Abstract
Background
The use of antibiotic stewardship programmes (ASPs) is increasing in Asia, but their effectiveness in reducing antibiotic consumption and their impact on clinical outcomes is not known.
Objectives
To determine the impact of ASPs conducted in Asia on the consumption of antibiotics and on patients’ clinical outcomes.
Methods
We systematically searched the Embase and Medline (PubMed) databases for studies that compared antibiotic consumption or clinical outcomes of patients in an Asian hospital or clinic with an ASP (intervention) with those in a similar setting without an ASP (control). Meta-analyses of all-cause mortality and hospital-acquired infection (HAI) were performed using random-effects models.
Results
The search identified 77 studies of which 22 and 19 reported antibiotic usage and cost, respectively. Among these, 20 (91%) studies reported reduced antibiotic usage and 19 (100%) reported cost savings in the intervention group. Duration of antibiotic therapy was reduced in six of seven studies in association with an ASP. Rates of all-cause mortality and HAI were not significantly different between the intervention and control groups. However, mortality rates were significantly improved by ASPs using drug monitoring, while HAI rates were also improved by ASPs that included infection control or hand hygiene programmes.
Conclusions
In Asia, ASPs reduce antibiotic consumption in hospital and clinic settings and do not worsen clinical outcomes. The findings strongly support the broad implementation of antimicrobial stewardship interventions in hospital and clinic settings in Asia.
Introduction
Antimicrobial resistance (AMR) is a growing global threat of huge concern to countries around the world.1,2 An especially alarming aspect is the rapid global spread of multiresistant bacteria causing common infections.3 Antibiotic stewardship programmes (ASPs) are considered an important approach for optimizing the use of antimicrobial drugs, especially in clinical settings. They have been defined by the IDSA as ‘coordinated interventions designed to improve and measure the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy and route of administration’.4 Other organizations and professional societies have adopted similar definitions.5,6 When mounting an ASP, the WHO has highlighted the need to engage professional and civil societies and patient organizations, and to take into consideration the local factors that drive sub-optimal use in different settings.7 Guidelines and recommendations for the proper development and implementation of ASPs have also been published.8,9
The effectiveness and economic impact of ASPs have been evaluated in previous systematic reviews10–12 and in specific settings including inpatient wards13 or critical care facilities.14 Some have focused on particular infections, e.g. Clostridium difficile.15 However, there is a lack of such studies from countries in Asia, which differ from western countries in many aspects. A recent international survey of ASPs in hospitals revealed that authorization to use restricted antibiotics was needed in 88% of ASPs implemented in Europe and 87% in North America, but only 38% in Asia.16 Another survey on non-compliance with antibiotic therapy for acute community infections reported that the proportion of respondents admitting non-compliance was highest in China (44%) and Japan (34.4%) and lowest in the Netherlands (9.9%) and Italy (11.2%).17 Other differences between Asian and western countries include public hygiene,18 communication between doctors and patients,19 people’s knowledge and attitudes about antibiotics,20 and so on. These differences are important determinants of the effectiveness of antimicrobial stewardship. This study aimed to use published data to assess the effectiveness of ASPs conducted in Asia in reducing the use of antibiotics, examine their impact on clinical outcomes and assess the findings within the framework of the recently published guidelines on proper implementation of ASPs by the IDSA.9
Methods
Search strategy and selection criteria
On 20 June 2016, we searched the Embase and Medline (PubMed) databases for three sets of terms (Appendix S1, available as Supplementary data at JAC Online): (i) ‘antimicrobial’ or equivalent terms such as ‘antibiotics’ or ‘antibacterial’; (ii) ‘stewardship’ and interventions of an ASP such as ‘formulary restriction’, ‘pre-authorization’ or ‘prospective audit’; and (iii) South Asia, South-East Asia or East Asia and individual countries within these areas.
We conducted this systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21
The titles and abstracts of studies satisfying these criteria were independently screened by two authors (C. F. L. and H. A.) who then reviewed the full texts of potentially relevant studies for eligibility. In this study, we defined an ASP as a hospital- or clinic-based programme that included an intervention or component the purpose of which was to reduce the transmission of AMR. Studies were included in our analysis if: (i) they compared outcomes in a hospital or clinic with an ASP (intervention) with those in a similar setting without an ASP (control); (ii) they reported the consumption of antibiotics or clinical outcomes in patients; and (iii) they had a hospital or a clinic setting. Studies reporting sufficiently detailed information were included in the meta-analysis. Conference abstracts, letters, editorials, review or perspective articles and qualitative studies were excluded. Selection results from the two reviewers were compared and a consensus was reached, with the involvement of a third author (S. F.) in case of discrepancies or disagreements. No language restriction was applied in screening articles. Non-English articles were read and translated as needed by the three authors, with the aid of Google translate if necessary.
Data extraction
We used a standardized form to record aggregate data on antibiotic consumption, duration of antibiotic treatment and the cost of antibiotics, as well as clinical outcomes such as mortality, hospital-acquired infections (HAIs) and length of stay in the hospital. Some studies reported two or more intervention groups, each individually compared with a control group. If the interventions were different (e.g. a three-arm randomized controlled trial), outcomes from all groups were included; otherwise if there was an intervention group with more than one intervention implemented (e.g. a factorial-design randomized controlled trial), the group using the most interventions was selected. For studies comparing the outcomes before and after the implementation of an ASP, if the pre- and/or post-implementation periods were divided into shorter time periods, we extracted the data in the period immediately before the implementation, which should be the most comparable with the post-implementation period, and the latest period after the implementation in order to examine the long-term effect. In the analysis, we included the results of all whole-group analyses whenever they were reported and included the results of subgroup analyses only when those of the whole-group analyses were not presented.
The interventions of an ASP were classified into the following categories: (i) education and training; (ii) pre-authorization, prospective audit, feedback, review, consultation and recommendation; (iii) drug control or monitoring, formulary restriction and intravenous-to-oral conversion; (iv) computerized system use; (v) intervention by infectious disease experts; (vi) antibiotic rotation; (vii) institutional guideline; (viii) national guideline; and (ix) infection control and hand hygiene programme.
Statistical analysis
Study characteristics such as country and type of intervention implemented in the ASP were summarized. The number and proportion of studies reporting an improved or deteriorated outcome in the ASP in relation to the control cohort were computed. Since the variation in the difference between the ASP and the control groups was usually not reported, we did not perform meta-analysis for continuous outcomes. For mortality and HAI, studies were included in the meta-analysis if the number of events and total cases of both the ASP and control cohorts were reported. In the meta-analysis, the reported results were pooled using a random-effects model. Statistical heterogeneity was assessed using the I2 statistic and tested by Cochran’s Q test.22 In cases in which substantial heterogeneity was found, subgroup analyses were performed by stratifying by the type of intervention. If the heterogeneity could not be reduced, further subgroup analyses by country, outcome or patient characteristics would be performed. We also examined how often ASPs used interventions recommended by the IDSA 2016 guidelines.9 We addressed some items that are hospital-wide for all patients (i.e. not limited to specific specialties such as paediatrics) in which no special investigation (e.g. rapid viral testing) was required. All analyses were conducted using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria) and the metafor package.23
Results
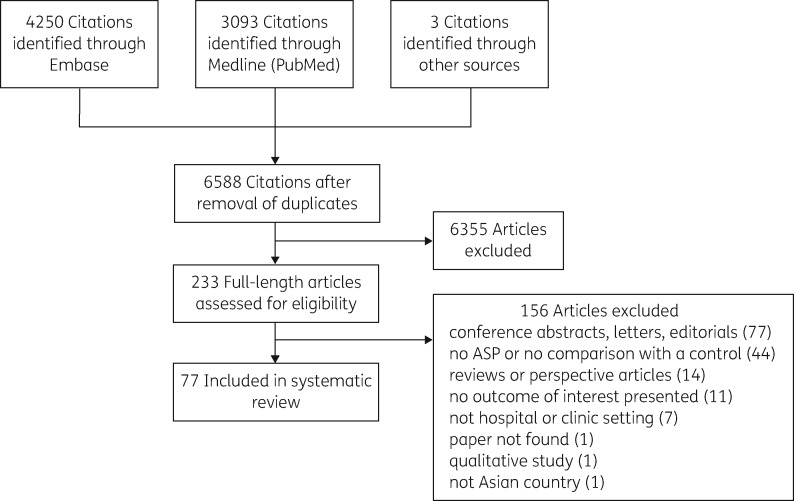
Our search identified 6588 unique article citations, of which 6355 were excluded based on screening of the title and abstract, and, when necessary, review of the full-length text (Figure 1). Seventy-seven studies met our inclusion criteria (Table S1) and were published between 1991 and 2016. They came from China (16), Japan (9), Thailand (9), Taiwan (8), Singapore (7), India (6), South Korea (5), Hong Kong (5), Vietnam (3), Bangladesh (2), Indonesia (2), Iran (2), Sri Lanka (1), Pakistan (1) and Cambodia (1).
Figure 1.
Flow diagram of study selection.
Most (67/77, 87%) of these studies involved only one hospital, but five studies involved 2 to 20 institutions (Table 1). Five studies evaluated the effectiveness of a national guideline for antibiotic utilization and four of them surveyed the use of antibiotics in 15 to 1625 hospitals or clinics; the other was a study that extracted antibiotic consumption data from the claims database of the Korean National Health Insurance system. These five studies did not mention how many hospitals implemented an ASP or what interventions were used in the ASP. Sixty studies assessed outcomes before and after implementation of the ASP. Eleven studies used control groups in different units within the same institute or in different institutes. Five studies compared the outcomes of the patients whose physicians either complied with, or did not comply with, ASP interventions. One study investigated the association between the use of hand cleaner and clinical outcomes. Most of the reported ASPs consisted of more than one intervention. The most common categories of ASP interventions were pre-authorization, prospective audit, feedback, review, consultation and recommendations (29), education and training (23) and institutional guidelines (18). Two additional interventions, financial penalties (the prescribing physicians being fined for inappropriate prescriptions) and public reporting (physicians’ performance data being publicly released), were used in two ASPs in China.
Table 1.
Characteristics of the selected studies
| Characteristic | n | % |
|---|---|---|
| Number of studies included | 77 | 100 |
| Number of hospitals or clinics involved | ||
| 1 | 67 | 87 |
| 2 | 1 | 1.3 |
| 3 | 2 | 2.6 |
| 15 | 2 | 2.6 |
| 20 | 1 | 1.3 |
| 65 | 1 | 1.3 |
| 226 | 1 | 1.3 |
| 1625 | 1 | 1.3 |
| all hospitals and clinics from the Korean National Health Insurance claim database | 1 | 1.3 |
| Mode of comparison | ||
| before versus after | 60 | 78 |
| comparative studies | 11 | 14 |
| cluster-randomized trials | 4 | 5.2 |
| non-randomized trials | 7 | 9.1 |
| compliant versus non-compliant participants | 5 | 6.5 |
| association with use of hand cleaner | 1 | 1.3 |
| Component or feature of the ASP | ||
| education, training, newsletters | 23 | 30 |
| pre-authorisation, prospective audit, feedback, review, consultation and recommendation | 29 | 38 |
| drug control or monitoring, formulary restriction | 14 | 18 |
| computerized system | 4 | 5.2 |
| intervention by infectious disease experts | 9 | 12 |
| antibiotic rotation | 3 | 3.9 |
| institutional guideline | 19 | 25 |
| national guideline | 5 | 6.5 |
| infection control and hand hygiene programme | 14 | 18 |
| others | 2 | 2.6 |
Consumption of antibiotics between the ASP and the control groups was compared in 22 studies. Twenty (91%) studies reported a reduction in the use of antibiotics associated with an ASP. Two studies reported a marginal increase in antibiotic consumption (0.1 DDDs per 1000 patient-days and 0.64 DDDs per 100 bed-days respectively). The estimated decrease in antibiotic usage ranged from 7.2 to 290 DDDs per 1000 patient-days in 12 studies that evaluated the absolute consumption, or 1.4 to 29.9 percentage points in five studies that assessed the proportion of patients prescribed antibiotics. Duration of antibiotic therapy was reported in seven studies and six of them showed a reduction ranging from 0.6 to 3.3 days. All 19 studies examining costs found that the ASP interventions reduced costs spent on antibiotics. Specifically, cost savings ranged from 12% to 73% in 12 studies recording the average antibiotic cost per patient or patient-day.
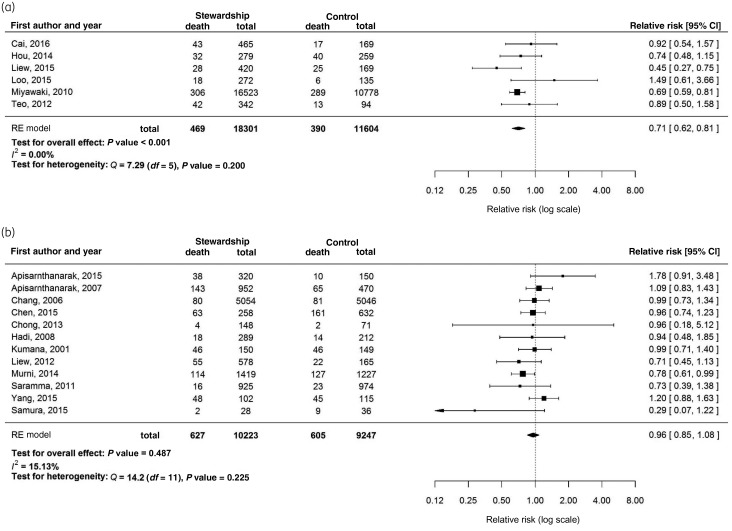
All-cause mortality of inpatients was reported in 21 studies. The point estimate of the mortality rate was higher in the control group than in the ASP group in 15 (71%) studies, but only 3 studies demonstrated a significant reduction in mortality by the ASP intervention. The pooled relative risk of mortality rate was 0.88 (95% CI = 0.77–1.001, P = 0.052) for 18 studies that provided the numbers of deaths and total cases in the ASP and control groups. Due to the significant heterogeneity (I2 = 45.59%, P = 0.010), a stratified meta-analysis was performed on the studies describing an ASP with and without drug control or monitoring, formulary restriction or intravenous-to-oral conversion. The pooled relative risk for 12 ASPs without drug control was 0.96 (95% CI = 0.85–1.08, P = 0.487), whereas for the 6 ASPs with drug control it was 0.71 (95% CI = 0.62–0.81, P < 0.001) (Figure 2). In a sensitivity analysis, by excluding the dominant study (Miyawaki et al. 201024), the pooled relative risk for the remaining five ASPs with drug control increased to 0.77 and became non-significant (95% CI = 0.56–1.07, P = 0.12). In particular, eight studies reported both mortality rate and antibiotic use. All of them demonstrated a reduction in antibiotic use. Only one study showed an increased mortality rate (8.2%–8.8%, relative risk = 1.07, P = 0.3) and the other seven had a relative risk ranging between 0.74 and 0.99.
Figure 2.
Forest plots of the impact of stewardship programmes on mortality rates. (a) Studies reporting a stewardship programme with drug control or monitoring, formulary restriction or intravenous-to-oral conversion. (b) Studies reporting a stewardship programme without drug control or monitoring, formulary restriction or intravenous-to-oral conversion.
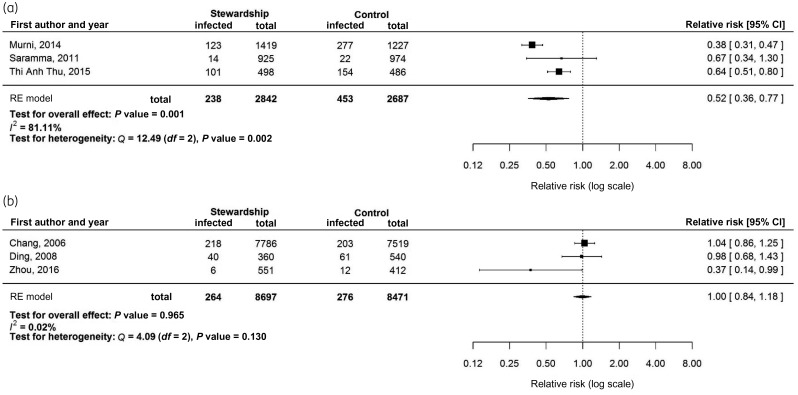
Thirteen studies compared HAI rates between the ASP and the control groups. All but one obtained a lower point estimate of the HAI rate in the ASP group than in the control group, but only three (23%) were significant. Six studies provided sufficient details for meta-analysis, which resulted in a significant protective effect (pooled relative risk = 0.66, 95% CI = 0.46–0.95, P = 0.025). However, there was significant heterogeneity (I2 = 88.33%, P < 0.001), so a stratified meta-analysis was conducted using studies with or without an infection control or hand hygiene programme. The pooled relative risk for the studies with an infection control or hand hygiene programme was 0.52 (95% CI = 0.36–0.77, P = 0.001), whereas studies without infection control no longer demonstrated protection against HAI (pooled relative risk = 1.00, 95% CI = 0.84–1.18, P = 0.965) (Figure 3). By excluding Chang et al.25 (2006), the pooled relative risk for studies without infection control remained non-significant (relative risk = 0.61, 95% CI = 0.24–1.52, P = 0.287). Seven studies reporting other infection or re-infection also demonstrated a protective effect by the ASPs, with the relative risk ranging between 0 and 0.68.
Figure 3.
Forest plots of the impact of stewardship programmes on hospital-acquired infection rates. (a) Studies reporting a stewardship programme with an infection control or hand hygiene programme. (b) Studies reporting a stewardship programme without an infection control or hand hygiene programme.
Among 15 studies reporting the length of hospital stay, eight studies (53%) showed an average reduction between 1.6 and 14 days associated with an ASP; four studies (27%) reported a prolongation of hospitalization of 0.1 to 4 days; and the remaining study found no difference.
We selected nine recommendations from the IDSA 2016 guideline. These recommendations are not limited to some particular medical specialty but are hospital-wide for all patients, and require no special investigation such as rapid viral testing. We assessed the proportions of antimicrobial stewardship interventions that implemented these recommendations (Table 2). Nineteen of the 77 studies followed the IDSA recommendation of using prospective audit and feedback (item I). Among the 23 studies including education as an intervention, as suggested by the IDSA, 19 studies also used other complementary interventions in tandem (item II). Also, none of the 19 studies reporting the cost of antibiotics mentioned that the calculation was based on purchasing data (item XXI). The IDSA guideline suggests against the use of antibiotic cycling; in this review, only three studies reported the use of antibiotic rotation (item VIII). However, some of the IDSA recommendations were not commonly followed. For example, only 1 out of 15 studies that included a form of review of the appropriateness of antibiotic prescription involved the prescriber in the review process (item VI) and no study mentioned the shortest effective duration of antibiotic therapy in their report (item XIII).
Table 2.
Selected recommendations from the IDSA 2016 guideline
| Item | Recommendations | Number of studies |
|---|---|---|
| I. Does the use of pre-authorization and/or prospective audit and feedback interventions by ASPs improve antibiotic utilization and patient outcomes? | Strongly recommend pre-authorization and/or prospective audit and feedback over no such interventions. | Nineteen studies reported the implementation of an intervention of pre-authorization, prospective audit and feedback or immediate concurrent feedback (the older terminology for prospective audit and feedback33). |
| II. Is didactic education a useful antibiotic stewardship intervention for reducing inappropriate antibiotic use? | Suggest that educational activities should be complemented by other stewardship activities. | Among the 23 studies that included education as an intervention, 19 studies used other stewardship interventions in tandem. |
| VI. Do strategies to encourage prescriber-led review of appropriateness of antibiotic regimens, in the absence of direct input from an antibiotic stewardship team, improve antibiotic prescribing? | Suggest the use of strategies to encourage prescribers to perform routine review of antibiotic regimens to improve antibiotic prescribing. | Fifteen studies reported some kind of review of the appropriateness of antibiotic prescription, mostly by infectious disease physicians, pharmacists, infection control teams or antimicrobial stewardship teams, or only reviewed summary statistics in monthly meetings. Only one study explicitly mentioned the involvement of the prescriber in the review process. |
| VII. Should computerized clinical decision support systems integrated into the electronic health record at the time of prescribing be incorporated as part of ASPs to improve antibiotic prescribing? | Suggest incorporation of computerized clinical decision support at the time of prescribing into ASPs. | Four studies reported the results of an ASP in which a computerized system was installed to guide or restrict antibiotic prescription. |
| VIII. Should ASPs implement strategies that promote cycling or mixing in antibiotic selection to reduce antibiotic resistance? | Suggest against the use of antibiotic cycling as a stewardship strategy. | Three studies reported the use of antibiotic rotation as a component of the ASP. |
| XI. Should ASPs implement interventions to increase use of oral antibiotics as a strategy to improve outcomes or decrease costs? | Strongly recommend ASPs implement programmes to increase both appropriate use of oral antibiotics for initial therapy and the timely transition of patients from intravenous to oral antibiotics. | Ten studies included conversion from intravenous to oral antibiotics, or restricted the use of some intravenous antibiotics as an intervention of the ASPs. Among them, seven studies reported cost savings while the other three did not evaluate antibiotic costs. Most studies reported reductions in the duration of therapy and/or length of stay in hospital. None of these ten studies showed an increase in mortality or infection rate. |
| XIII. Should ASPs implement interventions to reduce antibiotic therapy to the shortest effective duration? | Strongly recommend that ASPs implement guidelines and strategies to reduce antibiotic therapy to the shortest effective duration. | No study in this review defined or mentioned the shortest effective duration of the antibiotic therapy. |
| XX. Which overall measures best reflect the impact of ASPs and their interventions? | Suggest monitoring antibiotic use as measured by days of therapy (DOTs) in preference to DDD. | Fourteen studies reported antibiotic use measured in DDD, and 7 studies reported it in DOT. |
| XXI. What is the best measure of expenditures on antibiotics to assess the impact of ASPs and interventions? | Recommend measuring antibiotic costs based on prescriptions or administrations instead of purchasing data. | Nineteen studies reported the cost of antibiotics. None of them mentioned that the calculation was based on purchasing data. |
Discussion
We found that active ASPs are being conducted throughout Asia with a total of 77 studies from 15 countries meeting the selection criteria (Table 1). The primary objective of antimicrobial stewardship is to reduce the inappropriate use of antibiotics. The studies included in this review demonstrated that ASPs in Asia are associated with reduced usage of antibiotics with no deterioration in clinical outcomes. Almost all studies evaluating the consumption of antibiotics (20/22 studies) or the duration of antibiotic therapy (6/7 studies) reported a reduction in antibiotic use. Moreover, all 19 studies that provided the evaluation of antibiotic costs showed that the interventions decreased expenditure on antibiotics compared with the control group. These results were comparable with those of reviews of ASPs in western countries.10,13
All-cause mortality of inpatients was not significantly increased in the reviewed ASPs as shown in the meta-analysis (Figure 2), as well as in the eight programmes that reduced the amount of antibiotics used. This showed that the ASPs were successful in attaining the objective of reducing the use and hence cost of antibiotics without compromising clinical outcomes. This finding is important because of concerns that ASPs might result in inadequately treated infections and increased mortality.26 The above finding suggests that ASPs most likely reduce the use of antibiotics in patients who do not need them. It was also interesting to note that for some programmes with drug monitoring or control, formulary restriction or intravenous-to-oral conversion, the mortality rates were even further reduced. Similarly, Schuts et al.10 revealed that reduced mortality was associated with switching from intravenous to oral therapy, therapeutic drug monitoring and use of restricted antibiotics. A possible reason for this observation is that these ASP interventions reduce the risk of adverse events caused by antibiotics. Based on these findings, we believe future ASPs should include drug monitoring or control or formulary restriction to help improve the appropriate use of antibiotics. Moreover, for studies that included an infection control or hand hygiene programme, the rate of HAI was significantly decreased (Figure 3). This is not unexpected because proper infection control and hand hygiene has already been shown to save lives.27 Therefore, all ASPs should include infection control and a hand hygiene programme.
In this review, we compared the ASP interventions implemented in Asia against the recommendations made in the IDSA 2016 guidelines.9 We selected the IDSA guidelines for two reasons. First, these recommendations were recently developed by a panel of experts using a well-validated Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.28 Second, these recommendations are explicit, specific and directly related to ASP interventions.29 A large variety of antimicrobial stewardship interventions were reported in Asia. Some of them were in line with the IDSA recommendations9 (Table 2), such as adding complementary stewardship interventions to passive educational programmes (item II) and the use of prescribing rather than purchasing data in calculating the antibiotic costs (item XXI). However, some IDSA practices were seldom reported; for example, prescriber-led review of the appropriateness of antibiotics (item VI) and reduction to the shortest effective duration in antibiotic therapy (item XIII). It was not clear whether these practices were not performed or were not reported.
We found it difficult to report some results (Table 3). For reporting of continuous outcomes including the use of antibiotics, the duration of antibiotic treatment and the cost of antibiotics prescribed, many studies only provided the point estimates of the ASP and the control groups, or difference between them, without mentioning variation in the outcomes (standard deviation) or the estimates (standard error). This made it more difficult to pool the results from various studies in a meta-analysis. Previous reviews of antimicrobial stewardship studies also identified this under-reporting problem.10 Moreover, for reporting of antibiotic costs, some studies summarized the total cost spent in the respective periods instead of the average cost per patient in a cohort design comparing pre- and post-implementation of the ASP. Without a standardized measure, the antibiotic cost between the pre- and post-implementation periods could not be explicitly compared. The reporting of mortality rate was better in the sense that most studies (18/20) that reported the all-cause mortality provided the numbers of deaths and total cases in both groups, sufficient for pooling the results in a meta-analysis. However, the information on HAIs was under-reported. Among the 13 studies comparing the HAIs between the ASP and control groups, only six studies provided the numbers of deaths and relative denominators in the intervention and control groups, which are necessary for pooling the estimates in a meta-analysis. Moreover, some studies that compared the outcomes before and after the implementation of the ASP divided the pre- and post-implementation periods into two or more shorter time frames and presented the outcomes for each time frame. In this review, we extracted the data from periods immediately before the implementation and the latest period after the implementation for ease of comparing the results from different studies. However, it would be ideal if the aggregated results for the whole periods before and after the implementation of the ASP were also presented. Future studies on ASPs are recommended to provide additional aggregated outcomes for the pre- and post-implementation periods.
Table 3.
Suggested reporting of an ASP
| Area for improvement | Suggested reporting |
|---|---|
|
|
|
|
|
|
|
|
Our review has limitations. First, we only reviewed those ASPs published in full papers and some programmes might have been missed. We excluded conference abstracts and letters because details of the intervention and the results were usually not described. Second, we only included outcomes of the patients in hospitals or clinics. However, antimicrobial stewardship might have longer-term benefits and a complete evaluation of the impact of ASPs would extend beyond hospitals or clinics where the ASPs are implemented. These studies did not allow an assessment of the potential impact of ASPs on the community. Third, we did not assess the risk of bias of the selected studies.30 A large proportion (60/77 studies) of the studies included in our review were cohort studies comparing the pre- and post-implementation periods and hence the results may be confounded by other factors. These confounding factors, however, were not usually adjusted for in these studies. The number and characteristics of the healthcare workers involved are important considerations in ASPs as they induce human cost to the programmes, but most of the studies did not report this information. Nevertheless, two Hong Kong studies may shed some light on this issue: in an ASP with concurrent feedback and intravenous-to-oral conversion, the extra cost of the part-time audit nurse and the oral antibiotics was offset by the savings on intravenous drugs.31 In another ASP using education and concurrent feedback, the extra human costs were also offset by savings from antibiotic expenditure.32
In conclusion, this review shows that ASPs are being initiated in Asia although the number of hospitals reported in studies is relatively limited. Our findings strongly support the importance of expanding such programmes, using established guidelines and ensuring that aspects such as infection prevention and control are well integrated with antimicrobial stewardship activities. Finally, this study underscores the critical importance of providing professional training for hospitals in the region.
Supplementary Material
Acknowledgements
We thank Julie Au for technical assistance.
Funding
This project was supported by a commissioned grant from the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region (reference no. HKS-17-E11) and the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558).
Transparency declarations
B. J. C. has received research funding from Sanofi Pasteur for a study of influenza vaccine effectiveness. All other authors: none to declare.
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
C. F. L. designed the study. C. F. L., H. A., S. F. and P. W. conducted the literature search. C. F. L., S. F. and H. A. extracted the data from the included articles. C. F. L. analysed the data. C. F. L., B. J. C., K. F. and W. H. S. interpreted the results. C. F. L. wrote the first draft. All authors read and approved the final manuscript.
Supplementary data
Appendix S1 and Table S1 are available as Supplementary data at JAC Online.
References
- 1. Gould IM, Lawes T.. Antibiotic stewardship: prescribing social norms. Lancet 2016; 387: 1699–701. [DOI] [PubMed] [Google Scholar]
- 2. Wernli D, Haustein T, Conly J. et al. A call for action: the application of The International Health Regulations to the global threat of antimicrobial resistance. PLoS Med 2011; 8: e1001022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. Global Antimicrobial Resistance Surveillance System: Manual for Early Implementation. Geneva, Switzerland: WHO, 2015. http://apps.who.int/iris/bitstream/10665/188783/1/9789241549400_eng.pdf. [Google Scholar]
- 4. IDSA, USA. Promoting Antimicrobial Stewardship in Human Medicine https://www.idsociety.org/Stewardship_Policy/.
- 5. NICE, UK. Antimicrobial Stewardship: Systems and Processes for Effective Antimicrobial Medicine Use.https://www.nice.org.uk/guidance/ng15/chapter/1-recommendations. [DOI] [PMC free article] [PubMed]
- 6. Association for Professionals in Infection Control and Epidemiology, USA. Antimicrobial Stewardship http://www.apic.org/Professional-Practice/Practice-Resources/Antimicrobial-Stewardship.
- 7. WHO. The Evolving Threat of Antimicrobial Resistance: Options for Action. Geneva, Switzerland: WHO, 2012. http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf. [Google Scholar]
- 8. Dellit TH, Owens RC, McGowan JE Jr. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 9. Barlam TF, Cosgrove SE, Abbo LM. et al. Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: 1197–202. [DOI] [PubMed] [Google Scholar]
- 10. Schuts EC, Hulscher ME, Mouton JW. et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. [DOI] [PubMed] [Google Scholar]
- 11. Dik JW, Vemer P, Friedrich AW. et al. Financial evaluations of antibiotic stewardship programs-a systematic review. Front Microbiol 2015; 6: 317.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davey P, Peden C, Charani E. et al. Time for action-improving the design and reporting of behaviour change interventions for antimicrobial stewardship in hospitals: early findings from a systematic review. Int J Antimicrob Agents 2015; 45: 203–12. [DOI] [PubMed] [Google Scholar]
- 13. Wagner B, Filice GA, Drekonja D. et al. Antimicrobial stewardship programs in inpatient hospital settings: a systematic review. Infect Control Hosp Epidemiol 2014; 35: 1209–28. [DOI] [PubMed] [Google Scholar]
- 14. Kaki R, Elligsen M, Walker S. et al. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 2011; 66: 1223–30. [DOI] [PubMed] [Google Scholar]
- 15. Feazel LM, Malhotra A, Perencevich EN. et al. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69: 1748–54. [DOI] [PubMed] [Google Scholar]
- 16. Howard P, Pulcini C, Levy Hara G. et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother 2015; 70: 1245–55. [DOI] [PubMed] [Google Scholar]
- 17. Pechere JC, Hughes D, Kardas P. et al. Non-compliance with antibiotic therapy for acute community infections: a global survey. Int J Antimicrob Agents 2007; 29: 245–53. [DOI] [PubMed] [Google Scholar]
- 18. Jean SS, Hsueh PR.. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents 2011; 37: 291–5. [DOI] [PubMed] [Google Scholar]
- 19. Claramita M, Utarini A, Soebono H. et al. Doctor-patient communication in a Southeast Asian setting: the conflict between ideal and reality. Adv Health Sci Educ Theory Pract 2011; 16: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gualano MR, Gili R, Scaioli G. et al. General population's knowledge and attitudes about antibiotics: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2015; 24: 2–10. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12. [DOI] [PubMed] [Google Scholar]
- 22. Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–29. [Google Scholar]
- 23. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 24. Miyawaki K, Miwa Y, Tomono K. et al. Impact of antimicrobial stewardship by infection control team in a Japanese teaching hospital. Yakugaku Zasshi 2010; 130: 1105–11. [DOI] [PubMed] [Google Scholar]
- 25. Chang MT, Wu TH, Wang CY. et al. The impact of an intensive antimicrobial control program in a Taiwanese medical center. Pharm World Sci 2006; 28: 257–64. [DOI] [PubMed] [Google Scholar]
- 26. Han YY, Carcillo JA, Venkataraman ST. et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics 2005; 116: 1506–12. [DOI] [PubMed] [Google Scholar]
- 27. Pittet D. Clean hands reduce the burden of disease. Lancet 2005; 366: 185–7. [DOI] [PubMed] [Google Scholar]
- 28. Guyatt GH, Oxman AD, Schunemann HJ. et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011; 64: 380–2. [DOI] [PubMed] [Google Scholar]
- 29. CDC, USA. Core Elements of Hospital Antibiotic Stewardship Programs.2014. https://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. [DOI] [PMC free article] [PubMed]
- 30. Greenland S. Quality scores are useless and potentially misleading: reply to “Re: A critical look at some popular analytic methods”. Am J Epidemiol 1994; 140: 300–1. [DOI] [PubMed] [Google Scholar]
- 31. Seto WH, Ching TY, Kou M. et al. Hospital antibiotic prescribing successfully modified by ′immediate concurrent feedback′. Br J Clin Pharmacol 1996; 41: 229–34. [DOI] [PubMed] [Google Scholar]
- 32. Ng CK, Wu TC, Chan WM. et al. Clinical and economic impact of an antibiotics stewardship programme in a regional hospital in Hong Kong. Qual Saf Health Care 2008; 17: 387–92. [DOI] [PubMed] [Google Scholar]
- 33. Chung GW, Wu JE, Yeo CL. et al. Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence 2013; 4: 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.