.9 (2.0) | 7.8 (2.2) |
| Duration of exposure | ||
| n | 324 | 326 |
| mean (SD) days | 6.18 (2.81) | 6.15 (2.62) |
| median days | 5.00 | 5.50 |
| min, max days | 0.5, 14.0 | 0.5, 14.0 |
The types of ABSSSI were similarly distributed across the two treatment groups (Table 2). Baseline lesion size was similar between the two treatment groups with a median lesion size ∼300 cm2. Of the 660 enrolled patients, 490 (74.2%) had a positive ABSSSI culture. Of patients with positive cultures, Staphylococcus aureus was identified in 65.4% of patients treated with delafloxacin and 66.8% of those in the vancomycin/aztreonam group; MRSA infections were confirmed in 78 (32.1%) and 91 (36.8%) patients in the delafloxacin and vancomycin/aztreonam groups, respectively. Delafloxacin MIC50/90 was 0.008 and 0.25 mg/L (range 0.002–0.5 mg/L) for S. aureus; 0.12 and 0.25 mg/L (range 0.004–0.5 mg/L) for MRSA and 0.008 and 0.12 mg/L (range 0.002–0.5 mg/L) for MSSA. Approximately 40% of S. aureus isolates were levofloxacin non-susceptible (delafloxacin MIC range 0.004–0.5 mg/L); the majority were also MRSA. Of the MITT population, 40 (16.5%) in the delafloxacin and 43 (17.4%) in the vancomycin/aztreonam treatment groups were Gram-negative (Table S2). Overall, 15 patients (2.3%) had bacteraemia.
Table 2.
Summary of ABSSSI characteristics (ITT population)
| Characteristic | Delafloxacin, N = 331 | Vancomycin + aztreonam, N = 329 |
|---|---|---|
| ABSSSI category, n (%) | ||
| cellulitis/erysipelas | 128 (38.7) | 128 (38.9) |
| wound infection | 116 (35.0) | 116 (35.3) |
| major cutaneous abscess | 84 (25.4) | 83 (25.2) |
| burn infection | 3 (0.9) | 2 (0.6) |
| Erythema size (cm2; digital), mean ± SD (IQR) | 294.8 ±308.34 (121.5–332.7) | 319.1 ±314.03 (130.2–385.7) |
| Induration size (cm2; digital), mean ± SD (IQR) | 94.1 ±208.66 (22.3–94.8) | 120.7 ±219.6 (26.2–121.6) |
| Systemic signs, n (%) | ||
| lymph node enlargement | 285 (86.1) | 287 (87.2) |
| elevated C-reactive protein, >10× ULN | 131 (39.6) | 136 (41.3) |
| elevated white blood count, ≥10 000 cells/μL | 159 (48.0) | 165 (50.2) |
| fever, ≥38 °C | 78 (23.6) | 63 (19.1) |
| lymphangitis | 68 (20.5) | 55 (16.7) |
| Bacteraemia, n (%) | 6 (1.8) | 9 (2.7) |
| Local signs, n (%) | ||
| erythema/extension of redness | 329 (99.4) | 328 (99.7) |
| heat/localized warmth | 328 (99.1) | 326 (99.1) |
| pain/tenderness | 328 (99.1) | 327 (99.4) |
| swelling/induration | 323 (97.6) | 324 (98.5) |
| drainage/discharge | 209 (63.1) | 207 (62.9) |
| fluctuance | 175 (52.9) | 179 (54.4) |
| Pathogens identified at baseline (MITT), n (%)a | ||
| S. aureusb | 159 (65.4) | 165 (66.8) |
| MRSA | 78 (32.1) | 91 (36.8) |
| MSSA | 82 (33.7) | 74 (30.0) |
Patients with baseline pathogens: N = 243 for delafloxacin and N = 247 for vancomycin/aztreonam.
Patients with both MRSA and MSSA were counted only once. Percentages based on number of patients with baseline pathogens.
Clinical outcomes
Objective response
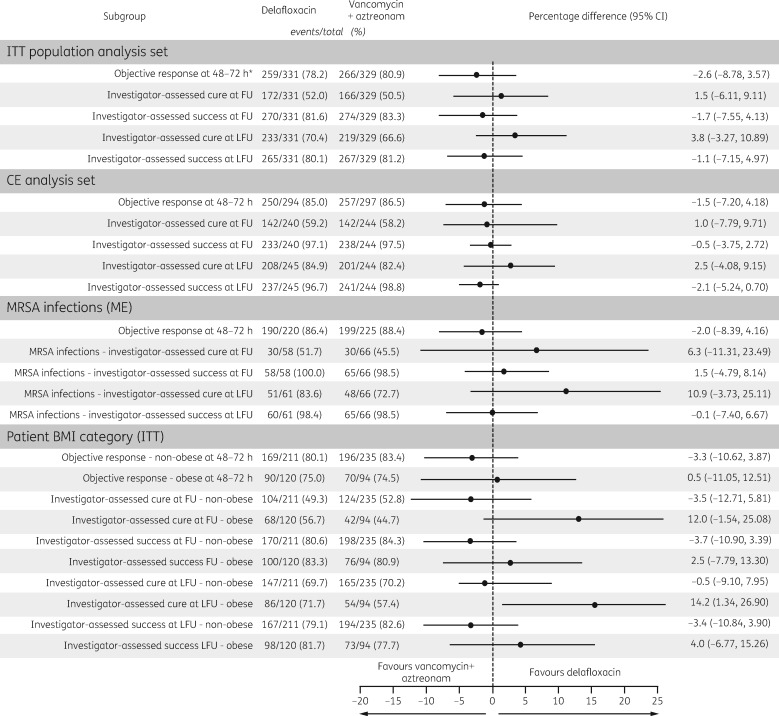
The percentage of responders at the 48–72 h objective response assessment in the ITT analysis set was similar between the two groups at 78.2% and 80.9% for delafloxacin and vancomycin/aztreonam, respectively, and non-inferiority was declared (Figure 2). Delafloxacin was comparable to vancomycin + aztreonam for the objective response at 48–72 h whether using the CE, ME or MITT analysis sets.
Figure 2.
Objective response and investigator-assessed response at FU and LFU by analysis set, MRSA infection at baseline and BMI category. *Primary endpoint. Cure = no remaining signs and symptoms. Improved = some remaining signs and symptoms, but no further antibiotics required. Success = cure + improved. ITT, all patients randomized; MITT, ITT patients with eligible pathogen; CE patients who completed activities as defined in the protocol; ME, CE patients with eligible pathogen. BMI was calculated as body weight (in kg)/h (in m2).
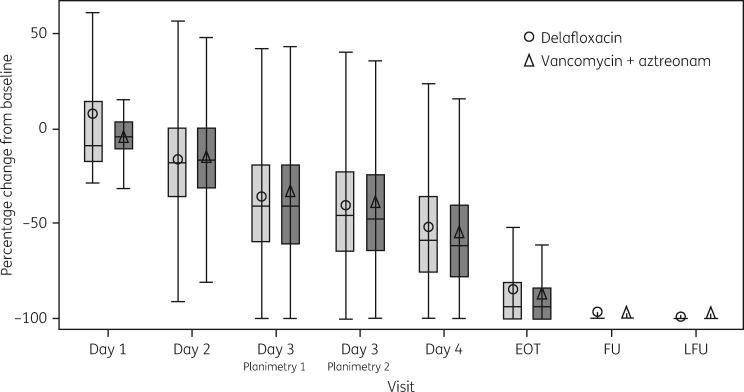
The mean percentage change from baseline in erythema was similar between the treatment groups at each LFU visit (Figure 3).
Figure 3.
Percentage change from baseline in reduction of erythema (digital planimetry) at each visit (ITT population).
Investigator assessment of response
Investigator-assessed cure rates and success rates were similar between study arms at the FU and LFU visits in the ITT population, meeting the non-inferiority criteria, and were comparable between the two treatment groups for the CE, MITT or ME analysis sets. The investigator-assessed response at the FU visit was also analysed by infection type and was found to be comparable between the delafloxacin and the vancomycin/aztreonam treatment groups (Table 3). Among patients with MRSA, the absolute difference in cure and clinical success at FU was 6.3% and 1.5% higher, respectively, for the delafloxacin group compared with the vancomycin/aztreonam group (Figure 2). The cure and success rates were numerically higher in obese patients at FU and LFU and statistically significantly higher cure at LFU for delafloxacin (71.7%) compared with vancomycin/aztreonam (57.4%). See Figure 2.
Table 3.
Investigator-assessed response at FU visit by infection type (ITT analysis set)
| Delafloxacin, N = 331 | Vancomycin + aztreonam, N = 329 | CI | |
|---|---|---|---|
| Cellulitis | |||
| cure | 86/128 (67.2%) | 78/128 (60.9%) | 6.3 (−5.52, 17.87) |
| success | 107/128 (83.6%) | 108/128 (84.4%) | −0.8 (−9.94, 8.37) |
| Abscess | |||
| cure | 44/84 (52.4%) | 40/83 (48.2%) | 4.2 (−10.93, 19.12) |
| success | 70/84 (83.3%) | 70/83 (84.3%) | −1.0 (−12.46, 10.47) |
| Wound | |||
| cure | 39/116 (33.6%) | 48/116 (41.4%) | −7.8 (−20.02, 4.72) |
| success | 90/116 (77.6%) | 94/116 (81.0%) | −3.5 (−13.97, 7.08) |
| Burn | |||
| cure | 3/3 (100%) | 0/2 (0.0%) | 100.0 (2.02, 100.00) |
| success | 3/3 (100%) | 2/2 (100%) | not evaluable |
Cure = complete resolution of symptoms. Success = cure plus improved and no further antibiotic needed.
The median baseline patient-reported pain score was 8.0 for either group. The mean reduction in pain was comparable between treatment groups, with a change from baseline to EOT of −5.4 (3.14) for delafloxacin and −5.1 (3.18) for vancomycin/aztreonam.
Microbiological efficacy
In the ME population at FU, microbiological responses were documented or presumed eradicated in 175 of 179 (97.8%) and 181 of 184 (98.4%) of patients treated with delafloxacin and vancomycin/aztreonam, respectively. Among patients with MRSA isolated at baseline, 100% and 98.5% of responses in the delafloxacin and vancomycin/aztreonam groups, respectively, were documented or presumed eradicated. Additionally, there was 100% documented or presumed eradication for the levofloxacin non-susceptible S. aureus isolates in the delafloxacin group. Per pathogen early objective response at 48–72 h and microbiological response rates at FU against pathogens that cause ABSSSI including Gram-positive pathogens including MRSA and Gram-negative pathogens were similar between delafloxacin and vancomycin/aztreonam patients (see Table 4). No superinfections were identified in either treatment group. New infections were detected in four patients including two in the delafloxacin group and two in the vancomycin/aztreonam group.
Table 4.
Per pathogen microbiological response
| By pathogen objective responders at 48–72 h, ME at 48–72 h analysis set |
Per pathogen microbiological response (documented or presumed eradication),a ME at FU analysis set |
|||
|---|---|---|---|---|
| delafloxacin, N = 220 | vancomycin + aztreonam, N = 225 | delafloxacin, N = 179 | vancomycin + aztreonam, N = 184 | |
| S. aureus | 123/145 (84.8%) | 134/150 (89.3%) | 115/117 (98.3%) | 119/121 (98.3%) |
| MRSA | 59/72 (81.9%) | 76/84 (90.5%) | 58/58 (100%) | 65/66 (98.5%) |
| MSSA | 65/74 (87.8%) | 58/66 (87.9%) | 57/59 (96.6%) | 54/55 (98.2%) |
| Streptococcus anginosus groupb | 28/30 (93.3%) | 33/37 (89.2%) | 22/22 (100%) | 26/27 (96.3%) |
| Staphylococcus epidermidis | 16/18 (88.9%) | 13/15 (86.7%) | 13/14 (92.9%) | 14/14 (100%) |
| Klebsiella pneumoniae | 10/11 (90.9%) | 9/10 (90%) | 9/9 (100%) | 9/9 (100%) |
| Escherichia coli | 3/5 (60%) | 9/9 (100%) | 4/4 (100%) | 7/7 (100%) |
| Streptococcus pyogenes | 5/7 (71.4%) | 2/5 (40%) | 5/5 (100%) | 4/4 (100%) |
| Staphylococcus lugdunensis | 6/7 (85.7%) | 3/3 (100%) | 7/7 (100%) | 1/1 (100%) |
| Staphylococcus haemolyticus | 5/5 (100%) | 2/2 (100%) | 5/5 (100%) | 2/2 (100%) |
Investigator-assessed response in ME at FU analysis set was the same as per pathogen microbiological response.
S. anginosus group includes S. anginosus, Streptococcus intermedius and Streptococcus constellatus.
Safety
Among the safety analysis set (n = 650), ≥1 TEAE was observed in 154 patients (47.5%) treated with delafloxacin and 193 patients (59.2%) in the vancomycin/aztreonam group (Table 5).
Table 5.
Summary of AEs affecting either treatment group: safety population
| Summary of AE, n (%) | Treatment group |
|
|---|---|---|
| delafloxacin, N = 324 | vancomycin + aztreonam, N = 326 | |
| Overall TEAEs | 154 (47.5) | 193 (59.2) |
| TEAEs affecting ≥5% of patients | ||
| diarrhoea | 27 (8.3) | 10 (3.1) |
| headache | 10 (3.1) | 25 (7.7) |
| infection | 28 (8.6) | 25 (7.7) |
| infusion-site extravasation | 28 (8.6) | 44 (13.5) |
| nausea | 24 (7.4) | 28 (8.6) |
| TEAEs by intensity | ||
| mild | 90 (27.8) | 126 (38.7) |
| moderate | 53 (16.4) | 60 (18.4) |
| severe | 11 (3.4) | 7 (2.1) |
| TEAEs related to study drug | ||
| total related to study drug | 78 (24.1) | 107 (32.8) |
| possibly | 56 (17.3) | 75 (23.0) |
| probably | 14 (4.3) | 23 (7.1) |
| definitely | 8 (2.5) | 9 (2.8) |
| TEAEs leading to early discontinuation of study drug | 3 (0.9) | 14 (4.3) |
| Related TEAEs leading to early discontinuation of study drug | 1 (0.3) | 8 (2.5) |
| Overall serious AEs | 12 (3.7) | 12 (3.7) |
| Deaths | 1 (0.3) | 1 (0.3) |
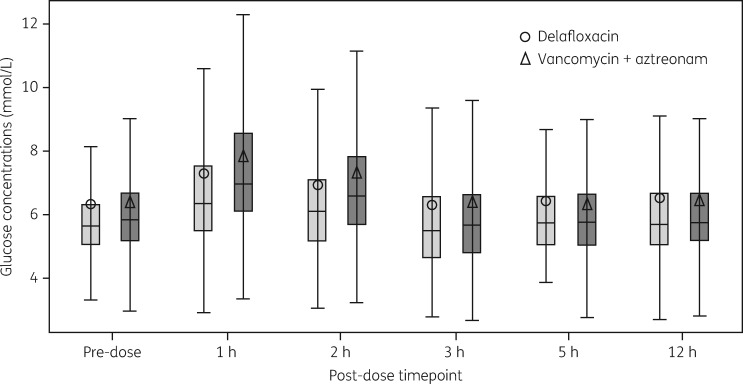
The majority of TEAEs were considered mild and unrelated to study drug in both groups, with a lower percentage of patients experiencing treatment-related TEAEs in the delafloxacin arm compared with vancomycin/aztreonam. The most common treatment-related AEs in delafloxacin-treated patients were gastrointestinal in nature reported in 12% of patients, primarily nausea and diarrhoea. Extensive analysis of AEs typically seen with fluoroquinolones was conducted. There were no cases of Clostridium difficile diarrhoea and there were no cases of tendinitis or tendon rupture, peripheral neuropathy or myopathy thought to be related to delafloxacin treatment. One delafloxacin-treated patient and two vancomycin/aztreonam-treated patients had a report of hypoglycaemia related to treatment; two delafloxacin patients and one vancomycin/aztreonam patient had reported hyperglycaemia potentially related to treatment. Intensive glucose monitoring for 12 h post-dose in patients who were also undergoing pharmacokinetic testing did not show differences between the two treatment groups (Figure 4). Treatment-related infusion reactions (extravasations, pain, oedema, phlebitis, swelling, etc.) occurred in 3% of patients in each treatment group. Renal failure was reported as potentially related to treatment in three patients on vancomycin/aztreonam compared with one report of renal impairment on delafloxacin. The occurrence of TEAEs resulting in premature study drug discontinuation was lower in the delafloxacin group compared with the vancomycin/aztreonam arm (0.9% and 4.3%, respectively).
Figure 4.
Box-plot of glucose concentrations (mmol/L): intense glucose analysis set. Note: the lower fence reflects the actual minimum value or 1.5× IQR below quartile 1, whichever is bigger; the upper fence reflects the actual maximum value or 1.5× IQR above quartile 3, whichever is smaller.
The rate of serious AEs was equal between the two treatment groups (3.7%), respectively. Of those, none in the delafloxacin treatment group and one in the vancomycin/aztreonam treatment group were considered to be potentially related to treatment. One death occurred in each treatment arm, with neither considered related to the study drug.
The groups were generally similar regarding changes from baseline in haematology and serum chemistry. Vital sign measurements, physical examination findings and ECGs were unremarkable. There were no increases in hepatic AEs in the delafloxacin treatment group when compared with vancomycin/aztreonam. In all patients regardless of baseline medical history or baseline laboratories, only three patients in the delafloxacin treatment group reported ALT >5× upper limit of normal (ULN) result at any time in the study, compared with five patients in the vancomycin/aztreonam treatment groups. Only two patients in either treatment group had AST >5× ULN any time during the trial (see Table S3). There were no reports of cases meeting the Hy's law definition in any patients during this study. Serum creatinine >2× ULN was seen in three vancomycin-treated patients any time during the trial, compared with no reports in delafloxacin-treated patients.
Discussion
This large, multicentre, double-blind, randomized, Phase 3 trial established that delafloxacin, an anionic fluoroquinolone, was non-inferior to vancomycin/aztreonam for the treatment of ABSSSIs. The non-inferiority of delafloxacin and vancomycin/aztreonam was demonstrated for the objective response at 48–72 h after initiation of treatment (FDA primary endpoint) and the investigator-assessed cure at FU (EMA primary endpoint). Moreover, across the various sensitivity analyses and secondary objectives, the delafloxacin and vancomycin/aztreonam arms were comparable. This study demonstrates that delafloxacin monotherapy is effective across a range of Gram-positive and -negative pathogens, and was comparable to vancomycin in the treatment of MRSA. Treatment with vancomycin requires a second antibiotic (aztreonam in this study) as initial empirical therapy if Gram-negative pathogens are suspected.
The focus of recent antibiotic development in ABSSSI has been on the coverage of Gram-positive infections, particularly MRSA. However, the risk for inappropriate antimicrobial therapy increases in skin infections when Gram-negative and mixed cultures are present.22,23 Other MDR bacteria are demonstrating similar trends, as seen with the rise in MRSA, and are increasing in prevalence in ABSSSIs. In addition, while Gram-negative pathogens can play an important role in long-standing polymicrobial infections, they are also increasingly found in monomicrobial skin and soft tissue infections (SSTIs).5,6 Gram-negative only cultures have been reported at a rate of 12.8% and mixed cultures in 10.6%–20.5% of patients hospitalized with serious skin infections.24,25
Initial therapy failure rates in skin infections have been shown to range from 16% to 43% depending on type of infection and results in additional days of hospitalization and increased costs.26,27 Combination therapy is used 40% of the time; however, the pathogen is never identified in 61% of cases. When choosing empirical therapy for infections with presumed Gram-negative bacteria, local epidemiology should be taken into account and consideration given to individual patient characteristics such as type of infection including certain SSTIs, comorbidities such as diabetes and compromised vascular profusion and by setting, e.g. residence in a nursing home or recent hospitalization.4,21–28
In this study, cure was defined by more stringent criteria, which required patients to be completely cured, and not merely improved for a positive investigator response. Other antibiotic studies in skin infections have defined a successful outcome as clinical improvement where no further antibiotic therapy is required. This aligns with the definition of success in this study. Whether using cure or success (cure + improved), delafloxacin was comparable to vancomycin/aztreonam in later clinical response at FU and LFU demonstrating a sustained clinical response.
Delafloxacin was well tolerated with a lower overall discontinuation rate compared with vancomycin/aztreonam. The safety profile reported in this study is consistent with previous clinical studies.16,17 Previous studies have shown that delafloxacin has minimal potential for drug interactions and no evidence of QT interval prolongation or phototoxicity.29–32 Taken together, delafloxacin has similar efficacy and safety compared with vancomycin/aztreonam for the treatment of ABSSSI. Delafloxacin offers the potential for the treatment of infections caused by Gram-positive pathogens including MRSA and Gram-negative pathogens, without the need for combination therapy.
Pharmacotherapy of obese patients presents additional challenges to physicians and pharmacists.33 A previous Phase 2 study noted better outcomes with delafloxacin in obese patients than vancomycin at FU and LFU.16 Unlike vancomycin, delafloxacin does not require weight-based dosing or drug monitoring. The results of this study demonstrated that, in obese patients (BMI ≥30 kg/m2), the investigator-assessed outcome at FU and LFU favoured delafloxacin, although it failed to meet statistical significance in all but investigator-assessed cure at LFU. However, the current study was not stratified for obesity at enrolment and a weight limit of 140 kg was instituted due to difficulties in blinding of vancomycin in an obese population.
Other limitations to this study include a low number of burn infections and surgical wounds and the number of Gram-negative pathogens was limited by use of the current ABSSSI definition that favours Gram-positive infections. There were a low number of older adults and non-whites and the rate of diabetes was lower than in the general population.
Delafloxacin was found to have comparable clinical activity to vancomycin in the treatment of patients with ABSSSI by early objective response, later clinical assessments and microbiological response including impact on MRSA. Additionally, delafloxacin was well tolerated. With both intravenous and oral formulations, delafloxacin is appropriate for the treatment of diverse skin infection types due to Gram-positive and -negative bacteria, including patients with MRSA.
Supplementary Material
Acknowledgements
An abstract of the data was presented at IDWeek 2015, San Diego, CA, USA, 2015 (Abstract no. 776).
Members of the PROCEED Study Group
Anna Barvinska, Lviv, Ukraine; Michal Chowers, Kfar Saba, Israel; Dennis Cortes, Miramar, FL, USA; Sadia Dar, Smryna, TN, USA; Oleksii Datsenko, Kharkiv, Ukraine; Robert Eyzaguirre, Long Beach, CA, USA; Brett Farley, Modesto, CA, USA; Janis Gardovskis, Riga, Latvia; Juan Pablo Horcajada Gallego, Barcelona, Spain; Osamah Hussein, Safed, Israel; Heidi Kabler, Las Vegas, NV, USA; Richard Keech, Anaheim, CA, USA; Lajos Kemény, Szeged, Hungary; Sergii Kosulnykov, Dnipropetroversusk, Ukraine; Oleksandr Kosynskyi, Dnipropetroversusk, Ukraine; Viktors Lovcinovskis, Daugavpils, Latvia; Christopher Lucasti, Somers Point, NJ, USA; Paul Manos, Oceanside, CA, USA; Carlos Munoz, Richmond, TX, USA; Maris Nalivaiko, Liepaja, Latvia; William Nseir, Nazareth, Israel; Steven O'Mara, Montgomery, AL, USA; William O'Riordan, Chula Vista, CA, USA; Scott Overcash, La Mesa, CA, USA; Ivan Puljiz, Zagreb, Croatia; John Pullman, Butte, MT; Galia Rahav, Ramat-Gan, Israel; Juan Diego Ruiz Mesa, Málaga, Spain; Shaukat Shah, Stockton, CA, USA; Vadym Shevchenko, Cherkasy, Ukraine; Warren Shu, Pasadena, CA, USA; Kaspars Snipe, Riga, Latvia; Sergiy Vasylyuk, Ivano-Frankiversusk, Ukraine; Anatoliy Zaichuk, Odesa, Ukraine.
These members enrolled patients in the study.
Funding
This work was funded by Melinta Therapeutics. In addition the editorial assistance (see below) was funded by Melinta Therapeutics.
Transparency declarations
J. P., B. F. and J. G. received payment for their services in enrolling patients. E. S., M. Q., L. L. and S. C. are employees of Melinta Therapeutics. R. L. was a consultant paid by Melinta Therapeutics.
Editorial assistance with this manuscript was provided by Strategic Healthcare Communications, Hillsborough, NJ, USA.
Author contributions
All authors participated in the execution and completion of the study and drafting and review of this manuscript. E. S., L. L., S. C. and M. Q. were responsible for development of the protocol. R. L. provided statistical and analytic support. J. P. and J. G. were investigators in the study.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Stevens DL, Bisno AL, Chambers HF. et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59: e10–52. [DOI] [PubMed] [Google Scholar]
- 2. Stevens DL, Bisno AL, Chambers HF. et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41: 1373–406. [DOI] [PubMed] [Google Scholar]
- 3. Barie PS, Wilson SE.. Impact of evolving epidemiology on treatments for complicated skin and skin structure infections: the surgical perspective. J Am Coll Surg 2015; 220: 105–16. [DOI] [PubMed] [Google Scholar]
- 4. Guillamet CV, Kollef MH.. How to stratify patients at risk for resistant bugs in skin and soft tissue infections? Curr Opin Infect Dis 2016; 29: 116–23. [DOI] [PubMed] [Google Scholar]
- 5. Bassetti M, Merelli M, Temperoni C. et al. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob 2013; 12: 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itani KM, Merchant S, Lin SJ. et al. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 2011; 39: 42–9. [DOI] [PubMed] [Google Scholar]
- 7. Moellering RC, Ferraro MJ.. Introduction: solving the clinical problem of vancomycin resistance. Clin Infect Dis 2012; 54 Suppl 3: S201–2. [DOI] [PubMed] [Google Scholar]
- 8. Stryjewski ME, Lentnek A, O’Riordan W. et al. A randomized Phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: the ASSURE study. BMC Infect Dis 2014; 14: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodvold KA, McConeghy KW.. Methicillin-resistant Staphylococcus aureus therapy: past, present and future. Clin Infect Dis 2014; 58 Suppl 1: S20–7. [DOI] [PubMed] [Google Scholar]
- 10. Lemaire S, Tulkens PM, Van Bambeke F.. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-Gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55: 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Bambeke F. Delafloxacin, a non-zwitterionic fluoroquinolone in Phase III of clinical development: evaluation of its pharmacology, pharmacokinetics, pharmacodynamics and clinical efficacy. Future Microbiol 2015; 10: 1111–23. [DOI] [PubMed] [Google Scholar]
- 12. McCurdy S, Lawrence L, Quintas M. et al. In vitro activity of delafloxacin and microbiological response against fluoroquinolone susceptible and non-susceptible S. aureus isolates from two Phase 3 studies of acute bacterial skin and skin structure infections (ABSSSI). Antimicrob Agents Chemother 2017; doi:10.1128/AAC.00772-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nilius AM, Shen LL, Hensey-Rudloff D. et al. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother 2003; 47: 3260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almer LS, Hoffrage JB, Keller EL. et al. In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against gram-positive and gram-negative organisms. Antimicrob Agents Chemother 2004; 48: 2771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harnett SJ, Fraise AP, Andrews JM. et al. Comparative study of the in vitro activity of a new fluoroquinolone, ABT-492. J Antimicrob Chemother 2004; 53: 783–92. [DOI] [PubMed] [Google Scholar]
- 16. O’Riordan W, Mehra P, Manos P. et al. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30: 67–73. [DOI] [PubMed] [Google Scholar]
- 17. Kingsley J, Mehra P, Lawrence LE. et al. A randomized, double-blind, Phase 2 study to evaluate subjective and objective outcomes in patients with acute bacterial skin and skin structure infections treated with delafloxacin, linezolid or vancomycin. J Antimicrob Chemother 2016; 71: 821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Center for Drug Evaluation and Research (CDER). Guidance for Industry Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment, August 2010. Silver Spring, MD: FDA, US Department of Health and Human Services.
- 19. EMA. Committee for Medicinal Products for Human Use (CHMP). Guideline on the Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections Draft. London, 18 February 2010. CPMP/EWP/558/95 rev 2. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/03/WC500079928.pdf.
- 20. Rybak M, Lomaestro B, Rotschafer JC. et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66: 82–98. [DOI] [PubMed] [Google Scholar]
- 21. Miettinen O, Nurminen M.. Comparative analysis of two rates. Stat Med 1985; 4: 213–26. [DOI] [PubMed] [Google Scholar]
- 22. Zilberberg MD, Micek ST, Kollef MH. et al. Risk factors for mixed complicated sin and skin structure infections to help tailor appropriate empiric therapy. Surg Infect (Larchmt) 2012; 13: 377–82. [DOI] [PubMed] [Google Scholar]
- 23. Lipsky BA, Napolitano LM, Moran GJ. et al. Economic outcomes of inappropriate initial antibiotic treatment for complicated skin and soft tissue infections: a multicenter prospective observational study. Diagn Microbiol Infect Dis 2014; 79: 266–72. [DOI] [PubMed] [Google Scholar]
- 24. Berger A, Oster G, Edelsberg J. et al. Initial treatment failure in patients with complicated skin and skin structure infections. Surg Infect (Larchmt) 2013; 14: 304–12. [DOI] [PubMed] [Google Scholar]
- 25. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65: 128–34. [DOI] [PubMed] [Google Scholar]
- 26. Russo A, Concia E, Cristini F. et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect 2016; 22 Suppl 2: S27–36. [DOI] [PubMed] [Google Scholar]
- 27. Tamma PD, Cosgrove SE, Maragakis LL.. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev 2012; 25: 450–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010; 65 Suppl 3: iii35–44. [DOI] [PubMed] [Google Scholar]
- 29. Litwin JS, Benedict MS, Thorn MD. et al. A thorough QT study to evaluate the effects of therapeutic and supratherapeutic doses of delafloxacin on cardiac repolarization. Antimicrob Agents Chemother 2015; 59: 3469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawrence L, Benedict M, Hart J. et al. Pharmacokinetics (PK) and safety of single doses of delafloxacin administered intravenously in healthy human subjects. In: Abstracts of the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2011. Abstract A2-045A. American Society for Microbiology, Washington, DC, USA.
- 31. Lawrence L, Benedict M, Litwin J. et al. A thorough Phase 1 QTc study of delafloxacin compared with placebo and moxifloxacin (MXF). In: Abstracts of the Fifty-second Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 2012. Abstract A1958. American Society for Microbiology, Washington, DC, USA.
- 32. Ferguson J, Lawrence L, Paulson S. et al. Assessment of phototoxicity potential of delafloxacin in healthy male and female subjects: a Phase 1 study. In: Abstracts of the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015. Abstract F-1198a. American Society for Microbiology, Washington, DC, USA.
- 33. Pai MP, Bearden DT.. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 2007; 27: 1081–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.