Abstract
Objectives: We compared the pharmacokinetics of moxifloxacin during rifampicin co-treatment or when dosed alone in African patients with drug-susceptible recurrent TB.
Methods: Patients in the intervention arm of the Improving Retreatment Success (IMPRESS) randomized controlled TB trial received 400 mg of moxifloxacin, with rifampicin, isoniazid and pyrazinamide in the treatment regimen. Moxifloxacin concentrations were measured in plasma during rifampicin-based TB treatment and again 4 weeks after treatment completion, when given alone as a single dose. Moxifloxacin concentration–time data were analysed using non-linear mixed-effects models.
Results: We included 58 patients; 42 (72.4%) were HIV co-infected and 40 (95%) of these were on efavirenz-based ART. Moxifloxacin pharmacokinetics was best described using a two-compartment disposition model with first-order lagged absorption and elimination using a semi-mechanistic model describing hepatic extraction. Oral clearance (CL/F) of moxifloxacin during rifampicin-based TB treatment was 24.3 L/h for a typical patient (fat-free mass of 47 kg), resulting in an AUC of 16.5 mg·h/L. This exposure was 7.8% lower than the AUC following the single dose of moxifloxacin given alone after TB treatment completion. In HIV-co-infected patients taking efavirenz-based ART, CL/F of moxifloxacin was increased by 42.4%, resulting in a further 30% reduction in moxifloxacin AUC.
Conclusions: Moxifloxacin clearance was high and plasma concentrations low in our patients overall. Moxifloxacin AUC was further decreased by co-administration of efavirenz-based ART and, to a lesser extent, rifampicin. The clinical relevance of the low moxifloxacin concentrations for TB treatment outcomes and the need for moxifloxacin dose adjustment in the presence of rifampicin and efavirenz co-treatment need further investigation.
Introduction
The WHO recommends moxifloxacin for the treatment of MDR TB1 and it is emerging as a key drug being investigated in shorter, novel drug regimens for the treatment of drug-susceptible and MDR TB.1–3 Moxifloxacin may be used for the treatment of drug-susceptible TB, if intolerance develops to one of the drugs used in standard first-line regimens or in patients with isoniazid monoresistance.4–6
The REMox7 and RIFAQUIN8 studies investigating moxifloxacin-containing regimens for shortening the treatment of drug-susceptible TB to 4 months failed to show non-inferiority for relapse or treatment failure after 18 months of follow up, compared with standard 6 month regimens.7,8Although there may be several reasons for these results,9,10 given that AUC/MIC is the driver of moxifloxacin efficacy, inadequate moxifloxacin concentrations in plasma and at sites of action against Mycobacterium tuberculosis, using standard 400 mg doses of moxifloxacin, have been suggested as a contributing factor.11 Furthermore, it is unclear whether a known drug interaction with rifampicin results in clinically significant decreases in moxifloxacin plasma concentrations that may have contributed to the outcomes of the REMox clinical trial as no drug concentrations were measured in the REMox study.7
Moxifloxacin is metabolized via glucuronide and sulphate conjugation by the cytosolic enzymes UDP-glucuronosyltransferase (UGT) and sulphotransferase. Moxifloxacin is a substrate of the drug transporter P-glycoprotein, involved in its absorption, distribution and elimination. Previous studies found that rifampicin co-administration decreased moxifloxacin plasma concentrations by up to 31%,12–15 due to rifampicin induction of glucuronosyltransferase, sulphotransferase and P-glycoprotein. However, rifampicin may also have the paradoxical effect of net inhibition of P-glycoprotein, which may result in higher absorption of co-administered drugs.16 There are no data in African patients with TB comparing moxifloxacin pharmacokinetics when dosed with or without rifampicin. Variable pharmacokinetics of standard first-line TB drugs have been described in African patients, in whom high levels of host genetic variability in drug-metabolizing and transporter enzymes and co-morbidities, including HIV, may result in suboptimal TB drug concentrations and treatment outcomes.17–19
In this study, we compared the pharmacokinetics of moxifloxacin when co-administered with rifampicin or dosed alone in African patients with drug-susceptible, recurrent TB, the majority of whom were HIV co-infected and on efavirenz-based ART.
Patients and methods
Study design and setting
We conducted a sequential-design, prospective pharmacokinetic sub-study within the ongoing Improving Retreatment Success (IMPRESS) open-label randomized controlled trial (NCT02114684), from October 2013 in KwaZulu-Natal, Durban, South Africa. The IMPRESS study was designed to determine whether a moxifloxacin-containing regimen, substituting moxifloxacin for ethambutol, of 24 weeks duration is superior to a standard control regimen of 24 weeks duration in improving recurrent TB treatment outcomes.
Participants
Patients in the intervention arm of the study, receiving moxifloxacin, who provided informed consent to be included in the pharmacokinetic sub-study had blood samples collected for pharmacokinetic analysis at pre-defined timepoints during the study. All participants recruited to the study were >18 years of age, had a past history of confirmed TB within the last 3 years, and had been diagnosed with sputum smear-positive, rifampicin-susceptible M.tuberculosis based on microscopy and GeneXpert technology. Both HIV-positive and -negative patients were included. Only patients with no predefined laboratory or clinical abnormalities were included.
Drug regimens
Patients randomized to the intervention arm of the study received daily 400 mg of moxifloxacin (Avelox®, Bayer Healthcare), weight-based rifampicin at 450 or 600 mg, and 225 or 300 mg of isoniazid, for patients 38–54 and ≥55 kg, respectively, during the 2 month intensive phase and 4 month continuation phase of TB treatment. During the intensive phase of treatment, pyrazinamide was used at 1500 and 2000 mg in patients between 38–54 and ≥55 kg, respectively. Patients who remained sputum smear or culture positive continued on pyrazinamide beyond 2 months of treatment, until sputum conversion. After the completion of TB treatment participants were given a single dose of moxifloxacin following a washout period of ∼4 weeks. All patients received at least 50 mg of pyridoxine with study drugs. There were no food restrictions in the pharmacokinetic study, although the time of the last meal was recorded in relation to drug dose and pharmacokinetic sample collection.
Follow-up
Patients were followed up for 24 months and clinical and safety monitoring was done every 2 months for the first 6 months, or as clinically indicated. Laboratory and safety investigations included haemoglobin as part of a complete blood count, renal and hepatic biochemistry, total protein and albumin determinations, and electrocardiogram monitoring. Sputum smear microscopy and culture were done at predefined timepoints in the study. HIV testing was done monthly in HIV-uninfected patients. HIV RNA viral load [Roche AmpliPrep-COBAS Taqman 48 Analyzer platform (Roche Molecular Diagnostics)] and CD4+ T cell count (FACSCalibur flow cytometer, Becton Dickinson Bioscience) were determined annually and viral load at 6 months. Adherence to TB treatment was measured using pill count, based on the number of tablets dispensed, physically returned, reported remaining or lost, as well as participant self-report of missed or incomplete doses in the 4 days prior to the day of study visit or pharmacokinetic sampling. HIV-co-infected patients received standard first-line ART containing efavirenz, emtricitabine and tenofovir. Treatment and prophylaxis for opportunistic infections and concomitant treatment used was recorded on case report forms. Patients requiring iron- or zinc-containing supplements or aluminium- and magnesium-containing antacids, known to affect the pharmacokinetics of moxifloxacin20,21 were counselled to take these at least 2–4 h before or after moxifloxacin dosing. Information relating to timing of dose for all drugs with known interaction potential with moxifloxacin was recorded on case report forms.
Pharmacokinetic sample collection
Plasma samples were collected prior to drug dose and at 2.5, 6 and 24 h after dose at months 1 and/or 2 during the intensive phase of TB treatment, at month 6 during the continuation phase of TB treatment and ∼4 weeks after the completion of TB treatment following a single dose of moxifloxacin. Plasma, collected in EDTA tubes, was centrifuged at 3000 rpm, placed on ice and immediately sent to the CAPRISA laboratory, to be stored in cryovials at –80°C within 1 h of collection. Moxifloxacin concentrations were quantified in clinical plasma samples using validated HPLC–MS/MS at the KwaZulu-Natal Research Institute for Tuberculosis and HIV (KRITH) pharmacology laboratory. The bioanalytical method was developed and validated according to FDA guidelines (2011).22 Sample preparation included protein precipitation with acetonitrile and subsequent dilution with water. Chromatographic separation was achieved using a Zorbax C18, 3.5 μm, 50 mm × 2.1 mm column and detection with an ABI Sciex 5500 QTrap mass spectrometer operated in positive mode. The following transitions were used; precursor ion → product ion (all in units of m/z): moxifloxacin, 402.1 → 358.2 and 402.1 → 364.1. The internal standard used was ciprofloxacin: 331.6 → 231.0 and 331.6 → 288.1. Moxifloxacin was analysed isocratically with a 22% acetonitrile/water/0.1% formic acid mobile phase. The injection volume was 2 μL and the total analytical run time was 5 min. The method was validated over the concentration range of 50–5000 ng/mL. Overall precision, based on quality control samples evaluated at low, medium and high concentrations, during the validation and analysis of samples ranged from 8.4% to 19.4% and accuracy ranged from 101.9% to 105%. Calculated carry-over at the lower limit of quantification (LLOQ) was 5.4%. The LC–MS/MS system was interfaced with a Dell® Windows® 7 computer running Analyst® software version 1.6.2, used for chromatographic data acquisition, peak integration and quantification of analytes.
Ethics
Ethics approval for the study was provided by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BFC029/13) and the Medicines Control Council of South Africa (MCC Ref:20130510).
Statistical analysis
The moxifloxacin concentration–time data were analysed using non-linear mixed-effects (NLME) modelling, implemented with the software NONMEM (version 7.3).23 Perl-speaks-NONMEM, Xpose and Pirana were used for model diagnostics and to track model development.24 Additional plots and post-modelling analysis were performed in R software25 via the RStudio interface.26
A stepwise modelling approach was employed by starting with a structural model to describe drug absorption, distribution and elimination processes and then exploring the effect of covariates such as weight, age, sex, rifampicin-based treatment, concurrent ART, adherence to TB treatment, intake of iron and/or magnesium and renal and hepatic function. In particular, for moxifloxacin, the effects of rifampicin co-administration on CL, absorption and bioavailability were investigated.
The tested structural models included one- and two-compartment disposition kinetics with first-order elimination or a semi-mechanistic model describing the effect of the liver both on systemic CL and first-pass extraction.27 For this latter approach, the moxifloxacin unbound fraction in plasma was assumed to be 50%28 and a value of 50 L/h was used for hepatic plasma flow in a typical patient.29 To characterize the absorption process, first-order lagged or transit compartment models were explored.30 Variability in pharmacokinetic parameters was included, assuming a lognormal distribution to describe changes between patients [between-subject variability (BSV)] and within the same patient but on different dosing occasions [between-occasion variability (BOV)]. An adjustment parameter was included in BOV in bioavailability to account for the variability in reported dosing time of the dose administered prior to the pharmacokinetic sampling day. Moxifloxacin concentration data below the nominal LLOQ of the assay were included in the analysis; values <10% of the LLOQ were censored and included by imputing half of the censoring threshold, as suggested by Beal.31 A combined additive and proportional error model was used to describe the residual unexplained variability, with the additive component bound to be at least 10% of the LLOQ.
Allometric scaling32 was used to account for the effect of body size on the disposition parameters (the exponent was fixed to 0.75 for clearance and 1 for volume parameters), including hepatic plasma flow in the semi-mechanistic model. Fat-free mass (FFM), and fat mass were calculated based on weight, height and sex as suggested by Janmahasatian et al.,33 and were explored as descriptors of body size along with total body weight, as previously recommended.32 Covariate effects were evaluated and included if they significantly improved the ability of the model to describe the data. Model improvements were evaluated by inspecting diagnostic plots, including visual predictive checks,34 and decreases in the objective function value (ΔOFV), which is assumed to have a χ2 distribution. Drops of more than 3.84 points for the addition of one parameter were considered significant at P<0.05. Finally, a non-parametric bootstrap with replacement (n = 300) was applied to assess the robustness of the parameter estimates and obtain the 90% CIs.
Results
Baseline characteristics
Moxifloxacin concentration–time data were available from 58 patients, 209 pharmacokinetic profiles and a total of 822 sampling timepoints. Median weight, FFM and age were 56.9 kg (IQR 51.1–65.2), 46.8 kg (IQR 42.5–50.3) and 37 years (IQR 31–42), respectively. Forty-one (70.7%) patients were male and 42 (72.4%) were HIV co-infected, with 40 (95%) on efavirenz-based ART (Table 1). Of the 209 pharmacokinetic profiles and 822 timepoints available, 204 pharmacokinetic profiles and 739 timepoints were included in the analysis for 58 patients. Reasons for exclusion of pharmacokinetic profiles and time-point data are available in Supplementary data (available at JAC Online).
Table 1.
Baseline characteristics of patients in the IMPRESS intervention arm pharmacokinetic study
| Variable | Result (N = 58) |
|---|---|
| Age (years), median (IQR) | 37 (31–42) |
| Male, n (%) | 41 (70.7) |
| Race (black African/Caucasian/coloureda), n (%) | 56 (96.6)/1 (1.7)/1 (1.7) |
| Weight (kg), median (IQR) | 56.9 (51.1–65.2) |
| Fat-free mass (kg) | 46.8 (42.5–50.3) |
| BMI (kg/m2), median (IQR) | 19.6 (18.0–23.3) |
| Alkaline phosphatase (IU/L), median (IQR) | 74.0 (58.0–97.0) |
| Total protein (g/L), median (IQR) | 77.0 (73.0–83.0) |
| Potassium (mmol/L), median (IQR) | 4.5 (4.2–4.9) |
| Bilirubin total (mmol/L), median (IQR) | 6.0 (5.0–9.0) |
| ALT (IU/L), median (IQR) | 18.0 (16.0–30.0) |
| AST (IU/L), median (IQR) | 27.0 (23.0–37.0) |
| Haemoglobin (g/dL), median (IQR) | 11.8 (10.4–12.7) |
| Platelets (109/L), median (IQR) | 407.0 (337.0–477.0) |
| Creatinine clearance (mL/min), median (IQR) | 121.0 (97.0–136.0) |
| HIV status (positive/negative), n (%) | 42 (72.4)/16 (27.6) |
| ART, n (%)b | |
| Efavirenz + emtricitabine + tenofovir | 40 (95.2) |
| Lopinavir/ritonavir + lamivudine + tenofovir | 2 (4.8) |
| CD4+ count (cells/mm3), median (IQR)b,c | 277.0 (139.0–384.0) |
| Viral load (log10 copies/mL)b,d | 3.3 (1.3–4.2) |
Mixed race.
Only for HIV-positive patients.
Four missing data.
Five missing data.
Moxifloxacin pharmacokinetics
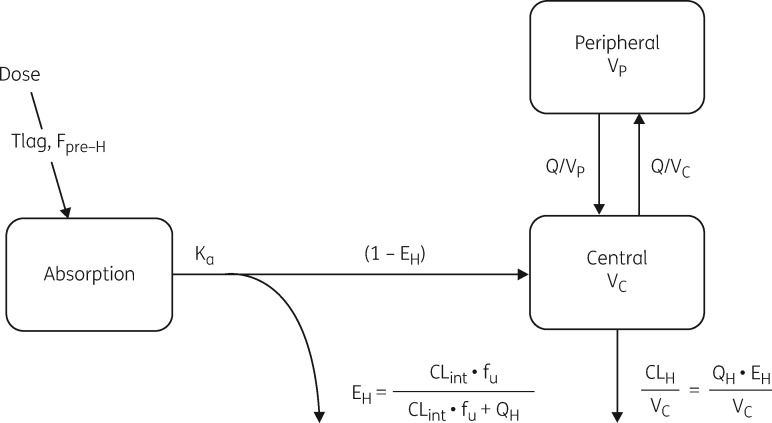
Moxifloxacin pharmacokinetics were best described using a two-compartment disposition model (when compared with one-compartment ΔOFV 45, two additional parameters, P<0.001), with first-order absorption and an absorption lag time, and elimination using the semi-mechanistic liver model describing first-pass extraction (ΔOFV 24 compared with simple first-order elimination from the central compartment, no additional parameters estimated). Since very little information was available in the absorption phase, a prior was added to improve parameter estimation and stabilize the model. Lognormal priors with 30% uncertainty were used, with a typical value of 0.75 h for the absorption lag time and 1.5 1/h for the absorption rate constant, as previously reported by Zvada et al.35 in a similar population. A schematic diagram of the final model is depicted in Figure 1 and a detailed description of the semi-mechanistic liver model is provided in the Supplementary data. The model parameter estimates are shown in Table 2; these include parameters of the hepatic model, i.e. intrinsic CL (which determines hepatic extraction) and pre-hepatic bioavailability (fraction absorbed and reaching the liver). For ease of interpretation, these values have been converted to oral clearance (CL/F) using the formulas in the Supplementary data (Appendix 2) and shown in Table 3.
Figure 1.
Schematic diagram of the semi-mechanistic model describing the pharmacokinetics of moxifloxacin in patients with drug-susceptible recurrent TB. Tlag, absorption lag time; Fpre-H, pre-hepatic bioavailability; Ka, absorption rate constant; EH, hepatic extraction; CLint, (hepatic) clearance intrinsic; fu, free (unbound) fraction of drug in plasma; QH, hepatic plasma flow; CLH, hepatic clearance; VC, volume of central compartment; VP, volume of peripheral compartment; Q, inter-compartmental clearance; QH, hepatic plasma flow.
Table 2.
Population parameter estimates of moxifloxacin pharmacokinetics
| Parameter description | Typical value (95% CI)a | Random variability (95% CI)a |
|---|---|---|
| Intrinsic CL during rifampicin-based TB treatment (L/h)b,c | 48.5 (44.1, 54.1) | BSV 14% (9.2, 19.4) |
| BOV 12.3% (0.1, 18.5) | ||
| Volume of central compartment (L)c | 126 (109.6, 134.5) | BSV 7% (0.1, 13.2) |
| Inter-compartmental CL (L/h)c | 2.04 (1.58, 4.71) | – |
| Volume of peripheral compartment (L)c | 30.5 (22.2, 54.4) | – |
| Pre-hepatic bioavailabilityd | 1 fixed | BOV 36.1% (26.9, 41.7) |
| Absorption lag time (h), priore | 0.55 (0.45, 0.74) | – |
| Absorption rate (Ka, 1/h), priore | 2.95 (1.21, 3.42) | BOV 104.5% (1.0, 121.0) |
| Hepatic plasma flow (L/h) | 50 fixed | |
| Moxifloxacin fraction unbound (%) | 50% fixed | |
| Change in intrinsic clearance while on single dose of moxifloxacin (%) | −29% (−37, −22) | |
| Change in pre-hepatic bioavailability while on single dose of moxifloxacin (%) | −23% (−33, −13) | |
| Change in intrinsic clearance while on efavirenz-based ART (%) | +42.4% (33, 58) | |
| Scaling factor for variability in bioavailability while on single dose of moxifloxacin (-fold)f | 0.62 (0.39, 0.89) | |
| Scaling factor for variability in bioavailability for unobserved doses (-fold)f | 2.5 (1.75,3.92) | |
| Proportional error (%) | 17.5 (12.3, 21.7) | |
| Additive error (mg/L) | 0.011 (0.005, 0.017) |
Obtained with a non-parametric bootstrap (n = 300).
Intrinsic CL of moxifloxacin when given at steady-state within rifampicin-based TB treatment and no efavirenz.
All CL and volume parameters have been allometrically scaled with FFM, and the typical values reported here refer to the typical patient, with FFM of 47 kg.
Pre-hepatic bioavailability is the fraction of the drug that is absorbed, crosses the gut wall unchanged, thus entering the portal vein and reaching the liver.
These parameters were estimated using a prior, as detailed in text.
These scaling factors modulate the size of the between-occasion variability in pre-hepatic bioavailability for the sections of data indicated (single dose and unobserved doses).
Table 3.
Typical values of oral clearance and exposure of moxifloxacin when given alone or with rifampicin-based TB treatment, with and without efavirenz-based ART
| Moxifloxacin scenario | On RIF-based TB treatment? | With EFV- based ART? | Intrinsic CL, L/h | Hepatic extraction (EH), % | Pre-hepatic bioavailability (Fpre-H), % | Oral CL (CL/F) (L/h) | Change in CL/F (%) | AUC (mg·h/L)a |
|---|---|---|---|---|---|---|---|---|
| Steady-state | yes | no | 48.5 | 33 | 100 (reference) | 24.3 | reference | 16.5 |
| Steady-state | yes | yes | 69.1 | 41 | 100 | 34.5 | +42.4 | 11.6 |
| Single doseb | no | no | 34.4 | 26 | 77 | 22.4 | −7.8 | 17.9 |
| Single doseb | no | yes | 49.0 | 33 | 77 | 31.8 | +31.3 | 12.6 |
RIF, rifampicin; EFV, efavirenz.
The typical values reported here refer to an individual with FFM of 47 kg (the median in our cohort).
AUC for a dose of 400 mg.
After completion of TB treatment.
The oral clearance of steady-state moxifloxacin when given as part of TB treatment with rifampicin, isoniazid and pyrazinamide was an estimated 24.3 L/h for a typical patient in the cohort (FFM of 47 kg). When comparing the pharmacokinetic profiles observed during TB treatment with those obtained after a single dose of moxifloxacin after completion of TB treatment, oral clearance was found to decrease by 7.8%, resulting in higher exposure. Interestingly, the model identified a dual effect: a 29% decrease in intrinsic CL (ΔOFV 29, one additional parameter, P<0.001) and a concomitant 23% decrease in pre-hepatic bioavailability (ΔOFV 23, one additional parameter, P<0.001). Additionally, a 42.4% increase in oral clearance was observed both during and after TB treatment in HIV-co-infected patients treated with efavirenz-based ART (ΔOFV 46, one additional parameter, P<0.001). The model described the effect of efavirenz as an increase in intrinsic clearance, i.e. hepatic enzymatic activity.
The effect of body size on clearance and volume parameters was captured with allometric scaling and best described using FFM as a body size descriptor (ΔOFV 14, as opposed to total body weight). After including the effect of body size, efavirenz and rifampicin-based TB treatment, there was small random BSV (14%) and BOV (12%) in moxifloxacin clearance. However, 36% BOV was found for pre-hepatic bioavailability (i.e. before the effect of hepatic extraction).This variability was smaller for the exposures observed in the single-dose visit, after the end of TB treatment. This was described in the model with a scaling factor estimated to be 0.62 for the BOV in pre-hepatic bioavailability (ΔOFV 9, one additional parameter, P = 0.003).
On the other hand, the pre-dose concentrations on the day of the pharmacokinetic visit were characterized by a larger variability than the profile obtained after the observed dose in the clinic. This was included in the model by including a scaling factor estimated to be 2.5 for the BOV in pre-hepatic bioavailability for all data obtained after an unobserved dose, taken by a patient prior to the observed dose in the clinic on the day of pharmacokinetic sampling (ΔOFV 48, one additional parameter, P<0.001). This larger variability was tested and included in the model to reflect the larger uncertainty affecting self-reported dosing information.
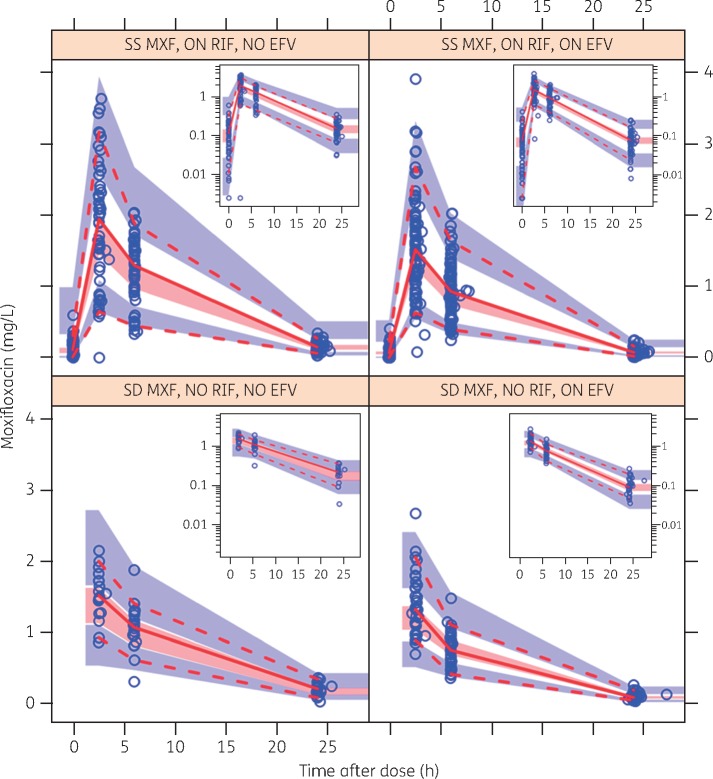
The visual predictive check in Figure 2 provides an overview of the concentrations observed in the study and shows that the model described the data adequately.
Figure 2.
Visual predictive check (VPC) stratified by co-administration with rifampicin and/or efavirenz. The dashed and solid lines are the 5th percentile, median and 95th percentile of the observed concentrations, while the shaded regions represent the corresponding 95% CIs for the same percentiles. The sub-plot in each stratum shows the same VPC with a logarithmic transformation applied to the y-axis. SS, steady-state; SD, single dose; MXF, moxifloxacin; RIF, rifampicin; EFV, efavirenz. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
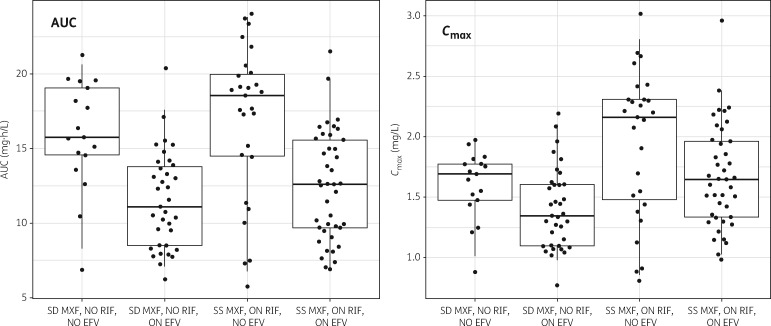
A summary of values of CL and model-predicted AUC for a typical patient in the different dosing scenarios (steady-state within TB treatment, or single dose alone, with or without concomitant efavirenz) is provided in Table 3. A visual depiction of the individual AUC and Cmax observed in our cohort is presented in Figure 3.
Figure 3.
Model-based moxifloxacin exposure stratified by co-administration of rifampicin and/or efavirenz. The left panel shows AUC0–∞ (for steady-state dosing) or AUC0–24 (for single dose), while the right panel shows Cmax. The dots are the model-predicted individual exposures observed in the study cohort and based on empirical Bayes estimates; geometric means were used to summarize multiple values from the same subject. The box summarizes the median and IQR, while the whiskers are the 2.5%–97.5% range. SS, steady-state; SD, single dose; MXF, moxifloxacin; RIF, rifampicin; EFV, efavirenz.
Discussion
In our cohort of drug-susceptible TB patients, we found low moxifloxacin concentrations (AUC) during concomitant rifampicin-based TB treatment. Higher concentrations (∼8%), but still low overall, were observed 1 month after discontinuation of rifampicin, when a single dose of moxifloxacin was administered for comparison. Notably, the increased exposure observed when a single dose of moxifloxacin was given alone after the end of TB treatment was found to be the result of two opposing effects: 30% decreased intrinsic clearance and decreased bioavailability. Moreover, in HIV-co-infected patients on efavirenz-based ART, clearance was increased by 42% when compared with HIV-uninfected patients or those on lopinavir/ritonavir-based ART. The effect of efavirenz, which lowered the AUC by 30%, was present both when moxifloxacin was given alone and when it was given within rifampicin-based TB treatment.
Our findings are in keeping with previous studies12–15,36 investigating the effect of rifampicin co-administration on moxifloxacin drug concentrations in healthy individuals and TB patients, showing lower concentrations of moxifloxacin due to rifampicin co-administration. However, these studies reported variable effects of rifampicin co-treatment on moxifloxacin exposure. Bioavailability studies,12–14 using cross-over or sequential study designs to limit inter-patient variability, and intensive pharmacokinetic sampling demonstrated much higher differences in steady-state moxifloxacin AUC (27%–31%) without concomitant rifampicin compared with studies in real-world settings,15,36 which may be limited by the heterogeneity between the groups compared and the small sample sizes reported. In our study, the model estimated an ∼30% decrease in intrinsic CL when moxifloxacin was given alone as opposed to during rifampicin-based TB treatment. On the other hand, the model also estimated a decrease in bioavailability for single-dose moxifloxacin pharmacokinetics when given alone after TB treatment completion. These two phenomena had opposite effects on moxifloxacin concentrations and the reasons are not entirely clear. Several explanations are plausible. Firstly, moxifloxacin AUC at steady-state has been shown to be moderately higher (∼30% for 400 mg once daily) than after the first or a single dose of moxifloxacin37 as used in our study, suggesting that the difference shown in pre-hepatic bioavailability may be a consequence of single-dose versus steady-state dosing. Secondly, it is possible that rifampicin, given concomitantly, increases the absorption of moxifloxacin; this may be due to net inhibition of P-glycoprotein by rifampicin during the absorption phase, as has been previously reported with digoxin.16 This suggests that the true effect of rifampicin co-treatment may be larger and closer to the 30% lower AUC demonstrated by previous studies,12–14 compared with moxifloxacin alone.
The higher clearance and lower moxifloxacin concentrations in HIV-co-infected patients on efavirenz-based ART has not been previously described. Efavirenz induces the activity of UGT,38,39 involved in moxifloxacin metabolism. There is evidence of efavirenz decreasing concentrations of other antiretroviral drugs metabolized by UGT, such as dolutegravir, by up to 57%.39 There have been conflicting reports of the effect of HIV co-infection on TB drugs, with some studies reporting decreased drug concentrations40,41 while others found non-significant or no changes.42 It is unclear whether the effect on clearance and AUC seen in those HIV-co-infected patients who are on efavirenz are due to the induction of UGT, HIV co-infection or a combination of these. There were two HIV-co-infected patients who were on lopinavir/ritonavir-based ART, and no decrease in moxifloxacin clearance could be observed in any of the seven pharmacokinetic profiles contributed by these patients, but the small numbers limit our ability to draw reliable conclusions about this observation. These findings are nevertheless concerning, given that this interaction may also impact moxifloxacin exposure in HIV-co-infected patients on efavirenz-based ART taking moxifloxacin-containing MDR TB treatment or in studies using moxifloxacin in novel drug regimens,3 and need confirmation in other studies.
The plasma concentrations of moxifloxacin achieved in our patients are low regardless of efavirenz or rifampicin co-treatment, when compared with previous reports.36,43–47 Moxifloxacin exhibits extensive inter-individual variability in pharmacokinetic parameters in healthy volunteers and patients with TB, with wide ranges in AUC, Cmax and CL/F values. AUC values between 8.5 and 140 mg·h/L and CL/F between 10 and 30.6 L/h have been reported in previous studies using 400 mg moxifloxacin doses.43,44,48,49 African populations have shown high levels of host genetic diversity and increased variation in drug metabolizing and transport enzymes, shown to result in lower drug concentrations and variation in drug response to other first-line TB drugs.17,18,50
We acknowledge several limitations. Firstly, we used relatively sparse pharmacokinetic sampling methods at each pharmacokinetic visit, a choice that may limit the precision of the individual estimates of exposure. However, we employed NLME modelling for the interpretation of the data; this analysis technique is designed to handle sparse data well, since it pools information across the entire population and is able to robustly identify population parameters, including typical values, variability and covariate effects.51 Furthermore, pharmacokinetic sampling around 2 and 6 h after dose has been shown to provide reasonably accurate estimates of moxifloxacin AUC in previous studies.52 A second limitation may be due to the fact that moxifloxacin pharmacokinetic parameters (CL/F and AUC) during TB treatment compared with dosing after completion of TB treatment may differ as a result of changes in patient physiology, increased weight and improved disease status. In our model, we have included the effect of body size to account for the effects of rifampicin and efavirenz on moxifloxacin pharmacokinetics. However, several other potential confounding factors, including genetic variability, may limit our model’s ability to robustly quantify the contribution of each separate factor. Thirdly, the study was not designed to determine the impact of the efavirenz interaction on moxifloxacin, hence efavirenz drug concentrations, known to have high variability in exposure,53 were not determined. Fourthly, our study used a single dose of moxifloxacin given after completion of TB treatment and compared with moxifloxacin at steady-state given concomitantly with rifampicin. It is possible that changes in the pharmacokinetics of moxifloxacin between single-dose and steady-state treatment may be responsible for the observed increase in pre-hepatic bioavailability. In this case, the actual effect of rifampicin co-administration would only be the ∼30% increase in intrinsic CL, resulting in decreases in moxifloxacin AUC similar to those reported in previous studies.12–14 Finally, pharmacokinetic sampling was not ideal, as hospitalization of ambulatory patients overnight, to minimize dosing errors and standardize sampling conditions, was not feasible within our study.
In conclusion, we found high CL and resulting low moxifloxacin concentrations (AUC) in South African patients with drug-susceptible, recurrent TB, further decreased by co-treatment with rifampicin and efavirenz-based ART. The clinical relevance of the low moxifloxacin concentrations is unclear, but the detected interactions, especially the efavirenz effect on moxifloxacin, warrants further investigation in studies to assess the need for dose adjustments and impact on TB treatment outcomes.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the study patients for their participation in the IMPRESS pharmacokinetic study; study nurses, counsellors and clinicians, the study co-ordinator Ms Dhineshree Govender and the IMPRESS study team for their work in the pharmacokinetic study. Special acknowledgement to Mrs Magashree Govender and the pharmacy team for support in the study. We thank Mrs Natasha Samsunder and laboratory staff of the CAPRISA, the pharmacology laboratory of the KwaZulu-Natal Research Institute for Tuberculosis and HIV (KRITH), and Global Clinical & Viral Laboratories, for laboratory support. Mr Day Munatsi and Mrs Precious Radebe are acknowledged for data management in the study and Dr Cheryl Baxter for technical editing of the final version of the manuscript. The Division of Clinical Pharmacology at the University of Cape Town acknowledges Novartis Pharma for their support of the development of pharmacometric skills in Africa.
Funding
This work was supported by grant number: 5R24TW008863 from the Office of Global AIDS Coordinator and the U. S. Department of Health and Human Services, National Institutes of Health (NIH OAR and NIH OWAR). Research reported in this publication was supported by the European & Developing Countries Clinical Trials Partnership (EDCTP) (TA.2011.40200.044), the South African Medical Research Council CAPRISA HIV-TB Pathogenesis and Treatment Research Unit the South African Medical Research Council under a Self-Initiated Research Grant. Study drug was donated by Bayer Pharmaceuticals. A. N. was the recipient of the University of KwaZulu-Natal College of Health Sciences Scholarship. H. M. is supported in part by the National Research Foundation of South Africa (Grant Number 90729). K. N. and N. P. were supported by the Columbia University-South Africa Fogarty AIDS260 International Training and Research Program (AITRP, grant # D43TW000231).The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the US government, EDCTP, NRF, Fogarty International Center, National Institutes of Health or the Medical Research Council.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to data, commented on drafts, and approved the final report. The corresponding author had final responsibility for the decision to submit for publication.
Transparency declarations
N. P. is the principal study investigator, on the Improving Retreatment Success Trial (IMPRESS) [NCT02114684]. Bayer Pharmaceuticals donated the study drug (moxifloxacin) used during the trial. S. E. is a member of the Global Respiratory Infection Partnership, sponsored by Reckitt and Benckiser. All authors have no other potential conflicts of interest to declare.
Author contributions
Study concept and design: A. N., N. P., K. N., H. M., P. D. Drafting of the manuscript: A. N., P. D., M. C. Statistical analysis: NLME modelling and data analysis: P. D., M. C., E. K.-P. General statistical support: N. Y.-Z. Acquisition, analysis or interpretation of data: all authors. Critical revision of the manuscript for important intellectual content: all authors.
Disclaimer
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the US government, EDCTP, NRF, Fogarty International Center, National Institutes of Health or the Medical Research Council.
Supplementary data
Descriptions of the reasons for exclusion of data and the moxifloxacin pharmacokinetic model can be found in the Supplementary data at JAC Online (https://academic.oup.com/jac/advance-access).
References
- 1. WHO. Global Tuberculosis Report2016. http://www.who.int/tb/publications/global_report/en/.
- 2. Moodley R, Godec TR.. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev 2016; 25: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dawson R, Diacon AH, Everitt D. et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015; 385: 1738–47. [DOI] [PubMed] [Google Scholar]
- 4. Potter J, Agrawal R, Barraclough C. et al. Moxifloxacin: an alternative to ethambutol for the treatment of presumed ocular tuberculosis. Ocul Immunol Inflamm 2015; 24: 1–7. [DOI] [PubMed] [Google Scholar]
- 5. WHO . Global Tuberculosis Report 2015. http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
- 6. Lee H, Jeong BH, Park HY. et al. Treatment outcomes with fluoroquinolone-containing regimens for isoniazid-resistant pulmonary tuberculosis. Antimicrob Agents Chemother 2016; 60: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gillespie SH, Crook AM, McHugh TD. et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014; 371: 1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jindani A, Harrison TS, Nunn AJ. et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014; 371: 1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nimmo C, Lipman M, Phillips PP. et al. Shortening treatment of tuberculosis: lessons from fluoroquinolone trials. Lancet Infect Dis 2015; 15: 141–3. [DOI] [PubMed] [Google Scholar]
- 10. Horsburgh CR Jr, Barry CE III, Lange C.. Treatment of tuberculosis. N Engl J Med 2015; 373: 2149–60. [DOI] [PubMed] [Google Scholar]
- 11. Alffenaar JW, Gumbo T, Aarnoutse R.. Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2015; 372: 576.. [DOI] [PubMed] [Google Scholar]
- 12. Nijland HM, Ruslami R, Suroto AJ. et al. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin Infect Dis 2007; 45: 1001–7. [DOI] [PubMed] [Google Scholar]
- 13. Weiner M, Burman W, Luo CC. et al. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob Agents Chemother 2007; 51: 2861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramachandran G, Hemanth Kumar AK, Srinivasan R. et al. Effect of rifampicin & isoniazid on the steady state pharmacokinetics of moxifloxacin. Indian J Med Res 2012; 136: 979–84. [PMC free article] [PubMed] [Google Scholar]
- 15. Manika K, Chatzika K, Papaioannou M. et al. Rifampicin-moxifloxacin interaction in tuberculosis treatment: a real-life study. Int J Tuberc Lung Dis 2015; 19: 1383–7. [DOI] [PubMed] [Google Scholar]
- 16. Reitman ML, Chu X, Cai X. et al. Rifampin's acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 2011; 89: 234–42. [DOI] [PubMed] [Google Scholar]
- 17. Chigutsa E, Visser ME, Swart EC. et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother 2011; 55: 4122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gengiah TN, Botha JH, Soowamber D. et al. Low rifampicin concentrations in tuberculosis patients with HIV infection. J Infect Dev Ctries 2014; 8: 987–93. [DOI] [PubMed] [Google Scholar]
- 19. Mboowa G. Genetics of sub-Saharan African human population: implications for HIV/AIDS, tuberculosis, and malaria. Int J Evol Biol 2014; 2014: 108291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stass H, Bottcher MF, Ochmann K.. Evaluation of the influence of antacids and H2 antagonists on the absorption of moxifloxacin after oral administration of a 400mg dose to healthy volunteers. Clin Pharmacokinet 2001; 40: 39–48. [DOI] [PubMed] [Google Scholar]
- 21. Stass H, Kubitza D.. Effects of iron supplements on the oral bioavailability of moxifloxacin, a novel 8-methoxyfluoroquinolone, in humans. Clin Pharmacokinet 2001; 40: 57–62. [DOI] [PubMed] [Google Scholar]
- 22. FDA. Guidance for Industry Bioanalytical Method Validation. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf.
- 23. Beal S, Sheiner L, Boeckmann A. et al. NONMEM Users Guides (1989-2009). Ellicott City, MD, USA: ICON Development Solutions, 2009. [Google Scholar]
- 24. Keizer RJ, Karlsson MO, Hooker A.. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna; 2014. http://www.R-project.org/. [Google Scholar]
- 26. RStudio Team. RStudio: Integrated Development Environment for R. RStudio, Inc, Boston, MA; 2015. http://www.rstudio.com/. [Google Scholar]
- 27. Gordi T, Xie R, Huong NV. et al. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br J Clin Pharmacol 2005; 59: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacGowan AP. Moxifloxacin (Bay 12-8039): a new methoxy quinolone antibacterial. Expert Opin Investig Drugs 1999; 8: 181–99. [DOI] [PubMed] [Google Scholar]
- 29. Cato A, Sutton L In: Cato III A, ed. Clinical Drug Trials and Tribulations, Revised and Expanded New York: Marcel Dekker Inc., 2002.
- 30. Savic RM, Jonker DM, Kerbusch T. et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26. [DOI] [PubMed] [Google Scholar]
- 31. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 32. Anderson BJ, Holford NH.. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32. [DOI] [PubMed] [Google Scholar]
- 33. Janmahasatian S, Duffull SB, Ash S. et al. Quantification of lean bodyweight. Clin Pharmacokinet 2005; 44: 1051–65. [DOI] [PubMed] [Google Scholar]
- 34. Holford N. The visual predictive check superiority to standard diagnostic (Rorschach) plots. In: PAGE. Abstracts of the Annual Meeting of the Population Approach Group in Europe, Pamplona, Spain, 2005. Abstract 738.
- 35. Zvada SP, Denti P, Sirgel FA. et al. Moxifloxacin population pharmacokinetics and model-based comparison of efficacy between moxifloxacin and ofloxacin in African patients. Antimicrob Agents Chemother 2014; 58: 503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pranger AD, van Altena R, Aarnoutse RE. et al. Evaluation of moxifloxacin for the treatment of tuberculosis: 3 years of experience. Eur Respir J 2011; 38: 888–94. [DOI] [PubMed] [Google Scholar]
- 37. Stass H, Kubitza D, Schuhly U.. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxyfluoroquinolone, after repeated oral administration. Clin Pharmacokinet 2001; 40: 1–9. [DOI] [PubMed] [Google Scholar]
- 38. Ji HY, Lee H, Lim SR. et al. Effect of efavirenz on UDP-glucuronosyltransferase 1A1, 1A4, 1A6, and 1A9 activities in human liver microsomes. Molecules 2012; 17: 851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song I, Borland J, Chen S. et al. Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir. Eur J Clin Pharmacol 2014; 70: 1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahmed R, Cooper R, Foisy M. et al. Factors associated with reduced antituberculous serum drug concentrations in patients with HIV-TB coinfection. J Int Assoc Physicians AIDS Care (Chic) 2012; 11: 273–6. [DOI] [PubMed] [Google Scholar]
- 41. Chideya S, Winston CA, Peloquin CA. et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 2009; 48: 1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Denti P, Jeremiah K, Chigutsa E. et al. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 2015; 10: e0141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peloquin CA, Hadad DJ, Molino LP. et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 2008; 52: 852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alffenaar JW, van Altena R, Bokkerink HJ. et al. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin Infect Dis 2009; 49: 1080–2. [DOI] [PubMed] [Google Scholar]
- 45. Zvada SP, Denti P, Geldenhuys H. et al. Moxifloxacin population pharmacokinetics in patients with pulmonary tuberculosis and the effect of intermittent high-dose rifapentine. Antimicrob Agents Chemother 2012; 56: 4471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruslami R, Ganiem AR, Dian S. et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 2013; 13: 27–35. [DOI] [PubMed] [Google Scholar]
- 47. Conde MB, Mello FC, Duarte RS. et al. A phase 2 randomized trial of a rifapentine plus moxifloxacin-based regimen for treatment of pulmonary tuberculosis. PLoS One 2016; 11: e0154778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manika K, Chatzika K, Zarogoulidis K. et al. Moxifloxacin in multidrug-resistant tuberculosis: is there any indication for therapeutic drug monitoring? Eur Respir J 2012; 40: 1051–3. [DOI] [PubMed] [Google Scholar]
- 49. Pranger AD, Kosterink JG, van Altena R. et al. Limited-sampling strategies for therapeutic drug monitoring of moxifloxacin in patients with tuberculosis. Ther Drug Monit 2011; 33: 350–4. [DOI] [PubMed] [Google Scholar]
- 50. McIlleron H, Willemse M, Werely CJ. et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis 2009; 48: 1547–53. [DOI] [PubMed] [Google Scholar]
- 51. Aarons L. Population pharmacokinetics: theory and practice. Br J Clin Pharmacol 1991; 32: 669–70. [PMC free article] [PubMed] [Google Scholar]
- 52. Magis-Escurra C, Later-Nijland HM, Alffenaar JW. et al. Population pharmacokinetics and limited sampling strategy for first-line tuberculosis drugs and moxifloxacin. Int J Antimicrob Agents 2014; 44: 229–34. [DOI] [PubMed] [Google Scholar]
- 53. Holzinger ER, Grady B, Ritchie MD. et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS Clinical Trials Group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22: 858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.