Abstract
Objectives
Patient- and procedure-related changes in modern medicine have turned CoNS into one of the major nosocomial pathogens. Treatments of CoNS infections are challenging owing to the large proportion of MDR strains and oxazolidinones often remain the last active antimicrobial molecules. Here, we have investigated a long-lasting outbreak (2010–13) due to methicillin- and linezolid-resistant (LR) CoNS (n = 168), involving 72 carriers and 49 infected patients.
Methods
Antimicrobial susceptibilities were tested by the disc diffusion method and MICs were determined by broth microdilution or Etest. The clonal relationship of LR Staphylococcus epidermidis (LRSE) was first determined using a semi-automated repetitive element palindromic PCR (rep-PCR) method. Then, WGS was performed on all cfr-positive LRSE (n = 30) and LRSE isolates representative of each rep-PCR-defined clone (n = 17). Self-transferability of cfr-carrying plasmids was analysed by filter-mating experiments.
Results
This outbreak was caused by the dissemination of three clones (ST2, ST5 and ST22) of LRSE. In these clones, linezolid resistance was caused by (i) mutations in the chromosome-located genes encoding the 23S RNA and L3 and L4 ribosomal proteins, but also by (ii) the dissemination of two different self-conjugative plasmids carrying the cfr gene encoding a 23S RNA methylase. By monitoring linezolid prescriptions in two neighbouring hospitals, we highlighted that the spread of LR-CoNS was strongly associated with linezolid use.
Conclusions
Physicians should be aware that plasmid-encoded linezolid resistance has started to disseminate among CoNS and that rational use of oxazolidinones is critical to preserve these molecules as efficient treatment options for MDR Gram-positive pathogens.
Introduction
CoNS are becoming some of the most prevalent nosocomial pathogens.1 As typical opportunistic bacteria, they now have a substantial impact on human health, particularly with the increased use of immunosuppressive therapies and indwelling or implanted foreign devices, which are essential in modern medicine. Indeed, as the dominant species of skin and of several mucosal microbiota, CoNS are at the forefront of important sources of endogenous infections. In addition, the treatment of CoNS infections is most often challenging due to the large proportion of methicillin-resistant isolates and increasing dissemination of strains with decreased susceptibility to glycopeptides.1 Consequently, antimicrobial therapy usually relies on a few last-resort antimicrobial molecules, including oxazolidinones.2
Linezolid is an oxazolidinone with activity against Gram-positive pathogens, including methicillin-resistant staphylococci and glycopeptide-resistant enterococci.3 Fifteen years after its approval for clinical use in the USA, linezolid remains extremely active against most Staphylococcusaureus and CoNS clinical isolates (mostly Staphylococcusepidermidis), with <1% and <2% resistant isolates, respectively, reported from surveillance studies.2,4–6 Due to its broad antimicrobial spectrum against Gram-positive bacteria, its favourable short-term safety profile, its pharmacokinetics/pharmacodynamics and its effectiveness, linezolid is widely used in critical care.7,8 Linezolid inhibits bacterial protein synthesis by reversibly binding and blocking the ribosomal peptidyl-transferase centre.7,9 The most frequently reported mechanism of resistance is a G2576U point mutation in the V domain of the 23S rRNA genes.6 However, the MIC of linezolid is directly related to the number of mutated 23S rRNA copies.10 Mutations in the ribosomal proteins L3 and L4 have also been reported to be responsible for linezolid resistance. The first known linezolid resistance trait transferable between Gram-positive bacteria is the cfr (chloramphenicol florfenicol resistance) gene. This gene encodes a 23S rRNA methylase that modifies the A2503 residue in domain V of 23S rRNA, thereby impeding binding to the ribosome of linezolid, phenicol, lincosamide, streptogramin A, pleuromutilin and the 16-member ring macrolides.11,12 Two other Cfr-like proteins, Cfr(B) and Cfr(C), have been recently described in Enterococcusfaecium and Clostridiumdifficile, respectively.13,14 More recently, a novel transferable resistance gene, optrA, which confers resistance to oxazolidinones and phenicols, has been described in enterococci isolated in China.15 The optrA gene has been mainly described in Enterococcus species and rarely in other Gram-positive bacteria, mostly of porcine origin, such as Staphylococcussciuri16,17 and Streptococcussuis.18
Nosocomial outbreaks with linezolid-resistant (LR) Enterococcusfaecalis,19E.faecium,20,21S.aureus22,23 and S.epidermidis24–32 have been sporadically reported. Infections caused by LR S.epidermidis (LRSE) remain uncommon and have rarely been associated with outbreaks.24,28–31 However, a long-lasting outbreak (23 ST2 isolates between 2004 and 2015) due to methicillin-resistant cfr-negative LRSE was described recently in Italy.29 Using WGS we have investigated the largest reported outbreak of linezolid- and methicillin-resistant CoNS, involving 72 carriers and 49 infected patients. By combining epidemiological, microbiological and genomic information we were able to show that this outbreak was related to (i) the dissemination of three different clones (ii) and to the transfer of two cfr-encoding plasmids.
Methods
Field investigation
A 750 bed tertiary care university teaching hospital (referred to as hospital PB in this study) located in a southern suburb of Paris serves as a European referral centre for liver transplantation. The hepatobiliary centre consists of an ICU and two medical–surgical hepatology (MSH) units. All diagnostic samples positive for LR-CoNS collected from patients between September 2010 and December 2013 were included in this study (n = 168). These samples were reassessed for diagnostic criteria to distinguish true infections from colonization. A summary of the clinical samples from which sequenced LRSE have been isolated is shown in Table S1 (available as Supplementary data at JAC Online). During the same period of time, linezolid usage has been assessed in hospital PB and compared with a case–control hospital (referred to as hospital B in this study), another 1000 bed tertiary care teaching hospital located 2 km from hospital PB.
Bacterial isolates and susceptibility testing
Diagnostic samples were processed according to standard methods. Isolates were identified at the species level using MALDI-TOF spectrometry (Maldi-Biotyper, Bruker Daltonique SA, Wissembourg, France). Antimicrobial susceptibilities were tested by the disc diffusion method for penicillin, oxacillin, kanamycin, tobramycin, gentamicin, erythromycin, clindamycin, pristinamycin, levofloxacin, chloramphenicol, tetracycline, fusidic acid, fosfomycin, trimethoprim/sulfamethoxazole, rifampicin and linezolid. Results of linezolid susceptibility testing by the disc diffusion method were primarily used to select LR isolates. MICs were determined by broth microdilution for linezolid and tedizolid, and by Etest (bioMérieux, La Balmes-les-Grottes, France) for vancomycin, teicoplanin, daptomycin, ceftobiprole and ceftaroline. Results were interpreted according to EUCAST as updated in 2015 (http://www.eucast.org/clinical_breakpoints/).
Linezolid resistance determinants
Total DNA was extracted using the UltraClean® microbial DNA isolation kit (MO BIO Laboratories, Carlsbad, CA, USA). Presence of the cfr gene was investigated by PCR with primers described in Table S2. Specific primers were used to amplify and sequence each copy of the 23S rRNA gene (rrlA to rrlF) and rplC, rplD and rplV genes encoding L3, L4 and L22 ribosomal proteins, respectively (Table S2). Linezolid-susceptible S.epidermidis ATCC 12228 was used as reference for nucleotide and amino acid sequence comparisons (GenBank accession number NC_004461).
Clonality analysis using repetitive element palindromic PCR (rep-PCR)
To evaluate their clonal relationship, all LRSEs were subjected to Diversilab™, a semi-automated rep-PCR method (bioMérieux, Marcy-l’Étoile, France). As recommended by the manufacturer, a cut-off for similarity of 95% defined a cluster.33–35
WGS procedure
WGS was performed on all cfr-positive LRSE (n = 30) and LRSE isolates representative of each rep-PCR-defined clone (n = 17). Library preparation was performed using a Nextera® XT DNA sample preparation kit (Illumina, San Diego, CA, USA). Sequencing was performed on an Illumina MiSeq sequencer with v3 chemistry using 2 × 75 bp paired-end reads at a raw cluster density of ∼1300000 clusters/mm2. Genome sequences were assembled using the Velvet software with an optimized k-value and a minimal coverage of eight.36 WGS results were used to genotype isolates by MLST (http://cge.cbs.dtu.dk/services/MLST/). To further determine variation amongst isolates that clustered in the same ST, we selected one strain from each ST as a reference (strains 1C6, 2E9 and 2F1 for the ST2, ST5 and ST22 lineages, respectively) and looked for SNPs. These SNPs were searched for by using the breseq software.37 DNA regions specific to each strain were searched by denovo assembly of the unmapped reads. Antibiotic resistance genes were identified through the Center for Genomic Epidemiology web tool (http://cge.cbs.dtu.dk/services/ResFinder/).38
Phylogenetic tree analysis using WGS data
Phylogenic analysis of the 47 LRSE strains together with 9 strains belonging to different STs was performed using core genome alignment and visualized using the pangenomic software package Harvest.39 Briefly, the core genome of the 56 S.epidermidis strains was aligned using the muscle algorithm with the parsnp tool. PhiPack software was used to filter recombination and phylogenetic reconstruction was done using FastTree2. Maximum likelihood using MEGA was used to infer phylogenetic relationships between strains belonging to the same ST.40
Self-transferability of cfr-harbouring plasmids
Self-transferability of cfr-carrying plasmids was analysed by filter-mating experiments between clinical cfr-positive LRSE (susceptible to fosfomycin) as donor and the linezolid-susceptible, fosfomycin-resistant Staphylococcuscapitis strain DAM as recipient. Trans-conjugants were selected on agar plates containing linezolid (1 mg/L) (Sigma-Aldrich, Lyon, France) and fosfomycin (100 mg/L) (Sigma-Aldrich).
Nucleotide sequence accession number
Sequencing reads from the 47 newly sequenced S.epidermidis strains have been deposited in the EMBL nucleotide sequence database (http://www.ebi.ac.uk/ena) under the study accession number (PRJEB22222).
Results
Description of the LR-CoNS outbreak
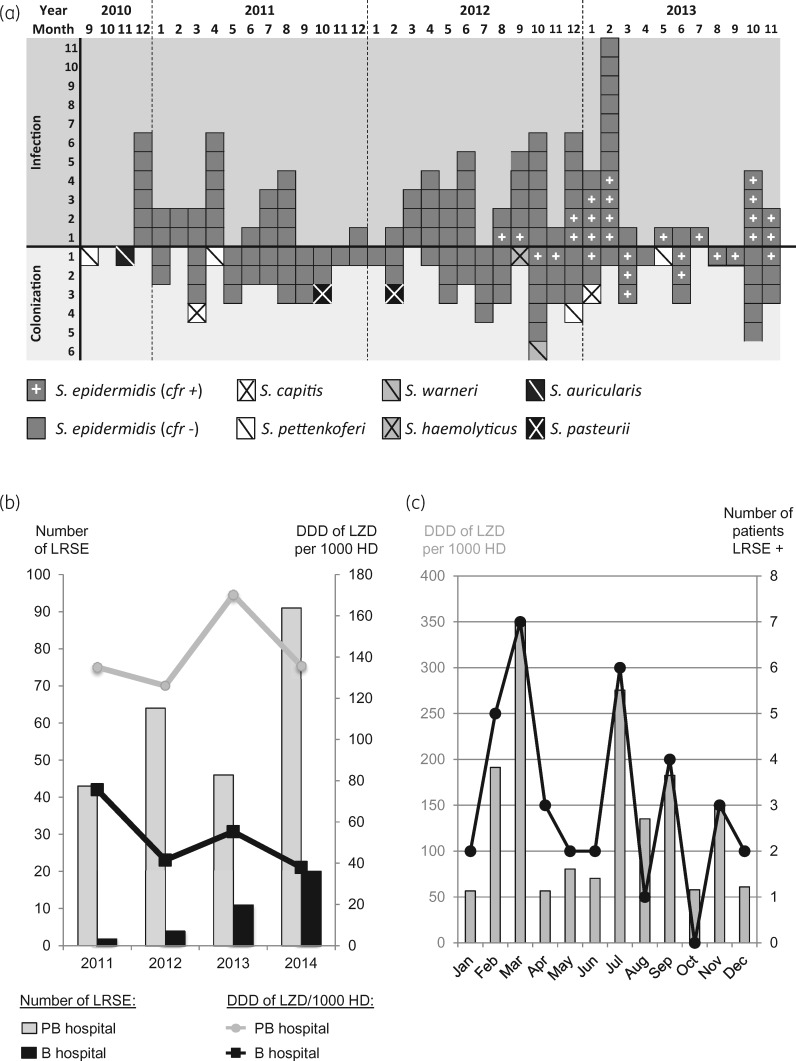
Between September 2010 and December 2013, 168 LR-CoNS were recovered from 121 patients hospitalized in the hepatobiliary centre of hospital PB, a 750 bed tertiary care university teaching hospital. Eighty-eight LR-CoNS collected from 49 patients were responsible for infections (Figure 1a). These isolates were all identified as S.epidermidis (Figure 1a). The 80 remaining LR-CoNS isolates (1 Staphylococcusauricularis, 2 S.capitis, 1 Staphylococcushaemolyticus, 4 Staphylococcuspettenkoferi, 1 Staphylococcuswarneri, 2 Staphylococcuspasteurii and 69 S.epidermidis) collected from 72 patients were considered to be colonizers (Figure 1a).
Figure 1.
Timeline of the outbreak caused by LR-CoNS and correlation with global linezolid consumption. (a) Time flow chart of LR-CoNS isolates recovered from September 2010 to December 2013. Each square corresponds to one isolate. Species identification and the presence of the cfr gene are indicated according to the legend under the chart. (b) Comparison of the number of LRSE isolates recovered from 2011 to 2014 in hospitals B and PB, two tertiary care university teaching hospitals located 2 km apart, and correlation with the defined daily doses (DDDs) of linezolid per 1000 days of hospitalization (HD). (c) Numbers of patients colonized and/or infected with LRSE per month in the ICU of hospital PB and correlation with linezolid consumption in this unit in 2014. LZD, linezolid.
During the study period, the number of isolated LRSE per year from clinical samples responsible for infection or colonization in hospital PB was 4- to 21-fold higher than those isolated in a case-control hospital (hospital B), a 1000 bed tertiary care teaching hospital located 2 km from hospital PB (Figure 1b). Monitoring linezolid prescriptions in the two hospitals showed that the higher number of LRSE is correlated (R2 = 0.80) with a higher consumption of linezolid with, from 2011 to 2014, an average of 141.8 and 29.2 defined daily doses per 1000 days of hospitalization in hospital PB and hospital B, respectively (Figure 1b). Furthermore, focusing on the ICU of hospital PB, the monthly number of patients colonized and/or infected with LRSE was correlated with the global consumption of linezolid in this unit (R2 = 0.89) (Figure 1c).
Susceptibility to last-resort antimicrobial families
All LR-CoNS were resistant, in addition to methicillin, to all aminoglycosides, quinolones and chloramphenicol (Figure S1). However, all strains remained susceptible to vancomycin, daptomycin and two β-lactams (ceftobiprole and ceftaroline) (Table 1). Although tedizolid, a second-generation oxazolidinone, was shown to be efficient against some LR isolates, especially Cfr-producing strains,41 all LR-CoNS were also resistant to tedizolid (Table 1). Accordingly, only therapies based on these last-resort antimicrobial families are likely to be effective, but they require parenteral administration.
Table 1.
Susceptibility of LR-CoNS (n = 168) to last-resort antimicrobial families (glycopeptides, daptomycin, fifth-generation cephalosporins and oxazolidinones)
| Antimicrobial class | Agent | MIC range (mg/L) | MIC50 (mg/L) | MIC90 (mg/L) | EUCAST MIC breakpoint (mg/L) |
|---|---|---|---|---|---|
| Glycopeptides | vancomycin | 0.75–4 | 1.5 | 2 | 4 |
| teicoplanin | 0.5–6 | 2.5 | 4 | 4 | |
| Lipopeptide | daptomycin | 0.12–1 | 0.25 | 0.5 | 1 |
| β-Lactams | ceftobiprole | 0.12–1 | 0.75 | 1 | 2 |
| ceftaroline | 0.06–0.5 | 0.25 | 0.5 | 1 | |
| Oxazolidinones | linezolid | 6 to >256 | 128 | 256 | 4 |
| tedizolid | 2 to >32 | 8 | 32 | 0.5 |
Clonality of LRSE isolates
We focused our analysis on LRSE, as they represented 100% of the strains responsible for infections (n = 88) and 93.5% (157/168) of the total LR-CoNS. Their clonal relationship was first evaluated using a semi-automated rep-PCR method. Using a 95% similarity threshold to discriminate clones, as recommended by the manufacturer, five distinct patterns were identified (C1–C5) (Figure S1A and B). C1, C2 and C3 were found among LRSE responsible for infections (n = 88) (Figure S1A), but also among LRSE considered as colonizers (Figure S1B). C4 and C5, with two isolates and one isolate respectively, were only identified among colonizers (n = 69) (Figure S1B). Among these LRSE, we identified 30 cfr-positive isolates but none was optrA, cfr(B) or cfr(C) positive.
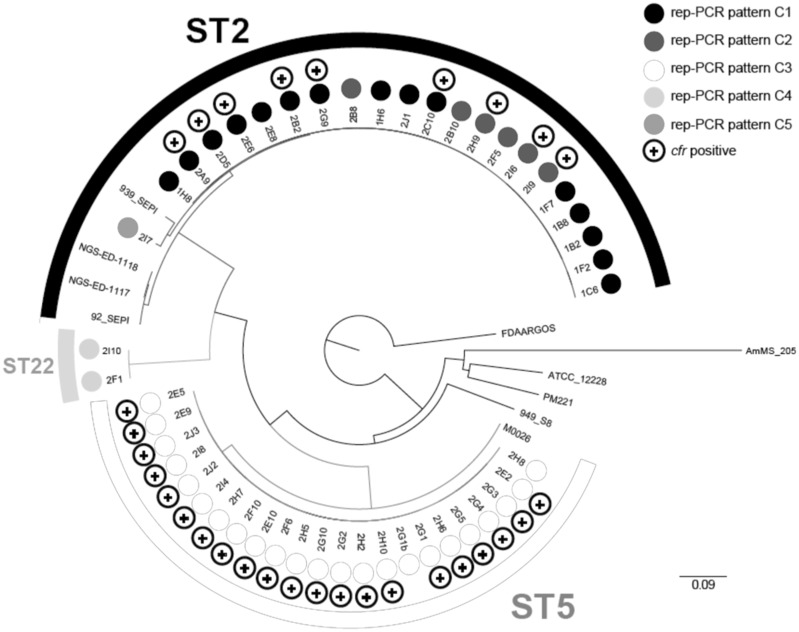
To unambiguously determine the clonal relationships within the outbreak, WGS was performed on the 30 cfr-positive LRSE and on 17 LRSE isolates representative of the five clones identified by rep-PCR (9, 3, 2, 2 and 1 isolates of clones C1, C2, C3, C4 and C5, respectively) (Figure S1A and B and Table S2). Maximum likelihood phylogeny on core-genome SNPs of these 47 LRSE and of 9 S.epidermidis reference genomes revealed that the 47 LRSE from the outbreak cluster in three distinct lineages belonging to MLST types ST2, ST5 and ST22 (Figure 2). LRSE isolates of the rep-PCR patterns C3 and C4 were all of ST5 and ST22, respectively. In contrast, isolates from C1, C2 and C5 were closely related and were all of ST2. Accordingly, a detailed analysis of those three clones revealed that clones C2 and C5 derived from clone C1, with which they share a common ancestor, having <13 SNPs along their core genome. Furthermore, clone C2 is not monophyletic (Figure 3a). A 13946 bp deletion within the SCCmec cassette region (Figure S2) occurred at least three times independently in the ST2 clone, leading to the modification in the pattern obtained by rep-PCR and to the misidentification of the two novel clones C2 and C5 (Figure 3a). The strain of clone C5 carries an additional SCCmec cassette region inserted elsewhere in the chromosome. This insertion is likely responsible for changes in the rep-PCR pattern as compared with clone C2. LRSE isolates of the rep-PCR patterns C3 and C4 are monophyletic and distantly related to the ST2 clone, in agreement with their different STs (ST5 and ST22, respectively). The 23 ST5 isolates share a common ancestor, having <11 SNPs along their core genome (Figure 3b), whereas the two ST22 isolates differ by only 6 SNPs (Figure 3c). Therefore, WGS revealed that the observed outbreak was mainly due to three clones of S.epidermidis referred to as ST2, ST5 and ST22 later in this article (Figure S3).
Figure 2.
Genetic relatedness and cfr expression of LRSE isolates. Maximum likelihood phylogenetic tree based on sequence variation in the core genome from the 47 LRSE isolates submitted to WGS with 9 strains belonging to different STs. Respective STs deduced from WGS data are indicated (ST2, ST5 and ST22). Presence of cfr is indicated by a + sign for cfr+ LRSE isolates.
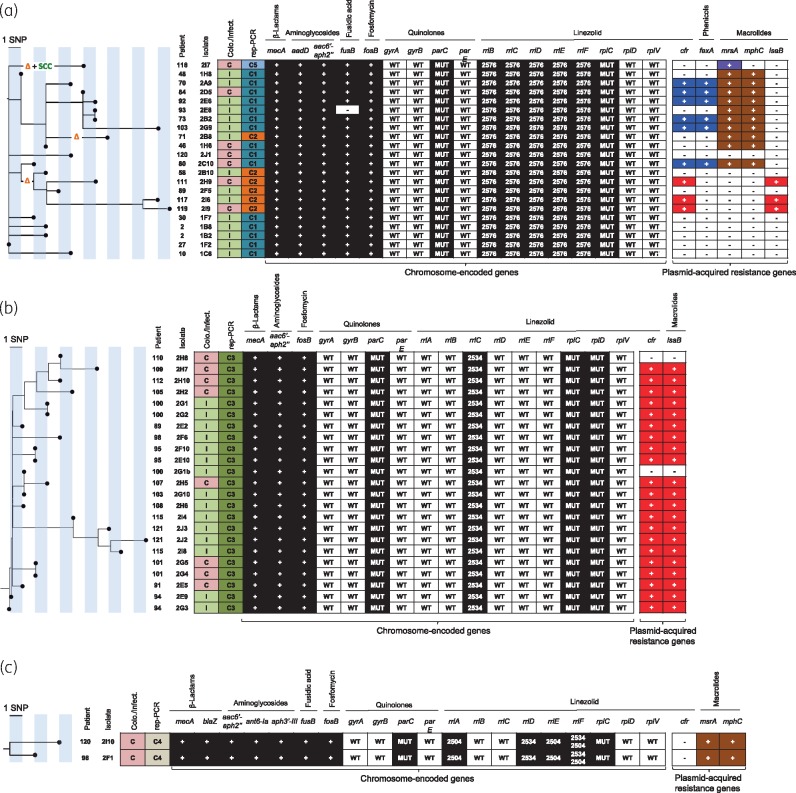
Figure 3.
Maximum likelihood phylogenetic tree based on sequence variation in the core genome. (a) ST2 LRSE isolates. (b) ST5 LRSE isolates. (c) ST22 LRSE isolates. The orange Δ indicates a deletion of 13946 bp in genes located in the SCCmec cassette region (Figure S2). The green ‘SCC’ indicates the presence of an additional SCC cassette. Patient and isolate numbers are indicated on the left of the tree. Colonization or infection is indicated in the first column (C in red box for colonization, I in green box for infection). Acquired resistance genes for β-lactams, aminoglycosides, fusidic acid, fosfomycin, linezolid, phenicols and macrolides are indicated as follows: + in a dark or coloured box, presence; - in a white box, absence. Mutations in chromosome-encoded genes involved in quinolone and linezolid resistance are represented. WT, WT gene; MUT, presence of a mutation known to be responsible for phenotypic resistance; 2576, G2576U point mutation in the V domain of the 23S rRNA gene (rrl); 2534, C2534U point mutation in the V domain of the 23S rRNA gene; 2504, U2504A point mutation in the V domain of the 23S rRNA gene. Plasmid-acquired genes boxed with the same colour (purple, blue, brown and red) are located on the same plasmid [blue, p-cfr-PBR-A plasmid of 38745 bp (Figure 4a); red, p-cfr-PBR-B plasmid of 40182 bp (Figure 4b)].
Chromosome-encoded resistance to linezolid
The G2576U point mutation was identified in the V domain of all five copies of 23S rRNA genes of the ST2 LRSE strains (Figure 3a). These mutations would be sufficient on their own to lead to a high level of resistance to linezolid. In addition, we also identified in ST2 LRSE two mutations in rplC encoding ribosomal protein L3 (L101V and M156T). These mutations have previously been described in LR isolates, although the L101V mutation was not shown to influence linezolid resistance.42
ST5 LRSE isolates harboured a single mutation (C2534U) in one copy of the 23S rRNA genes (rrlC) (Figure 3b), which is not sufficient to cause the high level of linezolid resistance of ST5 isolates. Accordingly, all ST5 LRSE isolates also harboured mutations in ribosomal proteins L3 (L101V, H146Q, V154L, A157R) and L4 (71insertG, R96T, N158S) that could have an additive effect on linezolid resistance. However, none of the L4 mutations was previously demonstrated to alter linezolid susceptibility.42 In particular, the N158S mutation has been found among linezolid-susceptible S.epidermidis isolates and is therefore probably a clonal marker rather than a resistance mutation.43
The two ST22 LRSE isolates are mutated (U2504A and/or C2534U) in four of the six copies of the 23S rRNA genes (rrlA, rrlD, rrlE and rrlF) (Figure 3c). Interestingly, the rrlF gene co-harbours two mutations that have rarely been identified in LR staphylococci. Mutations in the L3 ribosomal protein (L101V, G152D and D159Y) were also found. Interestingly, this linezolid resistance pattern is identical to that observed in ST22 LRSE isolates from Greece in 2010.44 However, no obvious link with Greece could be evidenced in the clinical cases of the two ST22 LRSE-infected patients from hospital PB.
cfr-carrying plasmids
The cfr gene has been detected in 7% (9/130) of the LRSE isolates of the ST2 clone, 91.3% (21/23) of the ST5 isolates and in none (0/2) of the ST22 isolates (Figure S3). Since the cfr gene has been detected on different plasmids, we analysed WGS data for plasmids carrying the cfr gene. Six ST2 cfr-positive isolates carried an identical 38745 bp cfr-harbouring plasmid, named p-cfr-PBR-A, which also harboured the fexA gene coding for phenicol resistance (Figure 4a). It is almost identical to the cfr-plasmid recovered from the first reported cfr-positive isolate of S.aureus in the USA in 2008,45 with only a 542 bp deletion and no SNP along the rest of the plasmid. In addition, p-cfr-PBR-A shows only three SNPs with a cfr-carrying plasmid recovered from a Staphylococcuscohnii isolated in China in 2013 (Figure 4a).46
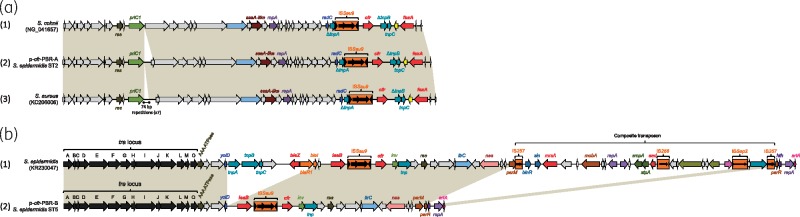
Figure 4.
Comparison of the two cfr-harbouring plasmids with related plasmids. (a) p-cfr-PBR-A: (1) corresponds to the cfr-carrying plasmid recovered from S. cohnii (GenBank NG_041657); (2) corresponds to the cfr-carrying plasmid recovered from ST2 LRSE isolates, p-cfr-PBR-A; (3) corresponds to the cfr-carrying plasmid recovered from S. aureus (GenBank KC206006). (b) p-cfr-PBR-B: (1) pSP01 cfr-encoding plasmid recovered from an S. epidermidis strain isolated in Italy; (2) corresponds to the cfr-carrying plasmid recovered from ST5 LRSE isolates, p-cfr-PBR-B. Common features are highlighted with beige shading. Gene names are as follows: res, resolvase; priC, primase C; ssa-like, secretory antigen Ssa-like; radC, DNA repair protein RadC; repA, replicase; tnp, transposase; cfr, chloramphenicol/florfenicol resistance; fexA, florfenicol/chloramphenicol efflux pump. Genes encoding antimicrobial resistance determinants are represented in red. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Most of the ST5 LRSE (21/23) and three cfr-positive ST2 isolates (all isolated in 2013) harbour a cfr-carrying plasmid of 40182 bp (p-cfr-PBR-B; Figure 4b). The p-cfr-PBR-B plasmid was closely related to the cfr-carrying pSP01 plasmid recovered from an S.epidermidis strain isolated in 2010 in Italy (Figure 4b).47 As shown in Figure 4(b), the p-cfr-PBR-B plasmid appears to be similar to the progenitor of the pSP01 plasmid. pSP01 shows only 24 SNPs over 40 kb with p-cfr-PBR-B, but carries two transposons inserted at nucleotide positions 16764 and 37532 bp of pSP01 and coding for three additional antimicrobial resistance determinants involved in resistance to β-lactams, macrolides and aminoglycosides (blaZ, mrsA and aad). p-cfr-PBR-Bs from ST5 and ST2 isolates were 100% identical, suggesting a transfer from an ST5 strain to an ST2 strain during the outbreak.
To strengthen our hypothesis of conjugative transfers of cfr-carrying plasmids, conjugation experiments were performed using an ST2 LRSE isolate encoding the p-cfr-PBR-A plasmid and an ST5 LRSE isolate encoding the p-cfr-PBR-B plasmid as donors and a WT S.capitis isolate (S.capitis DAM) naturally resistant to fosfomycin (MIC >1024 mg/L) as recipient. As shown in Table 2, both plasmids could be efficiently transferred, leading to multiple antibiotic resistances: to linezolid, clindamycin and chloramphenicol. The resistance to tedizolid observed for ST2 and ST5 clinical isolates was not transferred with the cfr-carrying plasmid, confirming a better stability of tedizolid towards cfr-related oxazolidinone resistance as compared with linezolid.41 Accordingly, the tedizolid resistance observed in all LRSE is related to the chromosomal mutations of 23S RNA genes and of L3 and/or L4 protein.
Table 2.
MICs for ST2 and ST5 Cfr-producing S. epidermidis isolates, S. capitis DAM and S. capitis DAM transconjugants
| Antimicrobial agenta | MIC (mg/L) |
||||
|---|---|---|---|---|---|
| S. epidermidis ST2 p-cfr-PBR-A | S. epidermidis ST5 p-cfr-PBR-B | S. capitis DAM (p-cfr-PBR-A)b | S. capitis DAM (p-cfr-PBR-B)b | S. capitis DAM | |
| Linezolid | >256 | >256 | 12 | 12 | 1 |
| Tedizolid | 32 | 3 | 0.25 | 0.25 | 0.25 |
| Ceftobiprole | 1 | 0.75 | 0.064 | 0.064 | 0.064 |
| Ceftaroline | 0.25 | 0.19 | 0.032 | 0.032 | 0.032 |
| Vancomycin | 3 | 1 | 1 | 1 | 1 |
| Teicoplanin | 2 | 0.75 | 0.25 | 0.25 | 0.25 |
| Daptomycin | 0.38 | 0.19 | 0.75 | 0.75 | 0.75 |
| Fosfomycin | 24 | 1 | >1024 | >1024 | >1024 |
| Clindamycin | >256 | >256 | >256 | >256 | 0.047 |
| Chloramphenicol | 256 | 64 | 256 | 64 | 4 |
| Erythromycin | 64 | 8 | 0.25 | 0.25 | 0.25 |
Antimicrobial agents for which susceptibility is impacted by Cfr-dependent 23S RNA methylation are in bold.
The two plasmids indicated in brackets were transferred into S. capitis DAM strain by conjugation.
Outbreak of cfr-positive LRSE
To determine the most likely transmission events during the disseminations of clones and plasmids, we combined clinical information (Table 3), the synoptic curve of infected or colonized patients, the distribution of cfr plasmids (Figure 5), the deep characterization of the isolates and the phylogeny of each clone (Figure 3).
Table 3.
Characteristics of patients (n = 23) with cfr-positive linezolid- and methicillin-resistant S. epidermidis (cfr-positive LRSE)
| Male, n (%) | 15 (65) |
| Age (years), mean (SD) | 59.1 (9) |
| SAPS 2 score, mean (SD) | 44.7 (15) |
| Diagnostic group, n (%) | |
| medical | 15 (65) |
| surgical | 8 (35) |
| Cancer, n (%) | 5 (22) |
| Hospitalized in ICU prior to isolation of LRSE, n (%) | 19 (83) |
| SOFA score, mean (SD) | 9.0 (4) |
| vasopressors, n (%) | 12 (52) |
| Prior antibiotic | |
| broad-spectrum >5 days, n (%) | 17 (74) |
| fluoroquinolones, n (%) | 3 (13) |
| linezolid | |
| length of use (days), median (IQR) | 18 (13–27) |
| total dose (mg), median, (IQR) | 10800 (7500–15900) |
| Total length of stay (days), mean (SD) | |
| hospital | 109.9 (69) |
| ICU | 36.8 (35) |
| Mortality, n (%) | 10 (43) |
| ICU | 7 (30) |
| another unit | 3 (13) |
| Infection with LRSE, n (%) | 14 (61) |
| Colonization with LRSE, n (%) | 9 (39) |
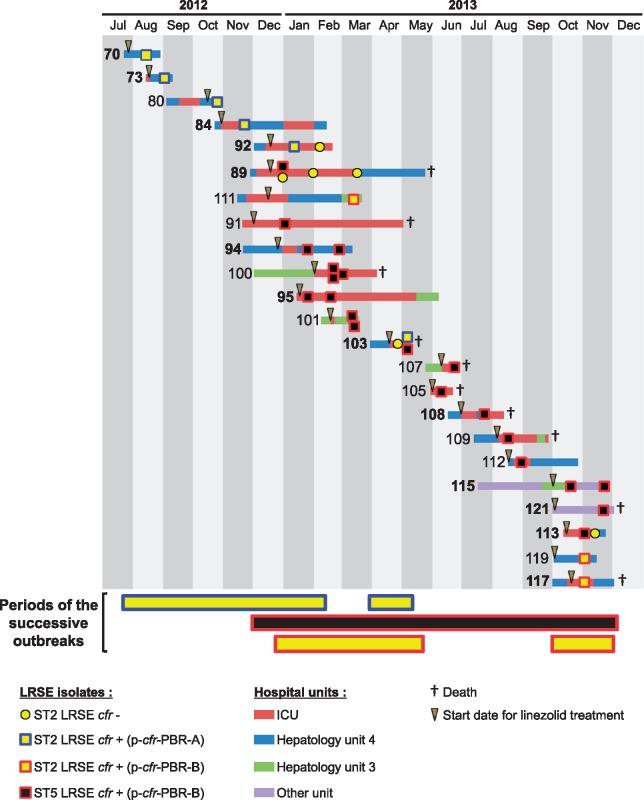
Figure 5.
Synoptic curve of patients infected or colonized with cfr-positive LRSE. The number in front of each line corresponds to the patient number. A patient number in bold signifies that the patient has been considered infected with a cfr-positive LRSE. A number not in bold indicates that the cfr-positive LRSE has been considered as a colonizer. The periods corresponding to the three successive outbreaks are indicated below the x-axis. The colour code corresponds to related strain (ST and cfr-encoding plasmid). Colour codes for hospitalization unit are indicated under the chart.
Phylogenetic analysis showed significant within-host diversity (e.g. Patients 2 and 100), which might result either from contamination with a diverse population or several events of contamination, making the reconstruction of the transmission chain difficult. Thus, we have concentrated our investigations on the transmission of cfr-carrying plasmids, which represents a major event in this outbreak. The synoptic curve showed that the vast majority of the patients were hospitalized either in the ICU or in one of the surgical units (HMS3 and HSM4) when they acquired the cfr-positive LRSE. In addition, we observed a classical ‘cascade’ of cfr-positive LRSE acquisition in patients, strongly suggesting classical bacterial dissemination via healthcare workers. The first outbreak involving the ST2 LRSE cfr+ (p-cfr-PBR-A) strain probably started in HSM4 in August 2012 with the suspected index case, Patient 70, and four secondary cases, Patients 73, 84, 92 and 103 (Figure 5). The phylogenetic analysis shows that the ST2 strain in patient 80 was probably acquired independently (meaning not from patient 73, 84, 92 and 103). The second outbreak, which involved the ST5 LRSE cfr+ (p-cfr-PBR-B) strain probably started in the ICU (Patient 89). From Patient 89 a cross-transmission probably occurred to Patients 91, 94, 95, 100, 101 and 103. Then, a cascade of cross-transmission for the ST5 LRSE (p-cfr-PBR-B) strain was observed to Patients 105, 107, 108, 109, 112, 113, 115 and 121. Concerning the third outbreak, which involved an ST2 LRSE cfr+ (p-cfr-PBR-B), microbiological and epidemiological data suggested an in vivo transfer of the plasmid p-cfr-PBR-B from the bacterial clone ST5 to the ST2 clone in a unique abscess of Patient 89, where an ST5 LRSE cfr+ (p-cfr-PBR-B) and a cfr-negative ST2 LRSE were isolated. Then, this ST2 LRSE cfr+ (p-cfr-PBR-B) strain was cross-transmitted to Patient 111. This strain was isolated 6 months later in Patients 117 and 119 while the ST5 LRSE (p-cfr-PBR-B) was the strain predominantly isolated from patients infected or colonized by LRSE. This resurgence of the ST2 LRSE cfr+ (p-cfr-PBR-B) strain might be explained by an indirect transmission route or by patients positive for LRSE who have not been detected.
Discussion
The characterization of this large outbreak showed that oxazolidinone resistance has already started to disseminate efficiently in the three most prevalent clones of S.epidermidis: ST2, ST5 and ST22.48 Since CoNS are opportunistic pathogens, they are mostly isolated from clinical samples recovered from immune-compromised or debilitated patients.1,49 Accordingly, such a large outbreak of LR-CoNS might only represent the tip of the iceberg of the global oxazolidinone resistance in staphylococci and could thus suggest that oxazolidinone resistance may have occurred at an unexpected level in CoNS of the skin flora. This last hypothesis is worrisome since we demonstrated that linezolid resistance was not only due to clonal dissemination but also to the in vivo transfer of two different self-conjugative plasmids carrying the cfr gene. Therefore, the risk of a rapid transfer of an oxazolidinone resistance determinant in more virulent species, such as S.aureus or even MRSA within the normal flora of non-infected patients, should be considered. Such transfer might lead to clinical issues regarding therapeutic options.50 Our results suggested that antibiotic stewardship measures aimed at controlling oxazolidinone prescription might help to avoid such a dramatic scenario. Indeed, since the dissemination of LRSE was correlated with the antimicrobial pressure occurring in the hospital (Figure 1b and c), restriction of linezolid prescriptions to experienced doctors in the ICU of hospital PB since December 2014 led to a decrease (−61.5%) in LRSE isolation (35 LRSE isolated from 15 patients in 2015 versus 91 LRSE isolated from 35 patients in 2014). However, this feature has to be confirmed since variations have been observed in LRSE isolation from 2010 to 2014.
With other MDR bacteria, such as carbapenemase-producing Enterobacteriaceae, specific infection control measures are implemented based on an efficient screening of colonized patients and then implementation of dedicated staff, a single room and limitation of patient transfer to avoid outbreaks. However, this requires the availability of accurate detection tests. Unfortunately, no commercially available assay addresses the detection of plasmid-mediated resistance to oxazolidinone, reinforcing the possible unrecognized dissemination of this resistance trait.
We confirmed that the transfer of the cfr-carrying plasmid alone does not alter tedizolid susceptibility (Table 2). Accordingly, and concomitantly with tedizolid approval in France in 2015, the clinical microbiology laboratory that is used by hospitals PB and B has implemented routine susceptibility testing of this drug in case of linezolid resistance. However, in this outbreak the in vivo transfer of the p-cfr-PBR-B plasmid always occurred in an ST2 LRSE isolate (Figures 3a and 5), which co-harboured chromosomal mutations in 23S rRNA genes and L3 and L4 ribosomal proteins (Figure 3a), leading also to tedizolid co-resistance (Tables 1 and 2). Thus, as opposed to oxazolidinones that could be orally administered, the few remaining active antimicrobial molecules (glycopeptides, daptomycin, ceftaroline and ceftobiprole) for the treatment of LRSE infections required parenteral injection. In addition, it has been shown for MRSA infections that high vancomycin MICs (≥1.5 mg/L) were associated with higher mortality rates and treatment failures.51 In our study, 82.9% of LRSE responsible for infections (73/88) had vancomycin MICs ≥1.5 mg/L, reducing the therapeutic options to daptomycin and ceftaroline and ceftobiprole. Consequently, dissemination of such clones might lead to prolonged hospitalization, increased cost for the hospital and in some instances increased mortality.
Finally, we demonstrated that WGS was crucial in the investigation of this outbreak and in accurately evidencing the transmission chain. Indeed, we have shown that molecular methods classically used for the typing of CoNS33–35 were not reliable (rep-PCR) or not discriminating enough. Our results strongly suggest that recognition of mobile genetic elements by rep-PCR that might occur by chance (like the SCCmec cassette here) leads to errors in the interpretation of clonality. This observation is of utmost importance since modifications in the SCCmec cassette are known to occur frequently in CoNS. Accordingly, rep-PCR results have to be considered with caution when analysing CoNS outbreaks.
Supplementary Material
Acknowledgements
We would like to thank Dr Liliana Mihaila for collecting some of the LR-CoNS isolates.
Funding
This work was supported by the Assistance Publique – Hôpitaux de Paris, a grant from the Université Paris Sud (EA 7361), the LabEx LERMIT supported by a grant from the French National Research Agency (ANR-10-LABX-33) and the LabEx IBEID.
Transparency declarations
None to declare.
Supplementary data
Tables S1 and S2 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Becker K, Heilmann C, Peters G.. Coagulase-negative staphylococci. Clin Microbiol Rev 2014; 27: 870–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mendes RE, Deshpande LM, Jones RN.. Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 2014; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs 1999; 8: 1195–202. [DOI] [PubMed] [Google Scholar]
- 4. Decousser JW, Desroches M, Bourgeois-Nicolaos N. et al. Susceptibility trends including emergence of linezolid resistance among coagulase-negative staphylococci and meticillin-resistant Staphylococcus aureus from invasive infections. Int J Antimicrob Agents 2015; 46: 622–30. [DOI] [PubMed] [Google Scholar]
- 5. Flamm RK, Mendes RE, Hogan PA. et al. Linezolid surveillance results for the United States (LEADER Surveillance Program 2014). Antimicrob Agents Chemother 2016; 60: 2273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu B, Kelesidis T, Tsiodras S. et al. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother 2013; 68: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diekema DJ, Jones RN.. Oxazolidinone antibiotics. Lancet 2001; 358: 1975–82. [DOI] [PubMed] [Google Scholar]
- 8. Kollef MH, Rello J, Cammarata SK. et al. Clinical cure and survival in Gram-positive ventilator-associated pneumonia: retrospective analysis of two double-blind studies comparing linezolid with vancomycin. Intensive Care Med 2004; 30: 388–94. [DOI] [PubMed] [Google Scholar]
- 9. Wilson DN, Schluenzen F, Harms JM. et al. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc Natl Acad Sci USA 2008; 105: 13339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Besier S, Ludwig A, Zander J. et al. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob Agents Chemother 2008; 52: 1570–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kehrenberg C, Schwarz S, Jacobsen L. et al. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol 2005; 57: 1064–73. [DOI] [PubMed] [Google Scholar]
- 12. Toh SM, Xiong L, Arias CA. et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol 2007; 64: 1506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Candela T, Marvaud JC, Nguyen TK. et al. A cfr-like gene cfr(C) conferring linezolid resistance is common in Clostridium difficile. Int J Antimicrob Agents 2017; 50: 496–500. [DOI] [PubMed] [Google Scholar]
- 14. Deshpande LM, Ashcraft DS, Kahn HP. et al. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2015; 59: 6256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Lv Y, Cai J. et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 2015; 70: 2182–90. [DOI] [PubMed] [Google Scholar]
- 16. Fan R, Li D, Wang Y. et al. Presence of the optrA gene in methicillin-resistant Staphylococcus sciuri of porcine origin. Antimicrob Agents Chemother 2016; 60: 7200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li D, Wang Y, Schwarz S. et al. Co-location of the oxazolidinone resistance genes optrA and cfr on a multiresistance plasmid from Staphylococcus sciuri. J Antimicrob Chemother 2016; 71: 1474–8. [DOI] [PubMed] [Google Scholar]
- 18. Huang J, Chen L, Wu Z. et al. Retrospective analysis of genome sequences revealed the wide dissemination of optrA in Gram-positive bacteria. J Antimicrob Chemother 2017; 72: 614–6. [DOI] [PubMed] [Google Scholar]
- 19. Ruggero KA, Schroeder LK, Schreckenberger PC. et al. Nosocomial superinfections due to linezolid-resistant Enterococcus faecalis: evidence for a gene dosage effect on linezolid MICs. Diagn Microbiol Infect Dis 2003; 47: 511–3. [DOI] [PubMed] [Google Scholar]
- 20. Rahim S, Pillai SK, Gold HS. et al. Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin Infect Dis 2003; 36: E146–8. [DOI] [PubMed] [Google Scholar]
- 21. Schulte B, Heininger A, Autenrieth IB. et al. Emergence of increasing linezolid-resistance in enterococci in a post-outbreak situation with vancomycin-resistant Enterococcus faecium. Epidemiol Infect 2008; 136: 1131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morales G, Picazo JJ, Baos E. et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis 2010; 50: 821–5. [DOI] [PubMed] [Google Scholar]
- 23. Sanchez GM, De la Torre MA, Morales G. et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010; 303: 2260–4. [DOI] [PubMed] [Google Scholar]
- 24. Bender J, Strommenger B, Steglich M. et al. Linezolid resistance in clinical isolates of Staphylococcus epidermidis from German hospitals and characterization of two cfr-carrying plasmids. J Antimicrob Chemother 2015; 70: 1630–8. [DOI] [PubMed] [Google Scholar]
- 25. Bonilla H, Huband MD, Seidel J. et al. Multicity outbreak of linezolid-resistant Staphylococcus epidermidis associated with clonal spread of a cfr-containing strain. Clin Infect Dis 2010; 51: 796–800. [DOI] [PubMed] [Google Scholar]
- 26. Karavasilis V, Zarkotou O, Panopoulou M. et al. Wide dissemination of linezolid-resistant Staphylococcus epidermidis in Greece is associated with a linezolid-dependent ST22 clone. J Antimicrob Chemother 2015; 70: 1625–9. [DOI] [PubMed] [Google Scholar]
- 27. Kelly S, Collins J, Maguire M. et al. An outbreak of colonization with linezolid-resistant Staphylococcus epidermidis in an intensive therapy unit. J Antimicrob Chemother 2008; 61: 901–7. [DOI] [PubMed] [Google Scholar]
- 28. Mihaila L, Defrance G, Levesque E. et al. A dual outbreak of bloodstream infections with linezolid-resistant Staphylococcus epidermidis and Staphylococcus pettenkoferi in a liver intensive care unit. Int J Antimicrob Agents 2012; 40: 472–4. [DOI] [PubMed] [Google Scholar]
- 29. Morroni G, Brenciani A, Vincenzi C. et al. A clone of linezolid-resistant Staphylococcus epidermidis bearing the G2576T mutation is endemic in an Italian hospital. J Hosp Infect 2016; 94: 203–6. [DOI] [PubMed] [Google Scholar]
- 30. O'Connor C, Powell J, Finnegan C. et al. Incidence, management and outcomes of the first cfr-mediated linezolid-resistant Staphylococcus epidermidis outbreak in a tertiary referral centre in the Republic of Ireland. J Hosp Infect 2015; 90: 316–21. [DOI] [PubMed] [Google Scholar]
- 31. Seral C, Saenz Y, Algarate S. et al. Nosocomial outbreak of methicillin- and linezolid-resistant Staphylococcus epidermidis associated with catheter-related infections in intensive care unit patients. Int J Med Microbiol 2011; 301: 354–8. [DOI] [PubMed] [Google Scholar]
- 32. Trevino M, Martinez-Lamas L, Romero-Jung PA. et al. Endemic linezolid-resistant Staphylococcus epidermidis in a critical care unit. Eur J Clin Microbiol Infect Dis 2009; 28: 527–33. [DOI] [PubMed] [Google Scholar]
- 33. Babouee B, Frei R, Schultheiss E. et al. Comparison of the DiversiLab repetitive element PCR system with spa typing and pulsed-field gel electrophoresis for clonal characterization of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2011; 49: 1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grisold AJ, Zarfel G, Strenger V. et al. Use of automated repetitive-sequence-based PCR for rapid laboratory confirmation of nosocomial outbreaks. J Infect 2010; 60: 44–51. [DOI] [PubMed] [Google Scholar]
- 35. Wang SH, Stevenson KB, Hines L. et al. Evaluation of repetitive element polymerase chain reaction for surveillance of methicillin-resistant Staphylococcus aureus at a large academic medical center and community hospitals. Diagn Microbiol Infect Dis 2015; 81: 13–7. [DOI] [PubMed] [Google Scholar]
- 36. Zerbino DR, Birney E.. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18: 821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deatherage DE, Barrick JE.. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol 2014; 1151: 165–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Treangen TJ, Ondov BD, Koren S. et al. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 2014; 15: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamura K, Peterson D, Peterson N. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28: 2731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Locke JB, Finn J, Hilgers M. et al. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob Agents Chemother 2010; 54: 5337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Long KS, Vester B.. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 2012; 56: 603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong A, Reddy SP, Smyth DS. et al. Polyphyletic emergence of linezolid-resistant staphylococci in the United States. Antimicrob Agents Chemother 2010; 54: 742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liakopoulos A, Spiliopoulou I, Damani A. et al. Dissemination of two international linezolid-resistant Staphylococcus epidermidis clones in Greek hospitals. J Antimicrob Chemother 2010; 65: 1070–1. [DOI] [PubMed] [Google Scholar]
- 45. Mendes RE, Deshpande LM, Castanheira M. et al. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob Agents Chemother 2008; 52: 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen H, Wu W, Ni M. et al. Linezolid-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents 2013; 42: 317–21. [DOI] [PubMed] [Google Scholar]
- 47. Brenciani A, Morroni G, Pollini S. et al. Characterization of novel conjugative multiresistance plasmids carrying cfr from linezolid-resistant Staphylococcus epidermidis clinical isolates from Italy. J Antimicrob Chemother 2016; 71: 307–13. [DOI] [PubMed] [Google Scholar]
- 48. Miragaia M, Thomas JC, Couto I. et al. Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J Bacteriol 2007; 189: 2540–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chessa D, Ganau G, Mazzarello V.. An overview of Staphylococcus epidermidis and Staphylococcus aureus with a focus on developing countries. J Infect Dev Ctries 2015; 9: 547–50. [DOI] [PubMed] [Google Scholar]
- 50. Cafini F, Nguyen le TT, Higashide M. et al. Horizontal gene transmission of the cfr gene to MRSA and Enterococcus: role of Staphylococcus epidermidis as a reservoir and alternative pathway for the spread of linezolid resistance. J Antimicrob Chemother 2016; 71: 587–92. [DOI] [PubMed] [Google Scholar]
- 51. van Hal SJ, Lodise TP, Paterson DL.. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 2012; 54: 755–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.