Abstract
Objectives
To investigate the performance of the two backbone drugs in the standard combination therapy regimen in the hollow-fibre system (HFS) model of pulmonary Mycobacterium avium complex (MAC) infection.
Methods
Six HFS were inoculated with human-derived monocytes infected with MAC, and treated with 15 mg/kg of ethambutol and 500 mg of azithromycin daily for 28 days to recapitulate the concentration–time profiles seen in the lungs of humans treated with these drugs and doses. The concentration–time profiles achieved were validated by sampling the central compartment at seven timepoints over 24 h. The total MAC burden, as well as the subpopulation resistant to 3 × MIC of each drug, was identified based on sampling the peripheral compartment of each system on days 0, 3, 7, 14, 21 and 28 of therapy. The experiment was performed twice.
Results
In non-treated control HFS, MAC grew from 5.0 to 8.53 log10 cfu/mL in 28 days. The dual therapy killed a maximum of 1.52 ± 0.43 log10 cfu/mL during the first 7 days, after which it failed. By day 28 there was no difference in MAC burden between the combination-therapy-treated and non-treated systems. Failure arose in parallel with the emergence of acquired ethambutol resistance. By day 28, 100% of the bacterial population was ethambutol resistant in the combination-therapy-treated HFS replicates.
Conclusions
The backbone combination of macrolide and ethambutol has poor MAC kill rates and is ineffective. Microbial kill is rapidly abrogated by acquired drug resistance. This backbone should be replaced.
Introduction
Mycobacterium avium complex (MAC) is the most common cause of lung disease caused by non-tuberculous mycobacteria in many countries.1–4 The currently recommended therapy for pulmonary MAC is a macrolide backbone (clarithromycin or azithromycin) and ethambutol, to which a rifamycin (rifampicin or rifabutin) may be added.5 It is known that the primary drug in the regimen is the macrolide, with ethambutol considered a vital companion drug. According to prescribing guidelines, patients should be treated until culture negative on therapy for 1 year.5 This factor alone indicates that the kill rates of the current regimen are expected by clinicians to be extremely poor, and thus the time to total sterilization of lesions takes years. The role of aminoglycosides as companion drugs is somewhat unclear and controversial.6 The dosing schedule of daily versus intermittent therapy has also been debated.7,8 In the accompanying meta-analysis of prospective clinical studies,9 we found that the standard regimen is associated with a success rate of only 55% after 6 months, and 22% after 1 year of therapy; this is worse than the outcomes for multidrug-resistant (MDR) tuberculosis and in the same range as for the streptomycin plus pyrazinamide dual therapy in the retreatment of tuberculosis in the East Africa study conducted 50 years ago.10 We have shown that as single therapy, azithromycin or ethambutol each have a transient effect. However, the drugs may work better in combination due to synergy: after all, therapy is given as combination therapy. Here, we were interested to examine the efficacy of this combination regimen in the hollow-fibre system of MAC (HFS-MAC). Our objective was to evaluate the kill rates of the combination azithromycin/ethambutol, and identify any role acquired drug resistance (ADR) may play.
The pharmacokinetic/pharmacodynamic (PK/PD) index associated with optimal microbial kill by azithromycin is the 0–24 h area under the concentration–time curve (AUC0–24) to MIC ratio.11 We have identified the optimal free-drug AUC0–24/MIC for MAC kill to be a ratio of 3.43. The maximal kill by azithromycin monotherapy was 0.6 log10 cfu/mL below day 0 bacterial burden; however, microbial kill lasted only 7 days and the systems exposed to concentrations achieved by the recommended standard doses developed ADR, which was the cause of therapy failure. The azithromycin AUC0–24/MIC associated with suppression of ADR was a ratio of 7.51.12 On the other hand, the PK/PD index associated with optimal kill by ethambutol was the peak/MIC ratio.13 The optimal peak/MIC ratio for the ethambutol effect was 13. However, ethambutol did not actually kill MAC to below the day 0 burden in the HFS-MAC, but merely constrained its growth, and was thus bacteriostatic. Here, we were interested if the dual-therapy regimen achieved microbial kill as high as the sum of the two, and if they protected each other from ADR.11,14
Materials and methods
Bacteria, media and other supplies
Prior to each experiment the stock culture of MAC (ATCC 700898) was thawed and grown to log phase at 37 °C in Middlebrook 7H9 broth supplemented with 10% OADC under shaking conditions. Human-derived THP-1 monocytes (ATCC TIB-202) were purchased from the ATCC (Manassas, VA, USA). Hollow-fibre cartridges were purchased from FiberCell (Frederick, MD, USA). RPMI 1640, heat-inactivated FBS and ethambutol were purchased from Sigma (St Louis, MO, USA). Azithromycin was purchased from the University of Texas Southwestern Medical Center campus pharmacy. Azithromycin and ethambutol were diluted using distilled water and filter sterilized.
Efficacy of the ethambutol/azithromycin combination regimen in the HFS-MAC
The MAC isolate we used in all our experiments has an ethambutol MIC of 8 mg/L, and an azithromycin MIC of 32 mg/L, based on our prior studies.11,12 MAC was grown to logarithmic growth phase (log-phase growth), as described previously.11,12,14,15 THP-1 cells were propagated in RPMI 1640 and 10% FBS (RPMI/FBS), as described in the past.14,15 The log-phase growth culture of MAC was adjusted to a bacterial density of 1.5 × 106 cfu/mL, and then used to infect 1.5 × 106 THP-1 cells/mL by co-incubating overnight at 37 °C under 5% CO2, for a multiplicity of infection (MOI) of 1:1. The infected monocytes were washed twice with warm RPMI 1640 by centrifugation at 100 g for 5 min to remove the extracellular bacteria. The THP-1 cells were examined in a haemocytometer for cell counts and viability after staining with trypan blue. Next, 1 mL of the infected THP-1 cells was lysed using PBS mixed with 0.5% Triton X-100. The cell lysate was serially 10-fold diluted and cultured on Middlebrook 7H10 agar and incubated for 14 days at 37 °C under 5% CO2, after which the colonies were counted to determine the actual MOI achieved.
The detailed description of the HFS-MAC for pulmonary disease has been published elsewhere.14,15 THP-1 cells, infected as described above, were inoculated into the peripheral compartment of each of six HFS-MAC (20 mL per HFS-MAC). The HFS-MAC were maintained at 37 °C under 5% CO2 with circulating RPMI/FBS. Three HFS-MAC were treated with the human equivalent dose of ethambutol (15 mg/kg) plus azithromycin (500 mg) once daily for 28 days.5 We recapitulated the concentration–time profiles of these two antibiotics as encountered in the lungs of patients, based on previous publications.16,17 The other three HFS-MAC served as the non-treated controls. In order to validate that the intended concentration–time profiles had been achieved, the central compartment of each HFS-MAC was sampled at 0, 1, 2, 6, 12, 18 and 23.5 h after the first drug infusion. The infected THP-1 cells in the peripheral compartment of the HFS-MAC were sampled on days 0, 3, 7, 14, 21 and 28 and processed as described above for a count of the total number of the viable THP-1 cells as well as to enumerate the bacterial burden in each system. The same samples were also cultured on Middlebrook 7H10 agar containing 3 × MIC of either ethambutol or azithromycin. The plates were incubated for 14 days at 37 °C under 5% CO2, after which the colonies were counted. The drug-supplemented agar was incubated for 21 days before the colonies were counted. GraphPad Prism (v. 6.05) was used to compute the kill rates of the combination regimen, and for an analysis of variance to compare the two-drug combination regimen with the non-treated controls at each timepoint. The HFS experiment was performed twice with three replicates for each regimen.
Measurement and analysis of drug concentration
Samples from the HFS-MAC central compartment were analysed for ethambutol and azithromycin concentration using validated liquid chromatography-tandem mass spectrometry methods, as described previously.12,14 There was no modification of the published method. Pharmacokinetic modelling was performed using ADAPT 5 software.18 Steps taken in the pharmacokinetic analysis were as outlined in prior publications.11,12,14,15,19
Results
The concentration–time profile of ethambutol achieved a biphasic shape with a half-life of 3 h for the first 12 h and of 8 h between 12 and 24 h, as occurs in patients. The ethambutol extracellular Cmax/MIC ratio achieved was 0.71, which translates to an intracellular Cmax/MIC of 13.13 Azithromycin achieved a half-life of 12 h, as encountered in patients. The extracellular azithromycin AUC0–24/MIC ratio achieved was 2.97, which translates to an intracellular AUC0–24/MIC ratio of 24 333.12
The THP-1 cell counts at each sampling timepoint are shown in Figure 1. There was no difference in the total THP-1 counts between the treated or non-treated systems at any of the sampling timepoints. Thus, the MAC continued to be intracellular throughout the experiment.
Figure 1.
THP-1 cell counts in the MAC-HFS. The number of viable monocytes did not differ between the systems treated with the two-drug combination or the controls that received no drug treatment. This shows that long-term exposure studies are possible in the MAC-HFS.
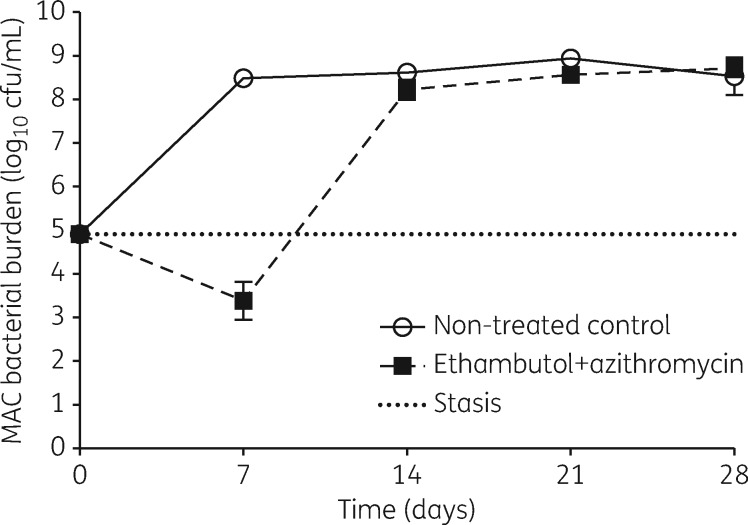
Figure 2 shows the day-to-day changes in the total MAC burden in the systems over the 28 day treatment period. The bacterial burden in non-treated HFS-MAC replicates grew at a rate of 0.18 ± 0.02 log10 cfu/mL/day to reach 8.53 ± 0.43 log10 cfu/mL. The combination of ethambutol and azithromycin killed, at a maximum, 1.52 ± 0.43 log10 cfu/mL below the starting bacterial burden. This is better than the sum of the maximal effect of each drug as monotherapy based on our prior studies.11,14 As shown in Figure 2, this was achieved on day 7. After day 7, the two-drug combination regimen failed as reflected by the change in the direction of the slope of the bacterial burden. By day 28, the MAC burden in the drug-treated systems had caught up with non-treated controls and had a bacterial burden of 8.72 ± 0.21 log10 cfu/mL. Accordingly, analysis of variance revealed a difference of − 5.10 log10 cfu/mL (95% CI − 6.65 to − 3.55 log10 cfu/mL) between untreated controls and the dual regimen on day 7, but there were no statistically significant differences on subsequent days. Thus, the effect of the combination regimen was transient.
Figure 2.
Efficacy of the ethambutol/azithromycin standard regimen in the MAC-HFS. The two-drug combination standard therapy failed to kill intracellular MAC beyond day 7.
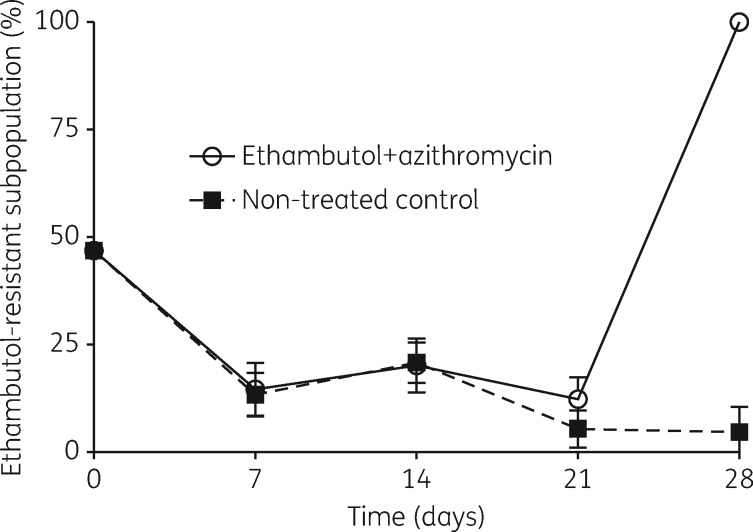
Why did the dual regimen fail? The MAC bacterial burden on drug-supplemented agar, intended to measure ADR, is shown in Figure 3. The figure shows that failure of the regimen coincided with the emergence of a sub-population resistant to 3 × MIC of ethambutol (i.e. low-level resistance). On the other hand, there was no change in proportion of a sub-population resistant to ethambutol at 3 × MIC relative to total population in the untreated HFS. Thus, the proportion of the population that was ethambutol resistant rose due to treatment with the combination regimen. However, there was no resistance to azithromycin 3 × MIC in any of these experiments. This means that resistance to ethambutol alone was enough to lead to failure, basically reverting to the pattern of azithromycin monotherapy observed in the past in the HFS-MAC.
Figure 3.
Emergence of drug resistance. For the first 3 weeks of the treatment the ethambutol-resistant sub-population decreased as a proportion of the whole population in both non-treated controls and the systems treated with the standard regimen. Interestingly there was an increase in the ethambutol-resistant subpopulation on day 28, and this was greater than the proportion present at the start of the experiment.
Discussion
The dual-therapy regimen of azithromycin and ethambutol achieved bactericidal activity that was higher than the sum of the two monotherapy regimens attained in our prior studies in the same system in the past.11,14 This means that the combination has some merit in terms of synergy. However, the extent of kill by the two drugs only just exceeded 1.0 log10 cfu/mL kill, and was transient. In fact, the period of downward decline in bacterial burden was not longer than for azithromycin therapy alone.11 This was despite the use of optimal exposures of the two drugs, which we have shown are not actually achieved in vivo by current standard dosing recommendations. In other words the backbone regimen failed even though we used the optimal exposures of these two drugs. This means that the backbone of the standard regimen has very poor kill rates, and this probably explains the poor and slow kill rates encountered in patients. The findings call for replacement of this regimen with another that can kill faster than 1.0 log10 cfu/mL in 1 week and has a sustained effect beyond 7 days. In an accompanying manuscript, we show that with addition of rifabutin to this combination, there is a higher depth of kill by the full triple-therapy regimen, which killed to 2 log10 cfu/mL below day 0, and did not fail until after 14 days.20
Failure of the dual combination coincided with emergence of low-level ethambutol resistance. First, the emergence of ADR was rapid, and halted microbial kill within 1 week of therapy. However, we did not perform sequencing of the drug-resistant isolates to confirm mutations associated with ethambutol resistance. Second, while we did not detect azithromycin ADR, failure of the combination regimen could suggest that low-level azithromycin resistance was nevertheless present, and after the effect of ethambutol was abrogated, reverted to the profile encountered with azithromycin monotherapy.14 It seems likely that the low-level azithromycin resistance could have been below 3 × MIC, as is the case with some efflux pumps that are associated with low-level resistance early during the ‘antibiotic resistance arrow of time’, which could explain our failure to detect that with our assay.11 Nevertheless, this points to a potentially important role for a third drug, such as a rifamycin. Perhaps the drugs we assume are central, such as azithromycin and ethambutol, contribute less in the regimen than is currently believed. However, if the contribution of rifamycins was equivalent in magnitude to those of azithromycin and ethambutol, the contribution would also only be transiently effective, and perhaps only contribute a few more weeks of effectiveness. This proposition is tested in an accompanying manuscript.20 Alternatively, failure could be ‘phenotypic resistance’ as a result of tolerance, due to non-replicating persistent MAC. It is known that MAC can form biofilms, in which persisters are enriched.21 In the future, we will examine the MAC transcriptome in HFS-MAC, to identify if there is a switch to a biofilm signature during the treatment with antibiotics.
There are some limitations to the study. First, we used only one standard laboratory strain of MAC. There could be diverse responses when a larger number of MAC strains are used, across a range of MICs. Since hollow-fibre studies are expensive, an alternative approach is to use a computer-aided clinical trial simulation incorporating the MIC distribution, which provides information on the target attainment probability of the dose across the MIC range. A second limitation is that we observed no emergence of azithromycin resistance. The reason could be the presence of very low-level azithromycin resistance in a sub-population, which was killed by 3 × MIC of azithromycin. Also we did not use molecular assays, which might have helped to detect the presence of a low-level resistant sub-population. Nevertheless, these limitations do not undermine our overall findings, which are that the two key drugs in the standard therapy regimen, ethambutol and azithromycin, even at optimal exposure, did not have a sustained effect against intracellular MAC. There is thus a need for the development of a new backbone for a treatment regimen that can kill effectively and faster than the currently used regimen.
Acknowledgments
Funding
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health New Innovator Award DP2OD001886-01 and the National Institute of Allergy and Infectious Diseases award R01AI079497.
Transparency declarations
T. G. is a consultant for LuminaCare solutions and is the founder of Jacaranda Biomed, Inc. All other authors have none to declare.
This article forms part of a supplement sponsored by the Baylor Research Institute.
References
- 1. Marras TK, Daley CL.. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002; 23: 553–67. [DOI] [PubMed] [Google Scholar]
- 2. Amorim A, Macedo R, Lopes A. et al. Non-tuberculous mycobacteria in HIV-negative patients with pulmonary disease in Lisbon, Portugal. Scand J Infect Dis 2010; 42: 626–8. [DOI] [PubMed] [Google Scholar]
- 3. Levy I, Grisaru-Soen G, Lerner-Geva L. et al. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg Infect Dis 2008; 14: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prevots DR, Shaw PA, Strickland D. et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med 2010; 182: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Griffith DE, Aksamit T, Brown-Elliott BA. et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 6. Kobashi Y, Matsushima T.. The effect of combined therapy according to the guidelines for the treatment of Mycobacterium avium complex pulmonary disease. Intern Med 2003; 42: 670–5. [DOI] [PubMed] [Google Scholar]
- 7. Griffith DE, Brown BA, Cegielski P. et al. Early results (at 6 months) with intermittent clarithromycin-including regimens for lung disease due to Mycobacterium avium complex. Clin Infect Dis 2000; 30: 288–92. [DOI] [PubMed] [Google Scholar]
- 8. Griffith DE, Brown BA, Girard WM. et al. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis 2001; 32: 1547–53. [DOI] [PubMed] [Google Scholar]
- 9. Pasipanodya JG, Ogbonna D, Deshpande D. et al. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium–intracellulare complex disease. J Antimicrob Chemother 2017; 72 Suppl 2: ii3–ii19. [DOI] [PubMed] [Google Scholar]
- 10. A controlled comparison of four regimens of streptomycin plus pyrazinamide in the retreatment of pulmonary tuberculosis. Tubercle 1969; 50: 81–114. [DOI] [PubMed] [Google Scholar]
- 11. Schmalstieg AM, Srivastava S, Belkaya S. et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother 2012; 56: 4806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deshpande D, Pasipanodya JG, Gumbo T.. Azithromycin dose to maximize efficacy and suppress acquired drug resistance in pulmonary Mycobacterium avium disease. Antimicrob Agents Chemother 2016; 60: 2157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conte JE Jr, Golden JA, Kipps J. et al. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob Agents Chemother 2001; 45: 2891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshpande D, Srivastava S, Meek C. et al. Ethambutol optimal clinical dose and susceptibility breakpoint identification by use of a novel pharmacokinetic-pharmacodynamic model of disseminated intracellular Mycobacterium avium. Antimicrob Agents Chemother 2010; 54: 1728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshpande D, Srivastava S, Meek C. et al. Moxifloxacin pharmacokinetics/pharmacodynamics and optimal dose and susceptibility breakpoint identification for treatment of disseminated Mycobacterium avium infection. Antimicrob Agents Chemother 2010; 54: 2534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peloquin CA, Bulpitt AE, Jaresko GS. et al. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob Agents Chemother 1999; 43: 568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu P, Allaudeen H, Chandra R. et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother 2007; 51: 103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Argenio DZ, Schumitzky A.. ADAPT II. A Program for Simulation, Identification, and Optimal Experimental Design. User Manual. Biomedical Simulations Resource Los Angeles, CA: University of Southern California, 1997.
- 19. Deshpande D, Srivastava S, Musuka S. et al. Thioridazine as chemotherapy for Mycobacterium avium complex diseases. Antimicrob Agents Chemother 2016; 60: 4652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshpande D, Srivastava S, Pasipanodya JG. et al. A novel ceftazidime/avibactam, rifabutin, tedizolid and moxifloxacin (CARTM) regimen for pulmonary Mycobacterium avium disease. J Antimicrob Chemother 2017; 72 Suppl 2: ii48–ii53. [DOI] [PubMed] [Google Scholar]
- 21. Yamazaki Y, Danelishvili L, Wu M. et al. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol 2006; 8: 806–14. [DOI] [PubMed] [Google Scholar]