Abstract
Background
Many countries are on the brink of establishing antibiotic stewardship programmes in hospitals nationwide. In a previous study we found that communication between microbiology laboratories and clinical units is a barrier to implementing efficient antibiotic stewardship programmes in Norway. We have now addressed the key communication barriers between microbiology laboratories and clinical units from a laboratory point of view.
Methods
Qualitative semi-structured interviews were conducted with 18 employees (managers, doctors and technicians) from six diverse Norwegian microbiological laboratories, representing all four regional health authorities. Interviews were recorded and transcribed verbatim. Thematic analysis was applied, identifying emergent themes, subthemes and corresponding descriptions.
Results
The main barrier to communication is disruption involving specimen logistics, information on request forms, verbal reporting of test results and information transfer between poorly integrated IT systems. Furthermore, communication is challenged by lack of insight into each other’s area of expertise and limited provision of laboratory services, leading to prolonged turnaround time, limited advisory services and restricted opening hours.
Conclusions
Communication between microbiology laboratories and clinical units can be improved by a review of testing processes, educational programmes to increase insights into the other’s area of expertise, an evaluation of work tasks and expansion of rapid and point-of-care test services. Antibiotic stewardship programmes may serve as a valuable framework to establish these measures.
Introduction
In Norway, implementation of antibiotic stewardship programmes (ASPs) is in its early stages.1 One of the core elements of ASPs is access to microbiology laboratory services.2,3 Microbiology laboratories are critical in surveillance of antibiotic resistance, development of empirical antibiotic treatment guidelines and guidance of clinical staff in the diagnosis and treatment of infections. Rapid delivery of microbiology test results has been shown to influence mortality, length of hospital stay and costs, as well as appropriateness of antibiotic prescribing and consumption, which are the main drivers for development of antibiotic resistance.4–7 In a previous study on antibiotic prescribing in hospitals, we found that clinicians perceived communication of microbiology test results as inadequate and a barrier to effective antibiotic stewardship.8
Processes involving communication between laboratories and clinical units are the most error-prone parts of laboratory testing.9,10 Up to 30% of adverse events in laboratory medicine impact patient care and up to 12% of the events cause actual or potential harm to patients.11,12 Taking into account the high volume of testing, such errors may significantly affect patient safety and public health globally, highlighting the need to review laboratory testing processes.
Norway has a dispersed geography with a variety of small, medium and large hospitals. There are 48 hospitals nationwide, of which 45 are public. Only 16 hospitals have on-site microbiology laboratories and hospitals without them send samples to the nearest hospital with such facilities. All laboratories are open during the daytime 6–7 days a week. Some hospitals provide a microbiology service in the evening, but none during the night. This evidently challenges communication between the microbiology laboratories and the clinical units.
Based on our previous findings that clinicians were dissatisfied with the communication between microbiology laboratories and clinical units, we proceeded to study the communication between the two from a laboratory point of view. In this study we investigate communication barriers between microbiology laboratories and clinical units, and how they can be addressed. To our knowledge this is the first published study on this topic.
Methods
Study design
A qualitative design, using semi-structured interview methodology, was chosen to study the question of communication barriers between microbiology laboratories and clinical units, and how these barriers can be addressed.13,14 In order to reduce any bias from social pressures between informants’ positions, individual interviews were preferred over focus groups.
Interviews
An interview guide was developed based on a literature review and on individual face to face conversations with four key informants (a manager, a doctor, a technician and a secretary), using open-ended questions.15–18 The informants were purposely sampled from a microbiology laboratory in western Norway. The interview guide covered the following topics: processing of specimens, roles, education/experience, communication, leadership and improvement measures.
Interviewees in the study were recruited by a request sent to the directors of research and development at the 16 hospitals in Norway with a microbiology laboratory. Eight laboratories responded positively. Inclusion continued until two criteria, saturation of empirical themes and diversity, were met.19,20 Ultimately, six laboratories were included, purposely selected based on hospital characteristics (teaching/non-teaching) and geography, securing representation from all four regional health authorities. A manager, a doctor and a technician were recruited from each of the six laboratories to obtain diversity of perspectives. The participating managers were mainly technicians by profession (Table 1).
Table 1.
Demographics of participants
| Governmental microbiology laboratories represented | 6 out of 16 |
| Regional health authorities represented | 4 out of 4 |
| Local/regional/university hospitals represented | n = 2/n = 2/n = 2 |
| Male/female | n = 4/n = 14 |
| Technician/doctor/manager | n = 6/n = 6/n = 6 |
| Aged 25–35/36–45/46–55/56–65 years | n = 4/n = 3/n = 6/n = 5 |
Interviews were performed between January and June 2015 at the interviewees’ workplace within working hours. They lasted from 46 to 86 min (mean 64 min), were recorded and transcribed verbatim. Author B. S., an infectious disease specialist and PhD student trained in qualitative methods, conducted and transcribed 15 interviews, whereas author A. L. B., a technician and MSc student, conducted and transcribed 3 interviews under supervision of authors B. S. and K. A.
Analysis
Thematic analysis was applied to the transcripts through the following steps:13,14,21 authors K. A., I. S. and B. S. (analytic team) read all the transcripts and independently listed emerging themes. Discussions led to an agreement on preliminary themes. Subsequently, author B. S. identified quotes reflecting each theme and developed preliminary subthemes and corresponding descriptions of subthemes. Preliminary themes, subthemes, descriptions and quotes were then discussed, resulting in elimination, reorganization, renaming and reformulation of some of them, before a final validation by the team. Translation of the results from Norwegian to English was conducted through discussions and agreements in the analytic team and co-author E. C.
Ethics
The study was approved by the Data Protection Officer at Haukeland University Hospital, representing the Norwegian Data Protection Authority (2013/6960). The Regional Committee for Medical and Health Research Ethics considered the study to fall outside the committee’s scope as no patient data were obtained. An informed consent form was signed by all interviewees.
Results
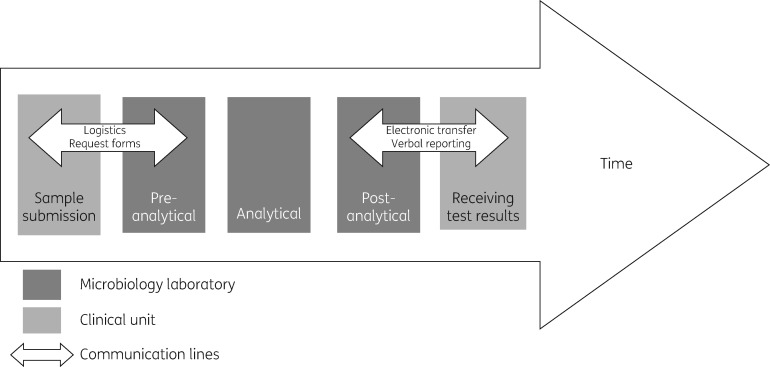
The interviewees describe the processing of specimens in three steps: pre-analytical, analytical and post-analytical. Technicians are mainly responsible for the pre-analytical and analytical steps, but may consult microbiologists when needed, and microbiologists mainly perform the post-analytical steps (Figure 1). All laboratories use electronic laboratory information systems (LIS) to store sample data and transfer test results to electronic medical records.
Figure 1.
Communication between microbiology laboratories and clinical units on specimen processing and test results.
In Norway, microbiologists are normally laboratory based, although some perform clinical ward rounds once or twice a week. Microbiology laboratories communicate with clinical units during all three steps, but more so on transition to and from the pre- and post-analytical steps. The interviewees described processing of specimens and corresponding communication with clinical units as illustrated in Figure 1.
Following data analysis, three main themes emerged that describe the barriers to communication between microbiology and clinical units: ‘disruption’, ‘lack of insight’ and ‘limited service provision’ (Table 2). These identified barriers subsequently identify potential channels to improve communication. ‘Disruption’ is easily identified at the pre- and post-analytic steps, whereas the themes ‘lack of insight’ and ‘limited service provision’ are additional barriers to communication that relate to processing of specimens (Figure 1).
Table 2.
Description of the identified themes
| Theme | Subtheme | Description | Quote |
|---|---|---|---|
| Disruption | specimen logistics | the process of specimen submission is difficult to follow, in part time consuming and poorly coordinated with the laboratory work processes, in particular for specimens from local hospitals | ‘My main concern regarding local hospitals is transport. Specimens are transported by bus for one and a half hours. It shouldn’t be a big problem, but submission of specimens must correspond with the bus schedule. During holidays such as Christmas and Easter, when everything is closed for days, time is spent figuring out how to submit the specimens as the hospital is not to spend money on taxis. So, occasionally important specimens are not submitted, before they are long overdue.’ (Manager) [D1] |
| ‘Specimens from local hospitals have to arrive by eight thirty for them to be processed in the Maldi in the morning. Frequently specimens arrive at nine thirty–ten, and we cannot sit and wait for them, but have to process the submitted specimens in order to give out their results. It’s a shame really, for the patients, that's for sure.’ (Technician) [D2] | |||
| request forms | inadequate information on microbiology request forms complicates and delays the initial specimen processing | ‘Very often there is only a name on the microbiology request form, or hardly that, a name and a date of birth. We do not know what kind of specimen it is, who has sent it, we don’t know anything. So the guessing game begins; we check up on the electronic patient record and make a lot of phone calls, which of course is error prone.’ (Technician) [D3] | |
| verbal reporting of test results | reporting test results over the phone represents a challenge in identifying the clinician concerned and making sure the significance of the result is acknowledged | ‘Yes, blood cultures can be challenging. If you’re not, if you can’t get hold of the requesting clinician, the result is pending out there somewhere. Nobody knows who the clinician concerned is, you know. We always make phone calls when blood cultures are positive. It may be fatal if we do not get hold of a doctor.’ (Technician) [D4] | |
| ‘Regarding significant test results, I may bypass nurses, … It may be crucial to talk to the clinician directly, to avoid information being misplaced.’ (Microbiologist) [D5] | |||
| IT systems | the laboratory and the wards have different and poorly integrated IT systems, and microbiology lab personnel are not familiar with the electronic patient record system | ‘I got a question I did not understand until someone told me that “their screen display is different from ours”, “Oh, is that so?”—I didn’t have a clue. I have never seen the electronical medical record. One of my colleagues had seen it, and she also found it difficult to interpret.’ (Technician) [D6] | |
| Lack of insight | microbiology | a majority of clinical personnel are perceived as having insufficient knowledge of microbiology | ‘I think that sometimes clinicians take a lot of specimens hoping that we can give them a diagnosis. For instance, nowadays we are inundated with throat specimens from the emergency department.’ (Microbiologist) [I1] |
| work processes | clinical personnel often lack insight into the laboratory's work processes, and microbiology lab personnel wish they had more insight into the clinical work processes | ‘They are used to getting clinical lab results within an hour or two, but with regard to microbiology results we have to explain to them that it takes one day for the bacteria to grow, and then another day for susceptibility testing. They don’t get it, and…’ (Microbiologist) [I2] | |
| ‘I don’t know how doctors interpret the test results. For instance, a urine specimen where numbers are low, do they interpret it as a urinary tract infection?’ (Technician) [I3] | |||
| the patient | microbiology lab personnel lack patient contact and insight into clinical conditions | ‘If you work at a microbiology laboratory and never have been on the wards, you will know that blood cultures are important as well as spinal fluid, but you don’t know HOW important until you’ve seen a patient suffering from meningitis, for instance. So I think this is an area that should be addressed.’ (Technician) [I4] | |
| Limited service provision | personnel resources | insufficient personnel resources limit opening hours and advisory services towards clinical staff | ‘You know, our opening hours are restricted. And every day, when we arrive at work there are missed calls on the phone. People have tried to call us during the evening, but there is no one there. Unfortunately, staffing and budgets do not allow us to be open 24/7, though I know that larger laboratories and some smaller labs elsewhere offer a better and wider range of services.’ (Manager) [S1] |
| ‘Some technicians do hold lectures in clinical units on how to obtain specimens among other things, which is good. However, we don’t do it often due to lack of time to prepare the lectures. Laboratory work comes first, which does not leave much time for preparation.’ (Manager) [S2] | |||
| diagnostic technology | insufficient diagnostic technology prolongs turnaround time | ‘If we were to have a MALDI-TOF, test results could be processed quicker, at least the ID of microbes. And, when molecular biological methods expand, with increased resources and equipment, it will contribute to a shorter turnaround time for test results. I suppose it will impact patient care and budgets in general. For instance, for MRSA patients who are isolated while waiting for the test results, rapid diagnostics make a difference.’ (Technician) [S3] |
Disruption
The interviewees describe communication with clinical units as disruptive. Firstly, disruption is related to logistics and request forms at the pre-analytic step. Secondly, there is disruption in verbal reporting of test results at the post-analytic step. Thirdly, communication is interrupted by poorly integrated laboratory and clinical IT systems (Figure 1).
Transfer of specimens from clinical units to the laboratories is complicated and time consuming, and specimen arrival is poorly coordinated with laboratory work processes. As a consequence, specimens go missing, show up after several days or arrive too late to be processed the same day (quotes D1 and D2 in Table 2). These are everyday challenges, but become more evident for specimens transferred from hospitals without a microbiology laboratory, particularly on weekends and holidays. These delays and their potential consequences for individual patients are of great concern to the interviewees.
‘My main concern regarding local hospitals is transport. Specimens are transported by bus for one and a half hours. It shouldn’t be a big problem, but submission of specimens must correspond with the bus schedule. During holidays such as Christmas and Easter, when everything is closed for days, time is spent figuring out how to submit the specimens as the hospital is not to spend money on taxis. So, occasionally important specimens are not submitted, before they are long overdue.’ (Manager) [D1]
Furthermore, incomplete information on microbiology request forms adds to the workload and delays initial specimen processing.
‘Very often there is only a name on the microbiology request form, or hardly that, a name and a date of birth. We do not know what kind of specimen it is, who has sent it, we don’t know anything. So the guessing game begins; we check up on the electronic patient record and make a lot of phone calls, which of course is error prone.’ (Technician) [D3]
The main challenge at the post-analytical step is reporting significant test results to clinical staff by phone. Identification of who is the treating doctor or nurse may be difficult. There is also uncertainty as to whether the results are acted upon [D4]. The concern of microbiology laboratory staff is that important information is left out when results are passed on from one person to another. Consequently, ward nurses, who are readily available by phone, may be bypassed in order to convey the results directly to the clinician concerned [D5]. Since clinicians may be hard to find, e.g. surgeons are often in the operating theatre, preliminary electronic results are provided to ensure transfer of important information to the clinical units.
All written exchange of information between laboratories and clinical units is based on electronic transfer, except for paper-based request forms for bacterial culture. However, information transfer is inadequate due to poorly integrated IT systems between the laboratories and the clinical units. Since technicians are not familiar with the electronic medical record and the final result displays differently in the two systems, oral communication around test results becomes complicated.
‘I got a question I did not understand until someone told me that “their screen display is different from ours”.—“Oh, is that so?”—I didn’t have a clue. I have never seen the electronical medical record. One of my colleagues had seen it, and she also found it difficult to interpret.’ (Technician) [D6]
This disruption in electronic information transfer leads to excess phone calls to the laboratory to clarify the results.
Lack of insight
When microbiology laboratory staff communicate with clinical staff, they perceive a mutual lack of insight into each other’s work: clinical staff lack insight into microbiology and laboratory work processes, and laboratory staff lack insight into patient-related issues and clinical work processes.
Firstly, microbiology laboratory staff report that many doctors and nurses do not fully understand the potential, but also the limitations, of microbiology tests. Furthermore, some of them have poor knowledge as to when to take a test and how to interpret the test results.
‘I think that sometimes clinicians take a lot of specimens hoping that we can give them a diagnosis. For instance, nowadays we are inundated with throat specimens from the emergency department.’ (Microbiologist) [I1]
Microbiology staff also express that clinical staff lack insight into the internal work processes of the laboratories. More specifically, they lack awareness of the need to provide good-quality specimens to the laboratory, and have limited knowledge of how specimens are processed in the laboratories, expressing their frustration over what they call ‘delayed test results’.
‘They are used to getting clinical lab results within an hour or two, but with regard to microbiology results we have to explain to them that it takes one day for the bacteria to grow, and then another day for susceptibility testing. They don’t get it, and …’ (Microbiologist) [I2]
At the same time, microbiology staff, especially technicians, would like to have more insight into clinical work processes, e.g. what are the daily routines on the wards? How are microbiology test results interpreted and applied [I3]? However, microbiologists with some clinical training sometimes act as interpreters. Technicians also express a lack of insight into patients and clinical conditions. They do not meet patients themselves, in contrast to technicians at a clinical biochemistry laboratory who obtain specimens on the wards. Furthermore, they have limited access to patients’ medical history and clinical condition.
‘If you work at a microbiology laboratory and never have been on the wards, you will know that blood cultures are important as well as spinal fluid, but you don’t know HOW important until you’ve seen a patient suffering from meningitis, for instance. So I think this is an area that should be addressed.’ (Technician) [I4]
The interviewees point out that it would have been valuable to have such insight when processing specimens or discussing test results with clinical staff.
Limited service provision
The overall aim of laboratory staff is to provide services beneficial for the patients. However, a barrier to optimal communication with clinical units is limited personnel resources and lack of updated diagnostic technology.
The workforce is too small to keep the laboratories open and provide a 24 h service and limited opening hours are of great concern to the interviewees.
‘You know, our opening hours are restricted. And every day, when we arrive at work there are missed calls on the phone. People have tried to call us during the evening, but there is no one there. Unfortunately, staffing and budgets do not allow us to be open 24/7, though I know that larger laboratories and some smaller labs elsewhere offer a better and wider range of services.’ (Manager) [S1]
They know that patients suffer from infectious diseases day and night and report that they frequently work late to complete test results, in order to meet requests from clinical staff.
The laboratory staff considers teaching and advisory services to be a significant part of their service provision to clinical units. For instance, ad hoc teaching on the phone on when and how to obtain specimens and choice of antibiotics is prioritized. To give lectures on microbiology for clinical staff is also considered essential; however, limited personnel resources restrict educational outreach [S2]. Services are further limited by lack of updated diagnostic technology, such as MALDI-TOFs, resulting in prolonged specimen turnaround time.
‘If we were to have a MALDI-TOF, test results could be processed quicker, at least the ID of microbes. And, when molecular biological methods expand, with increased resources and equipment, it will contribute to a shorter turnaround time for test results. I suppose it will impact patient care and budgets in general. For instance, for MRSA patients who are isolated while waiting for the test results, rapid diagnostics make a difference.’ (Technician) [S3]
Discussion
The results from this study highlight key communication barriers between microbiology laboratories and clinical units from a laboratory point of view. Disruption at the interface between laboratories and clinical units, mutual lack of insight into each other’s area of expertise and limited laboratory services are all barriers that need to be addressed in order to improve the communication.
To address disruption between laboratories and clinical units, the entire testing process from sampling of specimens to application of test results needs to be reviewed. For instance, specimen logistics are neither transparent nor adapted to technical developments within the laboratories, indicating a need for joint revision on submission of specimens. Communication of significant test results by phone is also found to be inadequate. As a consequence, vital test results may not be received by the treating clinicians, a situation that poses a patient safety threat. This highlights a need for common routines on who is to receive test results and how. Furthermore, there is a need for better integration between the IT systems at the laboratories and clinical units, especially at the post-analytical step, with improved presentation of test results in the electronic medical record.
It is not only processes at the interface between the microbiology laboratories and the clinical units that need to be addressed, but also processes within these units. A previous study showed that turnaround time could be significantly reduced by improved communication between staff running the MALDI-TOFs and staff at the molecular laboratory.22 Another study from across the United States found that follow-up of abnormal test results in clinical units is inadequate.23 A joint effort, where all the steps in the testing process are addressed, has the potential to reduce turnaround time. This would improve availability and timeliness of microbiology test results, which has been reported to be a barrier to implementing efficient antibiotic stewardship programmes.8
Limited microbiology services are also perceived as a barrier. Both limited personnel resources and lack of updated diagnostic technology prolong turnaround time and restrict advisory services and opening hours. However, new rapid microbiological techniques are evolving, to some extent replacing traditional culturing and susceptibility testing, which over time may reduce the need for personnel resources in the analytical processes.24 In addition, revision of testing processes and more adequate testing may improve workflow and workload, thereby releasing personnel resources to provide guidance to clinical staff.
Limited opening hours could be addressed by expansion of on-site and around the clock services, and the use of rapid diagnostic tests, such as immunochromatography- and PCR-based tests for detecting respiratory tract pathogens.25 However, the sensitivity and specificity of these tests vary and validation and quality control of these tests should be performed by a core microbiology laboratory. Although new rapid tests are evolving, traditional methods such as Gram staining of blood isolates may also impact patient treatment.24 Establishing these services may be of particular significance at hospitals without microbiologists, potentially reducing turnaround times of blood isolates significantly. To enhance quality control, external microbiologists may supervise local clinicians via tele-microbiology services, e.g. when interpreting Gram stains.18,26
The mutual lack of insight into each other’s area of expertise is a barrier closely related to disruption. Educational programmes with lectures combined with mutual internships for laboratory and clinical staff could contribute to a better understanding of complementary work processes and give laboratory staff insight into patient conditions. These perspectives also need to be integrated into the undergraduate education of laboratory and clinical staff.27
Establishing ASPs in hospitals can be an efficient framework to facilitate some of the suggested measures to improve communication and increase insight between microbiology laboratory and clinical staff. ASP teams are multidisciplinary and should preferably be staffed by microbiologists and infectious disease physicians.8,28 In performing antibiotic stewardship outreach visits in clinical units, microbiologists can enhance their role as interpreters of clinical processes and patient information to the technicians at the laboratory. Such visits also provide an opportunity to convey insight into microbiology and microbiological work processes to clinical staff, and teach them how to interpret test results.29 As microbiological methods evolve and become increasingly sophisticated, the need for professional guidance will increase.30 This may require a change in how some microbiologists execute their profession, from being predominantly laboratory based to working more closely with clinical staff.31
This study was performed in the Norwegian hospital setting; however, the findings are likely to resonate in all healthcare settings, although the specific challenges may vary. There is reason to believe that disruption in specimen logistics and verbal reporting of test results also poses a challenge for long-term care facilities and family physicians. In contrast to long-term care facilities, there are reports from family physicians indicating the presence of such barriers. Family physicians have an additional communication challenge in that they have to notify their patients of test results.32,33 A review of the communication between microbiology laboratory and clinical staff is therefore valuable in a variety of clinical settings.
In this study we performed individual interviews; however, it could be argued that observations in the laboratories would have added valuable information.34,35 Furthermore, according to the interviewees, medical secretaries at the laboratories may possess valuable experience regarding communication with clinical staff and could have been included as interviewees. The sample is dominated by females as they constitute the majority of the workforce in Norwegian laboratories. Finally, authors B. S. and A. L. B., being an infectious disease specialist and a technician, respectively, may have affected the response from the participants during interviews and interpretation of the results. However, by documenting preconceptions and performing analyses with a multidisciplinary scientific team, this limitation was managed.
Conclusions
In order to address the barriers to communication between microbiology laboratories and clinical units there is a need for a joint effort to improve disruption at the interface of the two units through a review of testing processes. We further recommend educational programmes to mutually increase insights into each other’s area of expertise, an evaluation of work tasks, and expansion of rapid and point-of-care test services to further improve laboratory services. ASPs may serve as a suitable framework to establish these measures, and thereby enhance communication between microbiology laboratories and clinical units.
Acknowledgements
We would like to thank the key informants, the interviewees and the directors of development and research in all the health trusts in Norway for contributing to the study. We also would like to thank Gørill Skaale Johansen, an engineer at the University of Bergen, for designing Figure 1.
E. C. is affiliated with the National Institute of Health Research Imperial Biomedical Research Centre and the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England and the NIHR Imperial Patient Safety Translational Research Centre.
Funding
This research was performed at the Norwegian Advisory Unit for Antibiotic Use in Hospitals without any external funding.
Transparency declarations
None to declare.
References
- 1. Norwegian Ministry of Health and Care Services. Actionplan on Antimicrobial Resistance in Health Care [in Norwegian]. 2016. https://www.regjeringen.no/contentassets/915655269bc04a47928fce917e4b25f5/handlingsplan-antibiotikaresistens.pdf.
- 2. Dellit TH, Owens RC, McGowan JE. et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–77. [DOI] [PubMed] [Google Scholar]
- 3. CDC. Core Elements of Hospital Antibiotic Stewardship Programs.http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html. [DOI] [PMC free article] [PubMed]
- 4. Huang AM, Newton D, Kunapuli A. et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57: 1237–45. [DOI] [PubMed] [Google Scholar]
- 5. Ly T, Gulia J, Pyrgos V. et al. Impact upon clinical outcomes of translation of PNA FISH-generated laboratory data from the clinical microbiology bench to bedside in real time. Ther Clin Risk Manag 2008; 4: 637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez KK, Olsen RJ, Musick WL. et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 2013; 137: 1247–54. [DOI] [PubMed] [Google Scholar]
- 7. Holmes AH, Moore LS, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 8. Skodvin B, Aase K, Charani E. et al. An antimicrobial stewardship program initiative: a qualitative study on prescribing practices among hospital doctors. Antimicrob Resist Infect Control 2015; 4: 24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta 2009; 404: 16–23. [DOI] [PubMed] [Google Scholar]
- 10. Laposata M, Dighe A.. “Pre-pre” and “post-post2 analytical error: high-incidence patient safety hazards involving the clinical laboratory. Clin Chem Lab Med 2007; 45: 712–9. [DOI] [PubMed] [Google Scholar]
- 11. Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med 2012; 32: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammerling JA. A review of medical errors in laboratory diagnostics and where we are today. Lab Med 2012; 43: 41–4. [Google Scholar]
- 13. Malterud K. Systematic text condensation: a strategy for qualitative analysis. Scand J Public Health 2012; 40: 795–805. [DOI] [PubMed] [Google Scholar]
- 14. Bradley EH, Curry LA, Devers KJ.. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007; 42: 1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caliendo AM, Gilbert DN, Ginocchio CC. et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57 Suppl 3: 139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphreys H, Nagy E, Kahlmeter G. et al. The need for European professional standards and the challenges facing clinical microbiology. Eur J Clin Microbiol Infect Dis 2010; 29: 617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhattacharya S. Clinical microbiology: should microbiology be a clinical or a laboratory speciality? Indian J Pathol Microbiol 2010; 53: 217-21.. [DOI] [PubMed] [Google Scholar]
- 18. Rhoads DD, Sintchenko V, Rauch CA. et al. Clinical microbiology informatics. Clin Microbiol Rev 2014; 27: 1025–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polit DF, Beck CT, Developing a sampling plan In: Kogut H, ed. Nursing Research: Generating and Assessing Evidence for Nursing Practice. Philadelphia, PA: Lippincott Williams & Wilkins, 2011; 337–64. [Google Scholar]
- 20. Baker SE, Edwards S.. How Many Qualitative Interviews Is Enough? http://eprints.ncrm.ac.uk/2273/4/how_many_interviews.pdf.
- 21. Pope C, Ziebland S, Mays N.. Qualitative research in health care. Analysing qualitative data. BMJ 2000; 320: 114–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kommedal O, Aasen JL, Lindemann PC.. Genetic antimicrobial susceptibility testing in Gram-negative sepsis - impact on time to results in a routine laboratory. APMIS 2016; 124: 603–10. [DOI] [PubMed] [Google Scholar]
- 23. Schiff GD, Hasan O, Kim S. et al. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med 2009; 169: 1881–7. [DOI] [PubMed] [Google Scholar]
- 24. Fournier PE, Drancourt M, Colson P. et al. Modern clinical microbiology: new challenges and solutions. Nat Rev Microbiol 2013; 11: 574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drancourt M, Michel-Lepage A, Boyer S. et al. The point-of-care laboratory in clinical microbiology. Clin Microbiol Rev 2016; 29: 429–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barenfanger J, Graham DR, Kolluri L. et al. Decreased mortality associated with prompt Gram staining of blood cultures. Am J Clin Pathol 2008; 130: 870–6. [DOI] [PubMed] [Google Scholar]
- 27. Engum SA, Jeffries PR.. Interdisciplinary collisions: bringing healthcare professionals together. Collegian 2012; 19: 145–51. [DOI] [PubMed] [Google Scholar]
- 28. Barlam TF, Cosgrove SE, Abbo LM. et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62: e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O’Brien MA, Rogers S, Jamtvedt G. et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007; issue 4: CD000409.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchan BW, Ledeboer NA.. Emerging technologies for the clinical microbiology laboratory. Clin Microbiol Rev 2014; 27: 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Read RC, Cornaglia G, Kahlmeter G.. Professional challenges and opportunities in clinical microbiology and infectious diseases in Europe. Lancet Infect Dis 2011; 11: 408–15. [DOI] [PubMed] [Google Scholar]
- 32. Hickner J, Graham DG, Elder NC. et al. Testing process errors and their harms and consequences reported from family medicine practices: a study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008; 17: 194–200. [DOI] [PubMed] [Google Scholar]
- 33. Elder NC, Graham D, Brandt E. et al. The testing process in family medicine: problems, solutions and barriers as seen by physicians and their staff: a study of the American Academy of Family Physicians’ National Research Network. J Pat Saf 2006; 2: 25–32. [Google Scholar]
- 34. Creswell JW. Qualitative Inquiry & Research Design: Choosing Among Five Approaches. Los Angeles, CA: Sage, 2013. [Google Scholar]
- 35. Yanes AF, McElroy LM, Abecassis ZA. et al. Observation for assessment of clinician performance: a narrative review. BMJ Qual Saf 2016; 25: 46–55. [DOI] [PubMed] [Google Scholar]