Abstract
Background: Previously we studied the antibiotic susceptibility of invasive Haemophilus influenzae collected in Canada from 1990 to 2006 and characterized isolates by serotype, MLST and ftsI gene sequencing for significant PBP3 mutations.
Objectives: To provide an update based on isolates collected from 2007 to 2014.
Methods: A total of 882 case isolates were characterized by serotype using slide agglutination and PCR. MLST was carried out to determine ST. Isolates were tested for β-lactamase production, presence of significant PBP3 mutations and antibiotic susceptibility by disc diffusion against 14 antibiotics. MIC values of three antibiotics were determined for 316 isolates using microbroth dilution.
Results: Non-typeable H. influenzae accounted for 54.6% of the isolates and 45.4% were serotypeable, predominantly type a (23.1%), type b (8.3%) and type f (10.8%). The overall rate of ampicillin resistance due to β-lactamase production was 16.4% and increased from 13.5% in 2007–10 to 19% in 2011–14. Significant PBP3 mutations were identified in 129 isolates (14.6%) with 23 (2.6%) also producing β-lactamase. MLST identified related STs (ST-136, ST-14 and ST-367) associated exclusively with genetically β-lactamase-negative, ampicillin-resistant isolates and confirmed previously reported associations between significant PBP3 mutations and ST.
Conclusions: A significant increase in β-lactamase-producing isolates was observed from 2007 to 2014; the rate of significant PBP3 mutations has increased since previously reported and 52.5% of non-typeable H. influenzae now show resistance markers. Resistance to trimethoprim/sulfamethoxazole was common and no resistance to fluoroquinolones or third-generation cephalosporins was found.
Introduction
Haemophilus influenzae is an important bacterial pathogen causing a wide variety of both invasive and respiratory infections. H. influenzae is divided into six serotypes based on polysaccharide capsule antigens that are denoted serotypes a–f, while strains that lack a polysaccharide capsule are designated non-typeable (NT-Hi). Prior to vaccination, serotype b (Hib) was a common cause of serious invasive disease, especially in children ≤5 years old.1 Since the introduction of the Hib conjugate vaccine, the rate of Hib disease has decreased dramatically and many reports now point to non-Hib and NT-Hi as responsible for the majority of invasive H. influenzae infections.2–5
Ampicillin resistance in H. influenzae, first described in the 1970s,6 is now a well-documented global phenomenon.7,8 Ampicillin resistance is divided into two main categories based on the production of β-lactamase enzymes. Isolates producing β-lactamase are termed β-lactamase positive, ampicillin resistant (BLPAR) and constitute the most common type of ampicillin resistance observed in H. influenzae, with most isolates producing either the TEM-1- or ROB-1-type β-lactamase.9 Isolates that do not produce β-lactamase, but contain alterations in PBP3, resulting in decreased affinity for β-lactams, are termed β-lactamase negative, ampicillin resistant (BLNAR).10 In addition to BLPAR and BLNAR, isolates that possess both β-lactamase as well as have significant PBP3 mutations are termed β-lactamase positive, amoxicillin/clavulanic acid resistant (BLPACR).10
Frequently, isolates that have significant PBP3 mutations have only low-level resistance and may not show the phenotypes of ampicillin or amoxicillin/clavulanic acid resistance when tested by disc diffusion or microbroth dilution methods. Such isolates are classified as genetically BLNAR/BLPACR (gBLNAR/gBLPACR), respectively.11
A previous study of 236 invasive H. influenzae collected from 1990 to 2006 in Canada revealed an overall β-lactamase-positive, ampicillin-resistant rate of 17.8%, with no significant difference between serotypeable versus NT-Hi strains.12 However, significantly more NT-Hi (12.9%) than serotypeable strains (2.3%) demonstrated resistance to trimethoprim/sulfamethoxazole.12 From the same collection of isolates, the rate of gBLNAR was 8.1% and the rate of gBLPACR was 0.9%.13 The present study also examines invasive isolates collected in the same manner as before and covers the period 2007–14 with the aim of providing an update on the current susceptibility of invasive H. influenzae in Canada.
Materials and methods
A total of 882 H. influenzae isolates from individual invasive disease cases in Canada from 1 January 2007 to 30 June 2014 were included in this study, and were collected in the same manner as described in our previous study.12 Isolates were submitted from Provincial and Territorial Public Health Laboratories and hospitals to the National Microbiology Laboratory (NML) as part of our laboratory surveillance activities. Since practice varies from province to province, the specimens received at the NML do not necessarily represent a systematic collection of all invasive case isolates across Canada. Most isolates were submitted for identification and serotyping and very few isolates were submitted solely for antibiotic susceptibility testing.
Serotyping of H. influenzae isolates was performed by slide agglutination using commercial antisera (Difco, Oakville, Ontario, Canada) and by PCR detection of serotype-specific genes and the capsule transport gene, bexA, as described by Falla et al.14 All non-typeable isolates were confirmed to be H. influenzae by partial 16S rRNA gene sequencing.15 MLST was carried out using methods reported by Meats et al.,16 and allele number and ST assignment was done through the H. influenzae MLST website, http://pubmlst.org/hinfluenzae/.
β-Lactamase production was detected using DrySlide nitrocefin (BBL, Becton Dickinson, Oakville, Ontario, Canada) and typed as TEM-1 or ROB-1 using primers described by Molina et al.17 Disc diffusion was carried out according to CLSI guidelines18 and the following antibiotic discs were tested: ampicillin (10 μg), amoxicillin/clavulanic acid (30 μg), cefaclor (30 μg), azithromycin (15 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), clarithromycin (15 μg), imipenem (10 μg), levofloxacin (5 μg), meropenem (10 μg), moxifloxacin (5 μg), tetracycline (30 μg) and trimethoprim/sulfamethoxazole (25 μg). Ampicillin, cefixime and ceftriaxone MICs were determined by microbroth dilution using Sensititre plates (Oxoid, Nepean, Ontario, Canada) according to manufacturer’s instructions. Control strain ATCC 49247 was used in each experiment for both disc diffusion and MIC determination.
Detection of gBLNAR and gBLPACR strains was done by PCR amplification and partial gene sequencing of the ftsI gene, which encodes PBP3.10 Amino acid substitutions in PBP3 associated with reduced susceptibility to β-lactams were determined through alignment with the β-lactamase-negative, ampicillin-susceptible strain Rd KW20 (GenBank accession number L42023) and grouped accordingly.19,20
Statistical analysis and P value determination were performed using the online z score calculator for two population proportions (www.socialstatistics.com).
Results
Production of β-lactamases and susceptibility of invasive H. influenzae to ampicillin and ceftriaxone
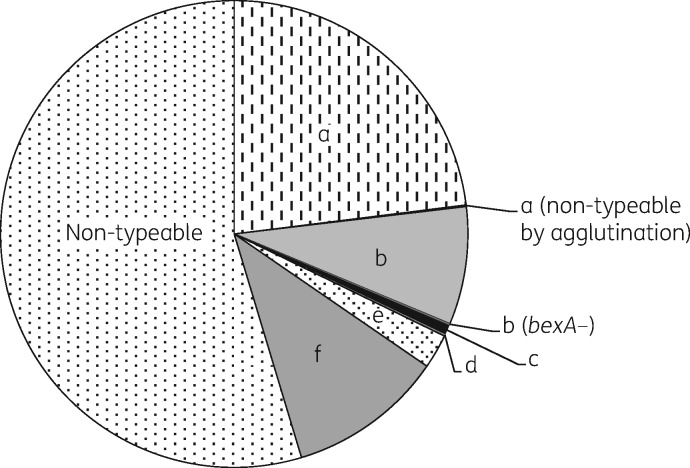
Figure 1 shows the serotype distribution for the 882 invasive H. influenzae isolates collected from 1 January 2004 to 30 June 2014.
Figure 1.
Serotype distribution of 882 invasive H. influenzae case isolates in Canada from 1 January 2007 to 30 June 2014.
Over twice as many NT-Hi isolates (103/482 or 21.4%) produced β-lactamase compared with serotypeable isolates (42/400 or 10.5%) and all were found to be resistant to ampicillin when tested by the disc diffusion method. TEM-1 type β-lactamase was found in 93.8% (n = 136), ROB-1 was found in 4.1% (n = 6) and 2.1% (n = 3) were negative for both TEM-1 and ROB-1. Among the serotypeable isolates, β-lactamase was most commonly found in Hib (25/73, 34.2%), followed by Hic (1/6, 16.7%), Hif (10/95, 10.5%), Hie (2/21, 9.5%) and Hia (4/204, 2.0%) (Table 1).
Table 1.
Antibiotic resistance profiles of 882 invasive H. influenzae case isolates in Canada
| Serotype | Number of isolates | β-Lactamase | Antibiotic resistance profilea |
|---|---|---|---|
| a | 198 | − | susceptible to all antibiotics tested |
| 1 | − | CLR R | |
| 1 | − | TET R | |
| 3 | + | AMP R | |
| 1 | + | AMP R, CHL R, SXT R, TET R | |
| b | 44 | − | susceptible to all antibiotics tested |
| 3 | − | SXT R | |
| 1 | − | CHL R, SXT R, TET R | |
| 19 | + | AMP R | |
| 4 | + | AMP R, CHL R, SXT R | |
| 1 | + | AMP R, CHL R, TET R | |
| 1 | + | AMP R, CHL R, CEC R, SXT R | |
| c | 5 | − | susceptible to all antibiotics tested |
| 1 | + | AMP R | |
| d | 1 | − | susceptible to all antibiotics tested |
| e | 18 | − | susceptible to all antibiotics tested |
| 1 | − | SXT R | |
| 2 | + | AMP R | |
| f | 83 | − | susceptible to all antibiotics tested |
| 1 | − | SXT R | |
| 1 | − | CLR R | |
| 10 | + | AMP R | |
| Non-typeable | 283 | − | susceptible to all antibiotics tested |
| 76 | − | SXT R | |
| 10 | − | CEC R | |
| 1 | − | TET R | |
| 1 | − | AMP Rb | |
| 7 | − | SXT R, CEC R | |
| 1 | − | SXT R, CLR R | |
| 85 | + | AMP R | |
| 14 | + | AMP R, SXT R | |
| 2 | + | AMP R, CEC R | |
| 1 | + | AMP R, SXT R, TET R | |
| 1 | + | AMP R, CHL R, SXT R, TET R |
R, resistant.
Antibiotics tested: AMP = ampicillin (10 μg), amoxicillin/clavulanic acid (30 μg); CEC = cefaclor (30 μg); azithromycin (15 μg), ceftriaxone (30 μg); CHL = chloramphenicol (30 μg), ciprofloxacin (5 μg); CLR = clarithromycin (15 μg), imipenem (10 μg), levofloxacin (5 μg), meropenem (10 μg), moxifloxacin (5 μg); TET = tetracycline (30 μg); SXT = trimethoprim/sulfamethoxazole (25 μg).
BLNAR isolate.
Ampicillin, cefixime and ceftriaxone MICs were determined for 316 of the 882 isolates. Originally, 317 isolates were chosen for MIC determination, but 1 gBLNAR did not grow on the MIC plate. The 316 isolates included all 23 gBLPACR, 105 gBLNAR as well as randomly selected BLPAR (n = 24) and BLNAS (n = 164) isolates and were comprised of 155 serotypeable and 161 NT-Hi (see below and Table 2). All β-lactamase-producing isolates had an ampicillin MIC >4 mg/L, while the corresponding values for the β-lactamase-negative isolates ranged from ≤0.125 mg/L to 4 mg/L. There was only one NT-Hi isolate that was β-lactamase negative, but ampicillin resistant, according to CLSI guidelines with an MIC = 4 mg/L and had significant PBP3 mutations Asp-350-Asn, Ser-357-Asn, Met-377-Ile, Ser-385-Ile, Leu-398-Phe and Asn-526-Lys (group III+) and was typed by MLST as ST-586.
Table 2.
Rate of gBLNAR and gBLPACR H. influenzae in Canada among serotypeable and non-typeable isolates
| β-Lactamase production | Total | Significant PBP3 mutations | No significant PBP3 mutations | gBLNAR rate (%) | gBLPACR rate (%) | |
|---|---|---|---|---|---|---|
| Serotypeable | + | 42 | 4 | 38 | 4/42 (9.5) | |
| − | 358 | 1 | 357 | 1/358 (0.3) | ||
| Non-typeable | + | 103 | 19 | 84 | 19/103 (18.4) | |
| − | 379 | 105 | 274 | 105/379 (27.7) |
Regardless of β-lactamase status, all 882 isolates were susceptible to ceftriaxone by disc diffusion and the 316 isolates tested for MICs were susceptible to both cefixime and ceftriaxone, according to CLSI guidelines.
Analysis of ftsI genes of invasive H. influenzae and the correlation to their ampicillin, cefixime and ceftriaxone MICs
Sequencing of the ftsI gene to determine significant PBP3 mutations associated with reduced susceptibility to β-lactams was carried out on all 882 isolates. Significant PBP3 mutations were found in five encapsulated isolates (1.3% of 400 isolates), including three Hib (all β-lactamase positive) and two Hif (one β-lactamase positive and one β-lactamase negative). In contrast, of the 482 NT-Hi isolates tested, 124 (25.7%) were found to contain significant PBP3 mutations (19 were β-lactamase positive) (Table 2). Of the 129 isolates with significant PBP3 mutations, 119 (92.2%) belonged to group II and were further classified into groups IIa (n = 22), IIb (n = 61), IIc (n = 9) and IId (n = 27) based on amino acid substitutions in addition to the Asn-526-Lys mutation (Table S1, available as Supplementary data at JAC Online). There were seven isolates belonging to group I (characterized by Arg-517-His), two group III+ (characterized by Ser-385-Thr, Leu-389-Phe and Asn-526-Lys) and one group III-like (characterized by Ser-385-Thr and Arg-517-His) (Table S1).
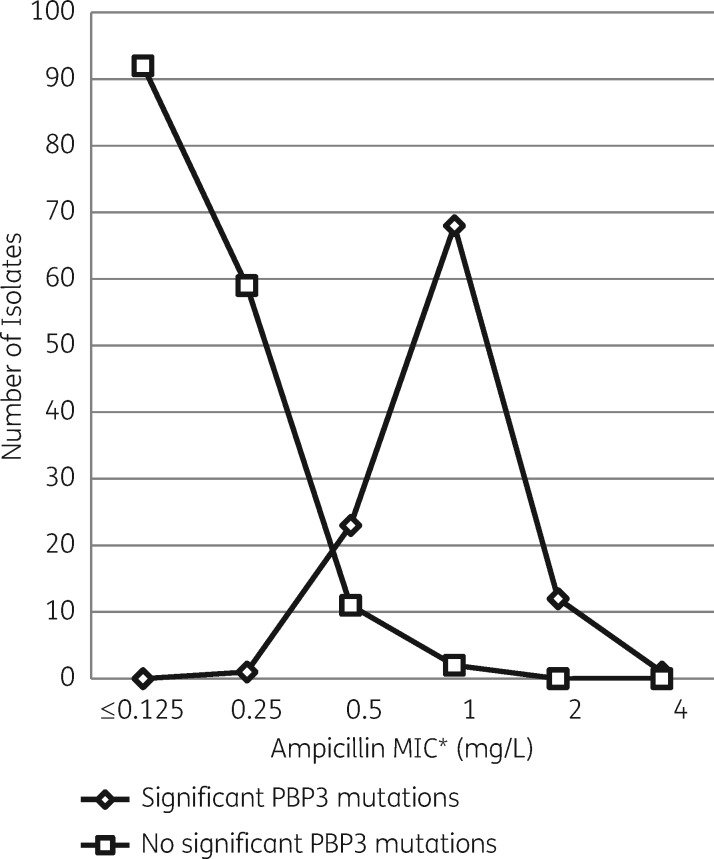
When the ampicillin MIC values of 269 β-lactamase-negative isolates (105 gBLNAR plus 164 BLNAS) were analysed, those with significant PBP3 mutations showed MIC values eight times higher than those without significant PBP3 mutations (Figure 2). The MIC50/MIC90 values for isolates with and without significant PBP3 mutations were 1/2 mg/L and ≤0.125/0.25 mg/L, respectively.
Figure 2.
Ampicillin MIC values for 269 β-lactamase-negative invasive H. influenzae case isolates with (n = 105) and without (n = 164) significant PBP3 mutations. *According to CLSI guidelines: ≤1 mg/L = susceptible, 2 mg/L = intermediate and ≥4 mg/L = resistant.18
Cefixime MICs for the 316 isolates ranged from ≤0.125 to 1 mg/L. The six isolates that showed the highest cefixime MIC values (either 0.5 or 1 mg/L) all had significant PBP3 mutations with three isolates belonging to group I, two group III+ and one group III-like. Ceftriaxone MIC values ranged from ≤0.03 to 0.25 mg/L. There were only two isolates with ceftriaxone MIC = 0.25 mg/L and both had significant PBP3 mutations and belonged to group III+.
Susceptibility of invasive H. influenzae to other antibiotics
Resistance to trimethoprim/sulfamethoxazole was significantly more common in NT-Hi (n = 100 or 20.7%) than in serotypeable isolates (n = 12 or 3.0%) (P < 0.05). Multi-drug resistance was also more common among NT-Hi: 26 (5.4%) versus 8 (2.0%) for serotypeable isolates (P < 0.05). Resistance to chloramphenicol, cefaclor, tetracycline and clarithromycin was also detected in small numbers (Table 1). No resistance to fluoroquinolones or to third-generation cephalosporins was observed.
Clonal analysis of invasive H. influenzae showing PBP3 mutations
Of the five serotypeable isolates showing significant PBP3 mutations, three were β-lactamase-positive Hib and belonged to three different, but related, STs (ST-95, ST-92 and ST-54): ST-92 is a double-locus variant (DLV) of ST-95, and ST-54 is a triple-locus variant (TLV) of ST-95 and a DLV of ST-92. There were two Hif isolates with significant PBP3 mutations that belonged to ST-124 (gBLNAR) and its single-locus variant (SLV) ST-698 (gBLPACR) (Table S2).
Of the 124 NT-Hi isolates that showed significant PBP3 mutations, 19 were also β-lactamase positive (gBLPACR). Ten of the 19 NT-Hi gBLPACR isolates (52.6%) belonged to two unrelated STs, ST-165 and ST-396, while the remaining nine isolates belonged to eight other unrelated STs (Table S2).
Of the 105 gBLNAR NT-Hi isolates, 60 or 57.1% were found to belong to 8 STs (ST-14, ST-136, ST-156, ST-201, ST-245, ST-367, ST-396 and ST-556), while the remaining isolates were found to belong to 27 different STs. Five STs, namely ST-14 (10/10), ST-136 (4/4), ST-201 (10/10), ST-367 (10/10) and ST-396 (11/11), were exclusively found in isolates with significant PBP3 mutations. ST-14, ST-136 and ST-367 are either DLV or TLV of each other and are part of the same clonal complex. Other STs associated with isolates with β-lactamase production and/or significant PBP3 mutations are described in Table S2.
Discussion
In this study of 882 invasive H. influenzae isolates, the majority (83%) were β-lactamase negative and susceptible to ampicillin and only one true BLNAR isolate was found. However, it is interesting to note that over half of NT-Hi (253/482 or 52.5%) were resistant to one or more antibiotics when tested by disc diffusion and/or contained significant PBP3 mutations, which is significantly higher than previously reported among Canadian invasive NT-Hi (56/147, 38.1%) (P < 0.05).12,13 In contrast, only 13% (52/400) of serotypeable isolates from this study showed antibiotic resistance and/or significant PBP3 mutations, with the exception of Hib, where 29 isolates (39.7%) showed antibiotic resistance and/or significant PBP3 mutations.
The previous study found that the overall rate of β-lactamase-producing isolates was 17.8%, but decreased from 21.4% in 1990–99 to 16.4% in 2000–06.12 However, in this study, even though the overall rate of β-lactamase production was lower (16.4%), the rate increased significantly throughout the study period, going from 13.5% in the years 2007–10 to 19% in 2011–14 (P < 0.05), with a high of 22.7% in 2014. Both studies found that more NT-Hi were β-lactamase positive compared with serotypeable isolates: 20.5% versus 13.5% in 1990–200612 and 21.4% versus 10.5% in this study.
In the previous study, 8.9% (21/236) of all invasive H. influenzae isolates tested were found to have significant PBP3 mutations;13 however, the rate has increased significantly to 14.6% (129/882) in the present study (P < 0.05). The rates of significant PBP3 mutations observed in this study fluctuated between 8.8% in 2007 and 19.2% in 2014, but did not appear to increase consistently with each year.
The majority of gBLNAR isolates belonged to group II based on the first-stage substitution Asn-526-Lys19 and displayed low-level resistance or reduced susceptibility to ampicillin, but remained susceptible to cefixime and ceftriaxone. When the MIC data were interpreted according to CLSI guidelines, only 1 out of 105 gBLNAR isolates tested could be classified as ampicillin resistant with MIC = 4 mg/L (group III+). However, using EUCAST breakpoints,21 an additional 12 would be considered ampicillin resistant with MIC = 2 mg/L (1 group I and 11 group II). Despite this, it is important to note that the gBLNAR isolates had MIC values eight times higher than those of BLNAS strains (Figure 2). Group II isolates with low-level ampicillin resistance are also predominant in Spain, Sweden, Norway and Korea,22–25 while the majority of Japanese isolates belong to group III and subgroups of group III.26 The group III+/III-like isolates in this study were all from the province of British Columbia and contained the second-stage substitution Ser-385-Thr.22 In addition, the group III-like isolate contained Arg-517-His and the group III+ isolates contained Leu-385-Phe and Asn-526-Lys. The Leu-389-Phe substitution is considered a third-stage mutation and is often associated with higher levels of resistance to β-lactams.22,23 The two group III+ isolates in this study had ampicillin MICs of 1 and 4 mg/L, cefixime MIC = 0.5 mg/L and ceftriaxone MIC = 0.25 mg/L. The group III-like isolate had ampicillin MIC = 1 mg/L, cefixime MIC = 1 mg/L and ceftriaxone MIC = 0.06 mg/L. Using CLSI guidelines, all were susceptible to cefixime and ceftriaxone; however, the two group III+ isolates would have been resistant to ceftriaxone using EUCAST guidelines. These results are consistent with previous reports of group III+ isolates showing decreased susceptibility to third-generation cephalosporins.24
The finding of only two group III+ isolates 2 years apart may seem insignificant, but it is nevertheless an important observation due to the potential for rapid emergence of these strains. A Norwegian study noted the Leu-389-Phe substitution in only 13% of high-level resistant gBLNAR isolates from 2006–10 and 93% in 2011–13.19 Japan has also reported a 2-fold increase in BLNAR isolates occurring between 2001 and 2004.27 Even though these studies are mainly focused on respiratory isolates, it is possible to assume that invasive isolates may follow a similar path.
The link between significant PBP3 mutations and MLST has been previously reported and shows a relationship between specific PBP3 substitution patterns and certain STs.25 Our data confirmed earlier findings that ST-14, ST-201, ST-367 and ST-396 were exclusively associated with isolates containing significant PBP3 mutations,24,25 and identified four isolates belonging to ST-136 (part of the same clonal complex as ST-14 and ST-367), also associated with isolates containing significant PBP3 mutations. All ST-367 (n = 10) and 9 out of 10 ST-14 isolates had identical PBP3 mutation patterns between amino acid positions 350 and 526 (Asp-350-Asn, Met-377-Ile, Ala-502-Val, Asn-526-Lys) and matched previously reported patterns for these STs,25 as did the four ST-136 isolates. All ST-396 isolates (n = 11) also had identical patterns (Ile-449-Val, Asn-526-Lys) as did ST-201 isolates (n = 10) (Asp-350-Asn, Gly-390-Glu, Asn-526-Lys) and again, both matched previously reported patterns for these STs.25
One limitation of this study is that not all invasive H. influenzae are captured due to our current passive laboratory surveillance system. As we have not changed our method of specimen collection since our last national study, isolates from this study can be compared with those from that previous study.12 Also, isolates were not pre-selected for inclusion in this study and hence may be regarded as a representative of invasive strains in Canada. Compared with our last reports,12,13 the key findings of this study are (i) the significant increase in β-lactamase-producing isolates (from 13.5% in 2007–10 to 19% in 2011–14), (ii) 14.6% of isolates with significant PBP3 mutations, (iii) >50% of NT-Hi are either resistant to one or more antibiotics and/or contained significant PBP3 mutations and (iv) confirmation of the relationship between ST and significant PBP3 mutations. Continued monitoring of antibiotic susceptibility and isolate characterization are important tools for detecting changes in disease-causing strains and providing guidance for empirical antibiotic treatment of patients.
Supplementary Material
Acknowledgements
Part of this work has been presented at CACMID—AMMI Canada 2015 Annual Conference (Abstract D03).
We would like to thank: the many staff of the Provincial Public Health Laboratories and hospital laboratories for submission of isolates; Dennis Law for assisting with the PCR serotyping; Ashley Muckle, MacKenzie Sato and Tracy Drew for their contributions to disc diffusion and MIC determination; and the staff at the National Microbiology Laboratory DNA Core Facility for the primer synthesis and sequencing work. This publication made use of the PubMLST web site (pubmlst.org) developed by Keith Jolley (Jolley & Maiden BMC Bioinformatics 2010; 11: 595) and sited at the University of Oxford. The development of that web site was funded by the Wellcome Trust.
Funding
This project was supported by internal funding.
Transparency declarations
None to declare.
Supplementary data
Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Watt JP, Wolfson LJ, O’Brien KL. et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009; 394: 903–11. [DOI] [PubMed] [Google Scholar]
- 2. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000; 13: 302–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsang RSW, Sill ML, Skinner SJ. et al. Characterization of invasive Haemophilus influenzae disease in Manitoba, Canada, 2000-2006: invasive disease due to non-type b strains. Clin Infect Dis 2007; 44: 1611–4. [DOI] [PubMed] [Google Scholar]
- 4. Adam HJ, Richardson SE, Jamieson FB. et al. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine 2010; 28: 4073–8. [DOI] [PubMed] [Google Scholar]
- 5. Shuel M, Hoang L, Law DK. et al. Invasive Haemophilus influenzae in British Columbia: non-Hib and non-typeable strains causing disease in children and adults. Int J Infect Dis 2011; 15: e167–73. [DOI] [PubMed] [Google Scholar]
- 6. Gunn BA, Woodall JB, Jones JF. et al. Ampicillin-resistant Haemophilus influenzae. Lancet 1974; ii: 845. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs MR. Worldwide trends in antimicrobial resistance among common respiratory tract pathogens in children. Pediatr Infect Dis 2003; S109–19. [DOI] [PubMed] [Google Scholar]
- 8. Sahm DF, Brown NP, Thornsberry C. et al. Antimicrobial susceptibility profiles among common respiratory tract pathogens: a GLOBAL perspective. Postgrad Med 2008; 120: 16–24. [DOI] [PubMed] [Google Scholar]
- 9. Farrell DJ, Morrissey I, Bakker S. et al. Global distribution of TEM-1 and ROB-1 β-lactamases in Haemophilus influenzae. J Antimicrob Chemother 2005; 54: 773–6. [DOI] [PubMed] [Google Scholar]
- 10. Ubukata K, Shibasaki Y, Yamamoto K. et al. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in β-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother 2001; 45: 1693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hotomi M, Fujihara K, Billal DS. et al. Genetic characteristics and clonal dissemination of β-lactamase-negative ampicillin-resistant Haemophilus influenzae strains isolated from the upper respiratory tract of patients in Japan. Antimicrob Agents Chemother 2007; 51: 3969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sill ML, Tsang RS.. Antibiotic susceptibility of invasive Haemophilus influenzae strains in Canada. Antimicrob Agents Chemother 2008; 52: 1551–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shuel ML, Tsang RS.. Canadian β-lactamase-negative Haemophilus influenzae isolates showing decreased susceptibility toward ampicillin have significant penicillin binding protein 3 mutations. Diagn Microbiol Infect Dis 2009; 63: 379–83. [DOI] [PubMed] [Google Scholar]
- 14. Falla TJ, Crook DWM, Brophy LN. et al. PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol 1994; 32: 2382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lau SK, Woo PC, Mok M. et al. Characterization of Haemophilus segnis, an important cause of bacteremia, by 16S rRNA gene sequencing. J Clin Microbiol 2004; 42: 877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meats E, Feil EJ, Stringer S. et al. Characterization of encapsulated and non-encapsulated Haemophilus influenzae and determination of phylogenetic relationship by multilocus sequence typing. J Clin Microbiol 2003; 41: 1623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molina JM, Cordoba J, Diosdado N. et al. Haemophilus influenzae and betalactam resistance: description of the blaTEM gene deletion. Rev Esp Quimioterap 2003; 16: 195–203. [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-sixth Informational Supplement M100-S26. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 19. Skaare D, Anthonisen IL, Kahlmeter G. et al. Emergence of clonally related multidrug resistant Haemophilus influenzae with penicillin-binding protein 3-mediated resistance to extended-spectrum cephalosporins, Norway, 2006 to 2013. Euro Surveill 2014; 49: 20986. [DOI] [PubMed] [Google Scholar]
- 20. Dabernat H, Delmas C, Seguy M. et al. Diversity of β-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob Agents Chemother 2003; 46: 2208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 6.0, 2016. www.eucast.org.
- 22. Resman F, Ristovski M, Forsgren A. et al. Increase of β-lactam-resistant invasive Haemophilus influenzae in Sweden, 1997-2010. Antimicrob Agents Chemother 2012; 56: 4408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park C, Kim K-H, Shin N-Y. et al. Genetic diversity of the ftsI gene in β-lactamase-nonproducing ampicillin-resistant and β-lactamase-producing amoxicillin/clavulanic acid-resistant nasopharyngeal Haemophilus influenzae strains isolated from children in South Korea. Microb Drug Resist 2013; 19: 224–30. [DOI] [PubMed] [Google Scholar]
- 24. Garcia-Cobos S, Arroyo M, Perez-Vazquez M. et al. Isolates of β-lactamase-negative ampicillin-resistant Haemophilus influenzae causing invasive infections in Spain remain susceptible to cefotaxime and imipenem. J Antimicrob Chemother 2013; 69: 111–6. [DOI] [PubMed] [Google Scholar]
- 25. Skaare D, Anthonisen IL, Caugant DA. et al. Multilocus sequence typing and ftsI sequencing: a powerful tool for surveillance of penicillin-binding protein 3-mediated β-lactam resistance in nontypeable Haemophilus influenzae. BMC Microbiol 2014; 14: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kishii K, Chiba N, Morozumi M. et al. Diverse mutations in the ftsI gene in ampicillin-resistant Haemophilus influenzae isolates from pediatric patients with acute otitis media. J Infect Chemother 2010; 16: 87–93. [DOI] [PubMed] [Google Scholar]
- 27. Shiro H, Sato Y, Toyonaga Y. et al. Nationwide survey of the development of drug resistance in the pediatric field in 2000-2001, 2004, 2007, 2010, and 2012: evaluation of the changes in drug sensitivity of Haemophilus influenzae and patients’ background factors. J Infect Chemother 2015; 21: 247–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.