Abstract
Background: Biapenem, a carbapenem antibiotic, has been shown to have synergistic bactericidal anti-TB activity when combined with rifampicin both in vitro and in the mouse model of TB chemotherapy. We hypothesized that this synergy would result in biapenem/rifampicin activity against rifampicin-resistant Mycobacterium tuberculosis.
Objectives: Our objective was to evaluate the synergy of biapenem/rifampicin against both low- and high-level rifampicin-resistant strains of M. tuberculosis, in vitro and in the mouse model.
Methods: Biapenem/rifampicin activity was evaluated using three strains of M. tuberculosis: strain 115R (low-level rifampicin resistance); strain 124R (high-level rifampicin resistance); and the drug-susceptible H37Rv parent strain. Biapenem/rifampicin synergy was evaluated in vitro by chequerboard titration. In vivo, we first conducted a dose-ranging experiment with biapenem against H37Rv in the mouse model. We then evaluated biapenem/rifampicin activity in mice infected with each M. tuberculosis strain.
Results: In vitro, synergy was observed between biapenem and rifampicin against H37Rv and strain 115R. In vivo, biapenem exhibited clear dose-dependent activity against H37Rv, with all biapenem doses as active or more active than rifampicin alone. Biapenem and rifampicin had synergistic bactericidal activity against H37Rv in the mouse model; no synergy was observed in mice infected with either of the rifampicin-resistant strains. Biapenem alone was active against all three strains.
Conclusions: Our preclinical experiments indicate that biapenem has potential for use as an anti-TB drug, including for use against rifampicin-resistant TB. Thus, biapenem has promise for repurposing as a ‘new’ – and desperately needed – drug for the treatment of drug-resistant TB.
Introduction
In its most recent Global TB Report, the WHO estimated that 480 000 new cases of MDR-TB, plus an additional 100 000 cases of rifampicin-resistant TB, occurred in 2015.1 MDR-TB is notoriously difficult to treat, in part due to the lack of new antibiotics. While the need for the development of new compounds/drugs is essential, repurposing of existing drugs has the potential for more rapid implementation in the clinic and thus is also of high priority. Recently, carbapenems, a class of β-lactam antibiotics primarily used for the treatment of infections caused by drug-resistant Gram-negative bacteria, have shown promise for use against mycobacterial infections.2–7
Traditionally, β-lactam antibiotics have not been effective against Mycobacterium tuberculosis, largely due to the presence of a highly active β-lactamase (BlaC).8 However, carbapenems are unique in that they are poor substrates for BlaC and they target a broader range of transpeptidases, enzymes that catalyse the cross-linking of peptidoglycan residues. Specifically, carbapenems inhibit d,d-transpeptidases, which catalyse the formation of 4→3 transpeptide linkages, and also inhibit the non-canonical l,d-transpeptidases, which catalyse the formation of 3→3 transpeptide linkages.3,9 The l,d-transpeptidases LdtMt1 and LdtMt2 are utilized extensively by M. tuberculosis in the formation of its peptidoglycan network;9,10 furthermore, lack of these enzymes is associated with altered cell physiology and morphology and reduced virulence.11,12 Thus, repurposing carbapenems to target mycobacterial l,d-transpeptidases represents a highly promising alternative treatment strategy for MDR-TB with the clear potential for implementation in the clinical setting.
Many carbapenems are hydrolysed by human renal dehydropeptidase-I (DHP-I) and in practice must be co-administered with a DHP-I inhibitor.13 Efforts to identify more stable carbapenems led to the development of biapenem, which is highly resistant to hydrolysis and thus does not require protection from DHP-I.14,15 Recent work by our group and others has demonstrated that biapenem has anti-TB activity, including activity against drug-resistant strains of M. tuberculosis;5,16 additionally, our group has found that biapenem is active in vivo against M. tuberculosis in the mouse model of acute TB.17 As pharmacokinetic studies in healthy volunteers indicate that mycobactericidal blood concentrations of biapenem can be obtained with safe dosing,18,19 it is now imperative to advance our understanding of biapenem as an anti-TB drug, especially for use as a desperately needed agent against drug-resistant M. tuberculosis. Our objective was to further characterize the in vitro anti-TB activity of biapenem and to validate the in vivo anti-TB activity of this carbapenem in mouse models of TB (including drug-resistant TB) chemotherapy.
Materials and methods
All experimental work was performed at the Johns Hopkins University School of Medicine, Baltimore, MD, USA. Work with live bacteria and infected animals was performed in biosafety level 3 facilities.
Ethics
All animal procedures were performed as per protocol MO15M25 approved by the Johns Hopkins University Animal Care and Use Committee in adherence to the national guidelines.
Bacterial strains and growth conditions
Rifampicin mono-resistant M. tuberculosis strains 115R and 124R and their parent strain H37Rv were used in this study. Strains 115R and 124R were isolated and characterized by Rosenthal et al. as described.20 Briefly, mice were infected with aerosolized cultures of M. tuberculosis H37Rv and rifampicin was administered once daily (5 days/week) by oral gavage for 8 weeks. Next, lungs were harvested and plated on Middlebrook 7H10 agar containing rifampicin as a selection agent. Two strains were isolated and labelled 115R and 124R. The MIC of rifampicin was 4 mg/L for 115R and 128 mg/L for 124R. Next, the rifampicin resistance-determining region was amplified and this DNA was sequenced. Strains 115R and 124R harbour point mutations in RpoB leading to L533P and S531L substitutions, respectively. All strains were grown in supplemented Middlebrook 7H9 broth as previously described.5
Drug preparation and administration
All drugs were purchased from Sigma. For in vitro experiments, drugs were first dissolved in water and then subsequently diluted when added to media. For in vivo experiments, drugs were dissolved in distilled water at concentrations to deliver the desired dose in 0.2 mL. Isoniazid and rifampicin were administered by oral gavage; biapenem was administered by subcutaneous injection. All drugs were prepared weekly and stored at 4 °C, except biapenem which was stored at −20 °C.
MIC, chequerboard titration assay and frequency of resistance to biapenem and rifampicin
The standard broth micro-dilution method21 was used to determine MICs of biapenem, rifampicin and isoniazid against M. tuberculosis strains. In summary, each strain was grown in Middlebrook 7H9 broth under the aforementioned conditions to exponential phase and the suspensions were used to inoculate 105 cfu into each well (96-well plate) containing a drug at 2-fold dilutions ranging from 64 to 0.06 mg/L. Broth without drug, but inoculated with bacteria, was used as a positive control for growth while the broth alone served as the negative control. As per CLSI guidelines,22 cultures were incubated at 37 °C and evaluated for growth by visual inspection at 14 days. The MIC90 was defined as the lowest concentration that inhibited growth by 90% compared with the no drug control. Data presented here are the average of two biological replicates. Chequerboard titration assays for rifampicin–biapenem combinations were performed as previously described5 to determine the fractional inhibitory concentration index (FICI). Combinations were considered synergistic if the FICI was ≤0.5, antagonistic if the FICI was >4.0 and to have no interaction if the FICI was >0.5 to 4.0. The frequency of spontaneous phenotypic resistance to biapenem (80 mg/L) and rifampicin (10 mg/L) was determined on drug-containing 7H10 agar as previously described.5
Animals
Female BALB/c mice (age 4–5 weeks) purchased from Charles River Laboratories were used in all experiments. The mice were housed in individually ventilated cages (up to five mice per cage). Room temperature was maintained (22–24 °C) with a 12 h light/dark cycle. All infections were done via the aerosol route within a Glas-Col Inhalation Exposure System, as per the manufacturer’s instructions using 10 mL of bacterial suspension. In all experiments, five mice per treatment group (and untreated control mice) were sacrificed at each stated time point. The mice were sacrificed by cervical dislocation under anaesthesia; lungs were dissected, transferred to sterile PBS, homogenized, and plated for quantitative cfu counts on 7H11 selective agar as previously described.5
Biapenem dose-ranging experiment in an early-phase acute infection model
Mice were infected with an M. tuberculosis H37Rv suspension of A600 ∼0.2. Treatment was initiated 3 days after infection with one of the following regimens: (i) no drug, negative control, bacterial counts should increase throughout the experiment; (ii) isoniazid at 10 mg/kg, a positive control with bactericidal activity in this model; (iii) rifampicin at 10 mg/kg, a positive control with bacteriostatic activity limiting bacterial growth in this model; and (iv) biapenem at 10 different dosing schemes: 50, 100, 200, 300 and 400 mg/kg administered once daily (q24h) or twice daily (q12h). Treatment was administered daily (7 days per week) for 4 weeks.
Biapenem activity in a late-phase acute infection model
Mice were infected with an M. tuberculosis H37Rv suspension of A600 ∼0.2. Treatment was initiated 2 weeks after infection with one of the following regimens: (i) no drug, negative control, bacterial counts should increase throughout experiment and mice should become moribund by 4 weeks after infection; (ii) isoniazid at 10 mg/kg, a positive control with bactericidal activity in this model; (iii) rifampicin at 10 mg/kg, a positive control with bactericidal activity in this model; and (iv) biapenem at 200 mg/kg twice daily (q12h). Treatment was administered daily (7 days per week) for 4 weeks.
Biapenem activity in mouse models of rifampicin-resistant TB
M. tuberculosis strains H37Rv, 115R and 124R were grown to exponential phase, and suspensions with an A600 of 0.02 were prepared for aerosol infections. Treatment was initiated 1 week after infection with one of the following regimens: (i) no drug, negative control, bacterial counts should increase throughout experiment; (ii) isoniazid at 10 mg/kg, a positive control with bactericidal activity in this model; (iii) rifampicin at 20 mg/kg, a positive control with bactericidal activity in this model; and (iv) biapenem at 300 mg/kg twice daily (q12h). Treatment was administered daily (7 days per week) for 8 weeks. For this experiment, mouse lung homogenates were plated on both plain and 0.04% activated-charcoal containing 7H11 selective agar plates to prevent any effects of drug carryover.23
Results
Biapenem exhibits activity in vitro against rifampicin-resistant M. tuberculosis
We recently observed in vitro synergy between rifampicin and biapenem against the M. tuberculosis reference laboratory strain H37Rv (FICI 0.24),5 and this synergy was confirmed in vivo in the mouse model of acute TB, where biapenem alone and rifampicin alone exerted bacteriostatic activity against M. tuberculosis, but when co-administered were highly and rapidly bactericidal.17 We therefore investigated if this dramatic increase in susceptibility to rifampicin in the presence of biapenem also occurred with rifampicin-resistant M. tuberculosis, specifically strain 115R with low-level rifampicin resistance and strain 124R with high-level resistance, as well as the parent H37Rv strain.20 Again, we observed synergy between rifampicin and biapenem against strain H37Rv (FICI 0.28) (Table 1). While rifampicin and biapenem did not exhibit synergy in activity against the high-level rifampicin-resistant strain 124R, synergistic activity was observed for these two drugs against the low-level rifampicin-resistant strain 115R (FICI 0.15). Thus, in vitro, biapenem at concentrations well below the maximum serum concentration obtained in humans (17.35 or 32 mg/L following single intravenous doses of 300 or 600 mg, respectively)14,19 has activity alone against M. tuberculosis, and synergy with rifampicin that may permit activity of both drugs against low-level rifampicin-resistant strains.
Table 1.
MICs of rifampicin and biapenem, alone and together, for M. tuberculosis strains
| Drug(s) | MIC (mg/L) for the following M. tuberculosis strains |
||
|---|---|---|---|
| H37Rv | 115R | 124R | |
| Isoniazid alone | 0.03–0.06 | 0.03–0.06 | 0.03–0.06 |
| Rifampicin alone | 0.256–0.512 | 2–4 | 64–128 |
| Biapenem alone | 2–4 | 4–8 | 8–16 |
| Rifampicin/biapenem combination (FICI, interpretation) | 0.0312/0.5 (0.28, synergy) | 0.25/1 (0.15, synergy) | 128/8 (1.0, indifference) |
In addition to the observed synergy between biapenem and rifampicin observed via the in vitro chequerboard experiment, we also found that the co-exposure to rifampicin and biapenem completely prevented the selection of M. tuberculosis strain H37Rv with phenotypic resistance (at 10 mg/L for rifampicin and 80 mg/L for biapenem) to these drugs (Table 2). Under these experimental conditions, co-exposure to rifampicin and biapenem also completely prevented the selection of M. tuberculosis strain 115R with resistance to either of these drugs at the concentrations tested, while co-exposure of strain 124R to these drugs could not prevent bacterial growth at the concentrations tested.
Table 2.
Frequencies of M. tuberculosis phenotypic resistance to biapenem and rifampicin
| Drug(s) (concentration in mg/L) | Frequency of phenotypic resistance of each of the following strains of M. tuberculosis |
||
|---|---|---|---|
| H37Rv | 115R | 124R | |
| Rifampicin (10) | 2.8 × 10−8 | 2.5 × 10−5 | NA |
| Biapenem (80) | 1.0 × 10−4 | 1.0 × 10−5 | 1.5 × 10−4 |
| Rifampicin (10)+ biapenem (80) | <5 × 10−9 | <5 × 10−9 | 8.0 × 10−5 |
Plates were inoculated with 2 × 108 cfu. NA, not applicable.
Biapenem exhibits antimicrobial activity in mouse models of acute TB
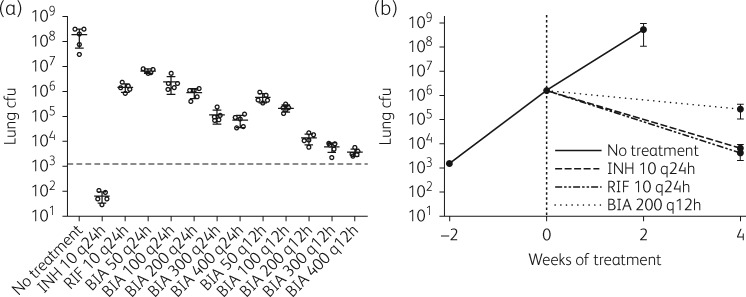
Our in vitro results indicate that biapenem should be evaluated for activity against rifampicin-resistant M. tuberculosis strains in vivo, in the mouse model of TB chemotherapy. However, the optimal dosing strategy for biapenem in this model is unknown. Therefore, we first assessed the in vivo activity of biapenem against M. tuberculosis in a dose-ranging experiment in a mouse model of early-phase acute TB in which treatment was initiated 3 days after infection when the bacterial count in the lungs was 3.08 (standard deviation 0.14) log10 cfu and the bacteria were actively replicating in the lungs. As expected, after 4 weeks of treatment the bacterial burden in the isoniazid-treated control mice decreased by approximately 1.5 log10 cfu, and the lung cfu count in the rifampicin-treated control mice increased, but to a limited extent compared with the untreated control mice (rifampicin has limited bacteriostatic activity in this infection model)24,25 (Figure 1a). Biapenem exhibited clear dose-dependent antimicrobial activity in the mouse lungs, with all biapenem dosing regimens as active or more active than rifampicin. Dosing twice daily resulted in greater antimicrobial activity for the same total daily dose; for example, 200 mg/kg twice daily was more active than 400 mg/kg once daily (Figure 1a). Furthermore, any daily dose greater than 200 mg/kg prevented the formation of gross lesions in the mouse lungs (Figure S1, available as Supplementary data at JAC Online).
Figure 1.
Biapenem activity in the mouse models of acute TB. (a) M. tuberculosis H37Rv cfu in the lungs of mice after 4 weeks of treatment with isoniazid (INH), rifampicin (RIF) or biapenem (BIA). The dose (in mg/kg) and rhythm (q24h or q12h) of administration is written after each drug abbreviation. Treatment was initiated 3 days after infection, and the broken horizontal line represents the lung cfu count at the start of treatment. Each data point represents lung cfu counts from one mouse, and error bars represent standard deviation of the mean. Gross pathology images of all mouse lungs from this experiment are presented in Figure S1. (b) M. tuberculosis H37Rv cfu in the lungs of mice after 4 weeks of treatment with isoniazid, rifampicin or biapenem in a late-phase acute model of mouse TB in which treatment is initiated 2 weeks after infection. The broken vertical line represents the day of starting treatment; each data point represents the mean and the error bars represent the standard deviation (five mice per group per time point).
Although the anti-TB activity of biapenem was clearly established in the dose-ranging experiment, the data suggested that biapenem, similarly to rifampicin, was not bactericidal against such actively multiplying M. tuberculosis in the early-phase acute infection model in which the overall bacterial burden is relatively low. We therefore evaluated the activity of biapenem (200 mg/kg administered twice daily) in a mouse model of late-phase acute TB in which treatment is initiated 2 weeks after infection, when the bacterial burden is higher in the mouse lungs. As expected in this model, all untreated control mice were moribund and thus were sacrificed by 4 weeks after infection. Also as expected, both isoniazid and rifampicin controls exhibited bactericidal activity, with both drugs killing approximately 2.5 log10 cfu in the mouse lungs after 4 weeks of treatment (Figure 1b), and biapenem was also bactericidal, killing approximately 1 log10 cfu in the mouse lungs after 4 weeks of treatment.
Biapenem exhibits antimicrobial activity in vivo against rifampicin-resistant M. tuberculosis
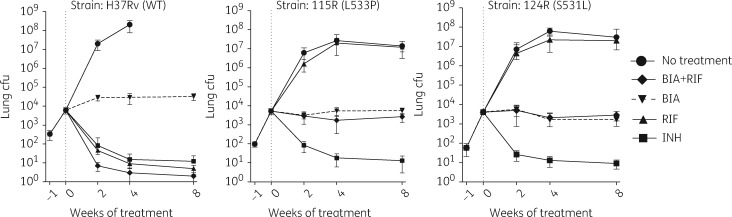
The positive activity of biapenem observed in the mouse models of acute infection further supported its potential as an anti-TB drug. We therefore moved forward to evaluate the in vivo activity of biapenem alone and in combination with rifampicin in mice infected with the low- and high-level rifampicin-resistant strains utilized in our in vitro experiments. BALB/c mice were infected by aerosol with each of the rifampicin-resistant strains (115R and 124R) as well as the H37Rv parent strain, and treatment was initiated 1 week after infection. In this experiment, biapenem was administered at 300 mg/kg twice daily, and rifampicin was administered at a high dose (20 mg/kg) to maximize exposure and the possibility to observe synergistic activity with biapenem against the rifampicin-resistant strains. In mice infected with the drug-susceptible H37Rv strain, isoniazid exerted bactericidal activity, as did high-dose rifampicin (Figure 2). We again observed that biapenem alone prevented bacterial multiplication, while biapenem plus rifampicin had bactericidal activity greater than either rifampicin or isoniazid alone. In mice infected with either of the rifampicin-resistant strains, rifampicin exhibited no antimicrobial activity (Figure 2). Administration of biapenem prevented multiplication of both rifampicin-resistant strains to the same degree that it prevented multiplication of the H37Rv parent strain. However, the combination of rifampicin and biapenem was not superior to biapenem alone against either of the rifampicin-resistant strains. Biapenem, alone or in combination with rifampicin, was able to prevent the formation of gross lung lesions (Figure S2).
Figure 2.
Assessment of efficacy of biapenem against rifampicin-resistant M. tuberculosis. Burden of M. tuberculosis in the lungs of mice treated with isoniazid (INH; 10 mg/kg), rifampicin (RIF; 20 mg/kg), biapenem (BIA; 300 mg/kg) and rifampicin plus biapenem and in untreated control mice (No treatment). Mice were infected with drug-susceptible (H37Rv) and rifampicin-resistant (115R, low-level resistance; 124R, high-level resistance) M. tuberculosis strains. The vertical broken line represents the start of treatment (Day 0). Data points represent mean lung cfu counts and error bars represent standard deviation (five mice per group per time point). Gross pathology images of all mouse lungs from this experiment are presented in Figure S2.
Discussion
With the WHO estimating that in 2015 approximately half a million people were newly diagnosed with MDR-TB,1 the need for treatment options effective against drug-resistant strains of M. tuberculosis is clearly critical. Here, we report that biapenem, a carbapenem antibiotic, has antimicrobial activity against M. tuberculosis, including rifampicin-resistant M. tuberculosis strains, both in vitro and in vivo, and thus has potential for repurposing as an anti-TB drug.
Our previous work demonstrated synergy between biapenem and rifampicin against M. tuberculosis H37Rv both in vitro5 and in vivo.17 In addition, cephalosporins were also observed to exhibit synergy with rifampicin against M. tuberculosis.26 The apparent synergy between rifampicin and the β-lactam subclasses cephalosporins and carbapenems against M. tuberculosis was unexpected. We and others have recently speculated on the potential mechanisms for the synergy.5,26 Here, while we observed in vitro synergy between biapenem and rifampicin against low-level rifampicin-resistant strain 115R and its drug-susceptible parent strain H37Rv, these two drugs did not exhibit synergy against the high-level rifampicin-resistant strain 124R (Table 1). In the case of strain 115R, co-exposure to rifampicin and biapenem resulted in an 8- to 16-fold decrease in the rifampicin MIC, from 2–4 mg/L to 0.25 mg/L, which is the MIC of rifampicin for drug-susceptible M. tuberculosis. In the mouse model, the combination of rifampicin and biapenem exhibited synergistic activity against the drug-susceptible H37Rv strain only (Figure 2). Importantly, biapenem alone exhibited similar activity against all three strains. Presumably, the exposures of rifampicin achieved in vivo were suboptimal for strains 115R and 124R to achieve the synergistic activity, as the high-dose rifampicin apparently had no activity against even the low-level rifampicin-resistant strain 115R. Understanding the nature of the observed synergy between rifampicin and biapenem thus warrants further investigation. Additionally, the 2- and 4-fold increases in MIC of biapenem for strains 115R and 124R were unexpected as the known mechanisms that confer resistance to rifampicin27 and biapenem28 are not known to be related. However, our observations are not unprecedented. An increase in MIC range of biapenem against drug-resistant M. tuberculosis was reported recently.29 A similar observation was also made earlier for meropenem.2
Another promising observation is that biapenem alone exhibited anti-TB activity across all infection models and against all M. tuberculosis strains evaluated. In TB treatment, drugs are always administered within a combination regimen, with different regimen components responsible for different anti-TB activities, including bactericidal activity against actively multiplying bacteria, bactericidal activity against slowly or non-replicating bacteria, and bacteriostatic activity to limit growth overall, preventing the selection of drug-resistant mutants.30 Our data indicate that biapenem may be able to contribute to each of these activities depending on the state of the M. tuberculosis infection and the companion drugs in the regimen, further supporting additional investigation into how to optimize the use of biapenem as an anti-TB drug.
Recently another carbapenem, meropenem (co-administered with amoxicillin/clavulanate), was shown to have early bactericidal activity (defined as a decline in sputum cfu counts during the first 14 days of administration) in TB patients.31 These emerging lines of evidence have kindled a wider interest in the use of β-lactam antibiotics and specifically carbapenems for use in TB treatment. Compared with meropenem, biapenem is known to be much more stable in the presence of human DHP-I,15 and in addition, we have previously demonstrated that biapenem is a much more potent inhibitor of the target M. tuberculosis transpeptidase LdtMt2 than is meropenem.17 Thus, it is possible that biapenem may even be more potent than meropenem against M. tuberculosis.
Due to interspecies differences in pharmacokinetics, experiments in mice are expected to underestimate the efficacy of carbapenems that may be obtained in humans.4,32 In mice, after a single subcutaneous administration (91 mg/kg) of biapenem, Cmax was 90 mg/L, but the elimination rate constant was ∼2 h.33 To keep the concentration of biapenem above the MIC, continuous/prolonged infusion is a more relevant approach.34 In contrast to mice, the bactericidal T>MIC target of 40% is readily attained in TB patients by administration of 2 g of meropenem every 8 h in combination with clavulanate, as illustrated by its impressive early bactericidal activity in a recent trial.31 Being more potent than meropenem, biapenem may attain the desired 40% T>MIC target at lower doses administered less frequently.35 Indeed, population pharmacokinetic/pharmacodynamic (PK/PD) model simulations indicate 600 mg of biapenem every 12 h would hit this target in plasma for virtually all patients infected with isolates with MIC ≤2 mg/L,36,37 which encompasses the MIC for biapenem in combination with clavulanate against our WT H37Rv strain.5 The maximum serum concentration of biapenem in humans of 17.35 or 32 mg/L following single intravenous doses of 300 or 600 mg, respectively,14,19 is above the biapenem MIC against M. tuberculosis strains, including the two rifampicin-resistant strains 115R and 124R. Therefore, biapenem by itself has the potential to be active against M. tuberculosis, including rifampicin-resistant isolates. Available PK/PD data suggest that biapenem may provide clinical efficacy comparable to meropenem while requiring less frequent dosing, which would be advantageous for TB programmes. It is therefore relevant and timely to investigate the anti-TB activity of biapenem, in comparison with meropenem, and also in combination with other anti-TB drugs, to provide a more in-depth understanding of how biapenem may be best utilized in TB treatment.
Supplementary Material
Acknowledgements
We thank Jacques Grosset for critical discussions.
Funding
This work was supported by National Institutes of Health (NIH) grants DP2OD008459 and R33AI111739.
Transparency declarations
None to declare.
Supplementary data
Figures S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. WHO. Global Tuberculosis Report. Geneva: WHO, 2016. [Google Scholar]
- 2. Hugonnet JE, Tremblay LW, Boshoff HI. et al. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 2009; 323: 1215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cordillot M, Dubee V, Triboulet S. et al. In vitro cross-linking of peptidoglycan by Mycobacterium tuberculosis L,D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother 2013; 57: 5940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhar N, Dubee V, Ballell L. et al. Rapid cytolysis of Mycobacterium tuberculosis by faropenem, an orally bioavailable β-lactam antibiotic. Antimicrob Agents Chemother 2015; 59: 1308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaushik A, Makkar N, Pandey P. et al. Carbapenems and rifampin exhibit synergy against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 2015; 59: 6561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar P, Arora K, Lloyd JR. et al. Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 2012; 86: 367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaganath D, Lamichhane G, Shah M.. Carbapenems against Mycobacterium tuberculosis: a review of the evidence. Int J Tuberc Lung Dis 2016; 20: 1436–47. [DOI] [PubMed] [Google Scholar]
- 8. Wang F, Cassidy C, Sacchettini JC.. Crystal structure and activity studies of the Mycobacterium tuberculosis β-lactamase reveal its critical role in resistance to β-lactam antibiotics. Antimicrob Agents Chemother 2006; 50: 2762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erdemli SB, Gupta R, Bishai WR. et al. Targeting the cell wall of Mycobacterium tuberculosis: structure and mechanism of L,D-transpeptidase 2. Structure 2012; 20: 2103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavollay M, Arthur M, Fourgeaud M. et al. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J Bacteriol 2008; 190: 4360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta R, Lavollay M, Mainardi JL. et al. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 2010; 16: 466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schoonmaker MK, Bishai WR, Lamichhane G.. Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to β-lactams. J Bacteriol 2014; 196: 1394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papp-Wallace KM, Endimiani A, Taracila MA. et al. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55: 4943–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry CM, Ibbotson T.. Biapenem. Drugs 2002; 62: 2221–34; discussion 2235. [DOI] [PubMed] [Google Scholar]
- 15. Hikida M, Kawashima K, Yoshida M. et al. Inactivation of new carbapenem antibiotics by dehydropeptidase-I from porcine and human renal cortex. J Antimicrob Chemother 1992; 30: 129–34. [DOI] [PubMed] [Google Scholar]
- 16. Zhang D, Wang Y, Lu J. et al. In vitro activity of β-lactams in combination with β-lactamase inhibitors against multidrug-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 2016; 60: 393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar P, Kaushik A, Lloyd EP. et al. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol 2017; 13: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mouton JW, Touzw DJ, Horrevorts AM. et al. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet 2000; 39: 185–201. [DOI] [PubMed] [Google Scholar]
- 19. Shah PM. Parenteral carbapenems. Clin Microbiol Infect 2008; 14Suppl 1: 175–80. [DOI] [PubMed] [Google Scholar]
- 20. Rosenthal IM, Tasneen R, Peloquin CA. et al. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother 2012; 56: 4331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gavan TL, Town MA.. A microdilution method for antibiotic susceptibility testing: an evaluation. Am J Clin Pathol 1970; 53: 880–5. [DOI] [PubMed] [Google Scholar]
- 22. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100-S15. CLSI, Wayne, PA, USA, 2005. [Google Scholar]
- 23. Tasneen R, Li SY, Peloquin CA. et al. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 2011; 55: 5485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nuermberger E. The role of the mouse model in the evaluation of new antituberculosis drugs In: Donald PR, van Helden PD, eds. Antituberculosis Chemotherapy. Basel, Switzerland: Karger, 2011; 145–52. [Google Scholar]
- 25. Kling A, Lukat P, Almeida DV. et al. Antibiotics. Targeting DnaN for tuberculosis therapy using novel griselimycins. Science 2015; 348: 1106–12. [DOI] [PubMed] [Google Scholar]
- 26. Ramón-Garcia S, González Del Rio R, Villarejo AS. et al. Repurposing clinically approved cephalosporins for tuberculosis therapy. Sci Rep 2016; 6: 34293.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller LP, Crawford JT, Shinnick TM.. The rpoB gene of Mycobacterium tuberculosis. Antimicrob Agents Chemother 1994; 38: 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar P, Kaushik A, Bell D. et al. Mutation in an unannotated protein confers carbapenem resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2017; 61: e02234-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horita Y, Maeda S, Kazumi Y. et al. In vitro susceptibility of Mycobacterium tuberculosis isolates to an oral carbapenem alone or in combination with β-lactamase inhibitors. Antimicrob Agents Chemother 2014; 58: 7010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med 1980; 1: 231–41. [PubMed] [Google Scholar]
- 31. Diacon AH, van der Merwe L, Barnard M. et al. β-Lactams against tuberculosis–new trick for an old dog? N Engl J Med 2016; 375: 393–4. [DOI] [PubMed] [Google Scholar]
- 32. Solapure S, Dinesh N, Shandil R. et al. In vitro and in vivo efficacy of β-lactams against replicating and slowly growing/nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 2013; 57: 2506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsumoto K, Kurihara Y, Kuroda Y. et al. Pharmacokinetics and brain penetration of carbapenems in mice. J Infect Chemother 2016; 22: 346–9. [DOI] [PubMed] [Google Scholar]
- 34. Kikuchi E, Kikuchi J, Nasuhara Y. et al. Comparison of the pharmacodynamics of biapenem in bronchial epithelial lining fluid in healthy volunteers given half-hour and three-hour intravenous infusions. Antimicrob Agents Chemother 2009; 53: 2799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takata T, Aizawa K, Shimizu A. et al. Optimization of dose and dose regimen of biapenem based on pharmacokinetic and pharmacodynamic analysis. J Infect Chemother 2004; 10: 76–85. [DOI] [PubMed] [Google Scholar]
- 36. Dong J, Xiong W, Chen Y. et al. Optimal dosing regimen of biapenem in Chinese patients with lower respiratory tract infections based on population pharmacokinetic/pharmacodynamic modelling and Monte Carlo simulation. Int J Antimicrob Agents 2016; 47: 202–9. [DOI] [PubMed] [Google Scholar]
- 37. Ikawa K, Morikawa N, Ikeda K. et al. Pharmacokinetic-pharmacodynamic target attainment analysis of biapenem in adult patients: a dosing strategy. Chemotherapy 2008; 54: 386–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.