Abstract
Background
Substantial heterogeneity in the epidemiology and management of Staphylococcus aureus bacteraemia (SAB) occurs in Latin America. We conducted a prospective cohort study in 24 hospitals from nine Latin American countries.
Objectives
To assess the clinical impact of SAB in Latin America.
Patients and methods
We evaluated differences in the 30 day attributable mortality among patients with SAB due to MRSA compared with MSSA involving 84 days of follow-up. Adjusted relative risks were calculated using a generalized linear model.
Results
A total of 1030 patients were included. MRSA accounted for 44.7% of cases with a heterogeneous geographical distribution. MRSA infection was associated with higher 30 day attributable mortality [25% (78 of 312) versus 13.2% (48 of 363), adjusted RR: 1.94, 95% CI: 1.38–2.73, P < 0.001] compared with MSSA in the multivariable analysis based on investigators’ assessment, but not in a per-protocol analysis [13% (35 of 270) versus 8.1% (28 of 347), adjusted RR: 1.10, 95% CI: 0.75–1.60, P = 0.616] or in a sensitivity analysis using 30 day all-cause mortality [36% (132 of 367) versus 27.8% (123 of 442), adjusted RR: 1.09, 95% CI: 0.96–1.23, P = 0.179]. MRSA infection was not associated with increased length of hospital stay. Only 49% of MSSA bloodstream infections (BSI) received treatment with β-lactams, but appropriate definitive treatment was not associated with lower mortality (adjusted RR: 0.93, 95% CI: 0.70–1.23, P = 0.602).
Conclusions
MRSA-BSIs in Latin America are not associated with higher 30 day mortality or longer length of stay compared with MSSA. Management of MSSA-BSIs was not optimal, but appropriate definitive therapy did not appear to influence mortality.
Introduction
Staphylococcus aureus is one of the most common causes of bloodstream infections (BSI) worldwide.1 An important challenge for the treatment of S. aureus-BSI is the presence of multidrug resistance. Indeed, MRSA isolates continue to pose important therapeutic challenges worldwide despite the availability of new antibiotics with anti-MRSA activity. The prevalence of MRSA in Latin America appears to be very heterogeneous, ranging from 6% in Central America to 80% in some South American countries.2 Several studies have shown that mortality rates among patients with BSI caused by MRSA are almost twice as high compared with those patients infected with MSSA isolates.3–8 For instance, a meta-analysis found that MRSA-BSI was associated with increased mortality compared with MSSA (pooled OR: 1.93, 95% CI: 1.54–2.42).3 In addition, other studies have shown more healthcare utilization parameters in MRSA-BSI compared with MSSA-BSI.9–11 MRSA-BSI was associated with a median increase in length of stay of 2 days and a mean excess hospital cost of US$6916 compared with MSSA in one study.9 All data available to date suggest that MRSA-BSI pose an important burden to healthcare services, particularly in developing countries where such systems lack robust resources.
Several studies have evaluated the burden of S. aureus infections in Latin America, but limited data are available on the clinical impact of MRSA infections in terms of outcomes, utilization of healthcare resources and evaluation of antibiotic management.2,12 Additionally, studies focusing on the molecular epidemiology of MRSA suggest a high degree of genetic heterogeneity among Latin American MRSA isolates.12–14 To assess comprehensively the clinical impact of MRSA-BSI and gain insights into the molecular epidemiology of these organisms in Latin America, we conducted a prospective cohort study to evaluate differences in 30 day attributable mortality among patients with clinically significant BSI due to MRSA compared with MSSA recruited from hospitals in nine Latin American countries. Secondary endpoints included the evaluation of parameters of healthcare utilization and appropriateness of treatment (MRSA versus MSSA).
Patients and methods
Study design
We conducted a prospective observational cohort study that included 24 hospitals in nine Latin American countries. Adult patients admitted to these hospitals with clinically significant bacteraemia caused by S. aureus (at least one positive blood culture was required) from January 2011 to July 2014 were included in the study. Each patient contributed only one episode, which was defined as the 14 day period after collection of the blood sample. Subsequent isolates obtained after the initial blood culture were collected and stored. Patients with polymicrobial bacteraemia or relapse of bacteraemia, those who were transferred from other institutions and those who withdrew from the hospital or died within the first 48 h after the diagnosis were excluded. All isolates were sent to a central laboratory in Bogota, Colombia, to confirm identification. Patients were prospectively followed up to determine outcomes up to 84 days after the initial blood culture.
Data collection
Data were obtained from patients’ medical records and entered into a pre-designed electronic case report form. Data gathered included demographics, comorbidities, microbiological information, antimicrobial therapy received during the 30 day period prior to the current episode, clinical information in the 48 h period before blood sampling, clinical and laboratory information on the current hospitalization, medical treatment provided and clinical outcomes. The place of acquisition was considered community-associated, healthcare-associated or hospital-associated (HA) based on standard definitions (see Supplementary data available at JAC Online). The Charlson weighted index of comorbidity and the McCabe–Jackson and APACHE II scores were used to evaluate comorbid conditions and severity of illness, respectively. The Pittsburgh bacteraemia score was used to predict mortality. A complicated BSI was defined as the presence of persistent positive blood cultures after 48–96 h, presence of endocarditis, deep tissue infection or metastatic infection. Clinical outcomes were measured at 7, 30 and 84 days after the initial blood culture. The primary outcome of the study was 30 day attributable mortality, but we also evaluated early mortality (7 days after positive blood culture) and late mortality (84 days after positive culture). Three categories of outcomes were considered: improved, unresolved and death. Death attributable to S. aureus-BSI was defined as the persistence of clinical signs and symptoms of sepsis without other identifiable cause of death or active infection at the time of death and positive blood cultures within 7 days of a patient’s death. Clinical outcomes were obtained from the charts by the investigators at each centre while patients remained hospitalized; for those discharged, every investigator contacted the treating physician to perform a clinical evaluation at the designated times. The healthcare utilization parameters evaluated included length of hospital stay, admission to an ICU, duration of stay in the ICU, readmission rate in the 84 day period after diagnosis and readmission owing to S. aureus infection. Adequate management of S. aureus-BSI was evaluated by determining the performance of four recommended standards of care: (i) removal of infected foci; (ii) repeat blood cultures after initial sample; (iii) use of proper antibiotic therapy; and (iv) performance of echocardiography.15,16 Antibiotic therapy was deemed appropriate if it was administered intravenously, and if it was given for at least 14 days for uncomplicated and for at least 28 days for complicated S. aureus-BSI. For MSSA-BSI, the appropriate empirical antibiotic therapy included use of β-lactam antibiotics with activity against MSSA with or without vancomycin, teicoplanin or daptomycin. For MRSA-BSI, the appropriate empirical antibiotic therapy included use of vancomycin, teicoplanin or daptomycin with or without a β-lactam. Appropriate definitive antibiotic therapy for MSSA-BSI included the use of β-lactams alone. Appropriate definitive therapy for MRSA-BSI included use of vancomycin, teicoplanin or daptomycin started within 4 days after blood sampling. We considered 4 days as the appropriate time for treating physicians to get susceptibility results from local laboratories.
Statistical analysis
For sample size calculation, we estimated that two-thirds of the BSIs will be MRSA.2 Mortality with MSSA was estimated at 25%.3–8 Thus, we estimated that a sample of 1201 participants with a 95% CI and 80% power would allow us to detect a difference of ≥8% in the 30 day attributable mortality between MRSA and MSSA. The required sample size will decrease if the ratio of MRSA to MSSA is closer to 1, or if the mortality among MSSA is lower than expected. Data analysis was performed using Stata v8.2 (College Station, TX, USA). Frequencies and percentages are presented for categorical variables. For numeric variables, mean and SD or median and IQR were calculated. In bivariate comparisons, we used Fisher’s exact test for categorical variable, and Student’s t-test or Mann–Whitney test for numeric variables as required. For multivariate analysis on mortality, adjusted relative risks were calculated using the generalized linear model, using link log, all variables tested in the bivariate analysis were evaluated in the multivariate analysis. Variables with a P < 0.05 were kept in the final model. A sensitivity analysis for 30 day mortality, evaluating all-cause mortality instead of attributable mortality was carried out. To model the length of hospital stay, we used multiple linear regression with log transformation of the outcome variable. All multivariate analyses were adjusted for clustering within hospital. The backward stepwise method based on P values was used to select variables for the final model.
Ethics
The study was registered at the Institutional Review Board of Universidad Peruana Cayetano Heredia in Lima, Peru with the code number 56868 in March 2010; an approval was received in April 2010. Local approvals at each hospital were also obtained. Owing to the observational nature of the study no signed inform consent was needed.
Results
Patient characteristics
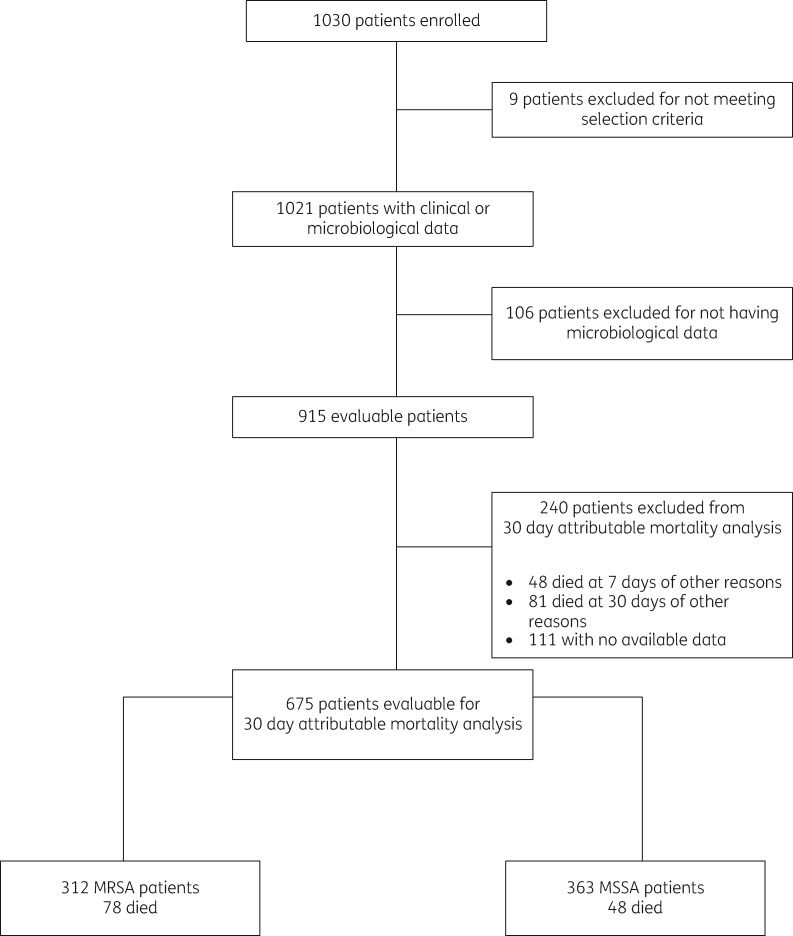
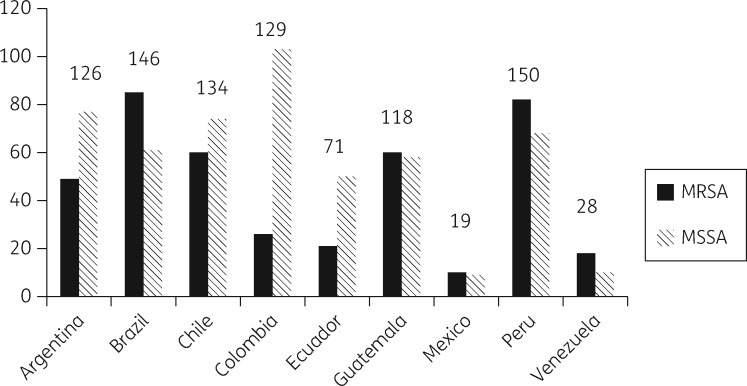
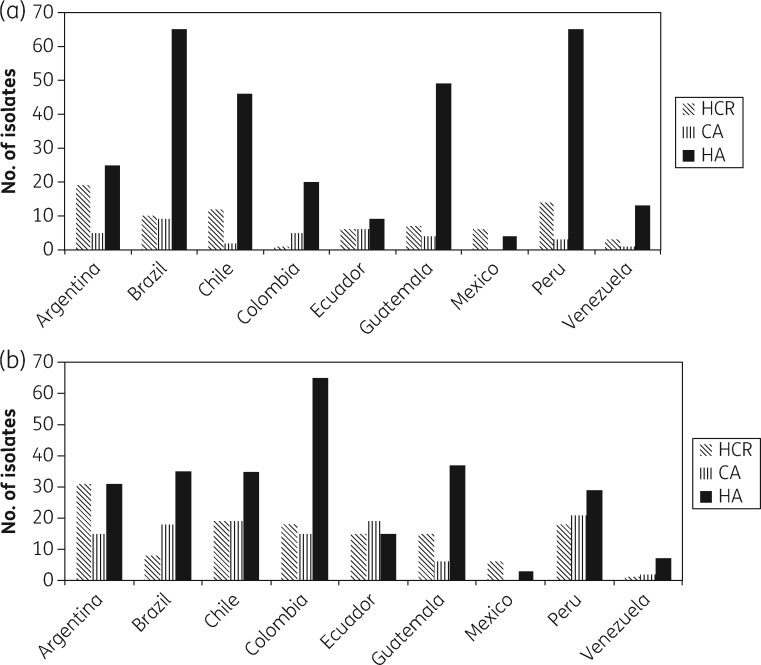
A total of 1030 patients were included in the study, nine patients were excluded for not meeting the selection criteria, and 106 were excluded for not having microbiological data. Of the remaining 915 evaluable patients (409 with MRSA, 44.7%), 240 patients were excluded from the primary analysis (Figure 1). The epidemiological and clinical characteristics of the 915 patients are presented in Table 1. Patients infected with MRSA-BSI were comparable to patients infected with MSSA-BSI in several parameters, including age, gender, source of bacteraemia, number of comorbidities, score values of Charlson, McCabe–Jackson, and APACHE II scales, duration of fever and rates of complicated BSI. However, patients with an MRSA-BSI acquired the infection more frequently in the hospital, more commonly had a comorbid condition in the 3 months preceding the BSI (particularly a neurologic disorders or were receiving immunosuppressive therapy), more frequently had a previous S. aureus infection (including previous MRSA infection), were more frequently hospitalized before the current episode, were more prone to have received antibiotics in the 30 day period preceding the current BSI, needed more mechanical ventilation, insertion of central venous catheters (CVCs) and surgical procedures and had a higher risk of dying as determined by the Pittsburgh score compared with patients with MSSA-BSIs. The distribution of S. aureus isolates by country is presented in Figure 2. MRSA predominated in Brazil, Guatemala, Peru and Venezuela, while MSSA was more frequently isolated from BSIs in Argentina, Chile, Colombia and Ecuador. Figure 3 shows the predicted place of acquisition of the S. aureus-BSI. Interestingly, both MRSA- and MSSA-BSIs were mostly HA. No resistance or heteroresistance to vancomycin was found among the MRSA strains; MIC50 and MIC90 of vancomycin were 1 mg/L, respectively.17
Figure 1.
Flow diagram of patients included in the study.
Table 1.
Clinical characteristics of patients included in the study infected with MRSA or MSSAa
| Characteristic | MRSA | MSSA | P valuea |
|---|---|---|---|
| No. (%) | 409 (44.7) | 506 (55.6) | |
| Age, years, mean (SD) | 59.2 (18.0) | 58.9 (18.7) | 0.815 |
| Male gender | 230 (56.2) | 298 (59.2) | 0.381 |
| Source of bacteraemia | |||
| primary | 259 (63.3) | 299 (59.4) | 0.246 |
| secondary | 150 (36.7) | 204 (40.6) | |
| Source of primary bacteraemia | |||
| CVC related | 181 (69.9) | 197 (65.9) | 0.320 |
| not CVC related | 78 (30.1) | 102 (34.1) | |
| Source of secondary bacteraemia | |||
| respiratory | 34 (8.3) | 43 (8.4) | 1.000 |
| skin | 38 (9.3) | 42 (8.2) | 0.638 |
| surgical | 19 (4.6) | 20 (3.9) | 0.624 |
| endovascular | 16 (3.9) | 32 (6.3) | 0.135 |
| bone/joint | 26 (6.3) | 34 (6.7) | 0.894 |
| urinary | 6 (1.5) | 10 (2.0) | 0.621 |
| abdominal | 15 (3.7) | 16 (3.1) | 0.715 |
| CNS | 4 (1.0) | 1 (0.2) | 0.178 |
| unknown | 18 (4.4) | 12 (2.4) | 0.095 |
| Place of acquisition | |||
| community | 35 (8.6) | 115 (22.9) | <0.001 |
| hospital acquired | 296 (72.4) | 257 (51.1) | |
| healthcare related | 78 (19.1) | 131 (26.0 | |
| Presence of any comorbidity in the 3 month period before diagnosis of BSI | |||
| cancer | 56 (13.6) | 90 (17.7) | 0.103 |
| transplant | 2 (0.5) | 6 (1.2) | 0.310 |
| AIDS | 3 (0.7) | 9 (1.8) | 0.244 |
| myocardial infarction | 26 (6.3) | 25 (4.9) | 0.386 |
| heart failure | 82 (20.0) | 93 (18.2) | 0.554 |
| peripheral vascular disease | 68 (16.6) | 53 (10.4) | 0.008 |
| diabetes | 122 (29.7) | 130 (25.9) | 0.159 |
| lung disease | 52 (12.7) | 55 (10.8) | 0.409 |
| burn | 5 (1.2) | 8 (1.6) | 0.782 |
| liver disease | 37 (9.0) | 48 (9.4) | 0.909 |
| kidney disease | 154 (37.5) | 204 (40.0) | 0.455 |
| neurological disorder | 95 (23.1) | 53 (10.4) | <0.001 |
| gastric/duodenal ulcer | 14 (3.4) | 25 (4.9) | 0.324 |
| connective tissue disease | 16 (3.9) | 22 (4.3) | 0.868 |
| previous surgery | 123 (29.9) | 104 (20.4) | 0.001 |
| immunosuppressive therapy | 46 (11.2) | 87 (17.1) | 0.014 |
| other comorbidities | 157 (38.2) | 213 (41.8) | 0.280 |
| Number of comorbidities, median (IQR) | 2 (2–3) | 2 (1–3) | 0.138 |
| Previous S. aureus infection | 29 (7.1) | 17 (3.3) | 0.014 |
| Previous MRSA infection | 17 (4.1) | 6 (1.2) | 0.005 |
| Previous hospitalization | 164 (39.9) | 164 (32.2) | 0.044 |
| Days since last hospitalization, median (IQR) | 47 (22–83) | 31 (16–63) | 0.009 |
| Use of antimicrobials 30 days before admission | 233 (56.7) | 123 (24.1) | <0.001 |
| Ward of admission | |||
| medical | 256 (63.7) | 372 (74.4) | 0.003 |
| surgical | 76 (18.9) | 60 (12.0) | |
| obstetrics/gynaecology | 3 (0.8) | 2 (0.4) | |
| emergency room | 42 (10.5) | 49 (9.8) | |
| other | 25 (6.2) | 17 (3.4) | |
| Information 48 h before blood sampling | |||
| mechanical ventilation | 62 (15.1) | 46 (9.0) | 0.015 |
| CVC in place | 250 (60.8) | 246 (48.2) | 0.001 |
| parenteral nutrition | 27 (6.6) | 17 (3.3) | 0.062 |
| surgical procedure | 51 (12.4) | 29 (5.7) | 0.001 |
| dialysis | 111 (27.0) | 139 (27.3) | 0.827 |
| severe sepsis | 90 (22.2) | 75 (15.0) | 0.007 |
| Charlson comorbidity scale, median (IQR) | 2 (1–3) | 2 (1–3) | 0.485 |
| Charlson score >2 | 157 (38.2) | 195 (38.2) | 1.0 |
| McCabe–Jackson scale | 0.957 | ||
| rapidly fatal | 60 (14.8) | 72 (14.4) | |
| ultimately fatal | 143 (35.3) | 174 (34.8) | |
| non-fatal | 202 (50.0) | 254 (50.8) | |
| APACHE II score, median (IQR) | 0 (0–14) | 0 (0–12) | 0.341 |
| APACHE II score >0 | 67 (16.8) | 58 (12.3) | 0.066 |
| Pittsburg score, median (IQR) | 1 (0–3) | 1 (0–2) | <0.001 |
| Pittsburg score >1 | 187 (45.5) | 190 (37.3) | 0.013 |
| Transthoracic echocardiography performed | 162 (39.9) | 243 (48.5) | 0.015 |
| Transthoracic echocardiography for CVC-related bacteraemia | 70 (39.1) | 91 (46.4) | 0.246 |
| Duration of fever, mean (SD), days | 8.5 (22.5) | 6.4 (8.2) | 0.102 |
| Total WBC×103, mean (SD) | 12.0 (11.0) | 10.1 (9.5) | 0.006 |
| Creatinine, mg/dL, median (IQR) | 1.1 (0.6–3.3) | 1.3 (0.7–4.3) | 0.058 |
| Glucose, mg/dL, mean (SD) | 111.7 (93.3) | 105.8 (94.6) | 0.351 |
| C-reactive protein, mg/dL, mean (SD) | 20.5 (52.2) | 24.4 (59.0) | 0.309 |
| Complicated bacteraemia | 64 (15.6) | 80 (15.7) | 0.736 |
| Focus of complicated bacteraemia | |||
| infective endocarditis | 22 (5.4) | 31 (6.1) | 0.672 |
| others | 46 (11.2) | 52 (10.2) | 0.668 |
Values are n (%) unless noted otherwise. WBC, white blood cells.
χ2 or Fisher’s exact test for categorical variables, Student’s t-test when means are displayed and Kruskal–Wallis test when medians are displayed.
Figure 2.
Distribution of S. aureus-BSIs by country. Total number of isolates is shown above each pair of bars.
Figure 3.
Place of acquisition of S. aureus-BSIs by country. (a) MRSA and (b) MSSA. HCR, healthcare associated; CA, community associated; HA, hospital associated.
Primary and secondary outcomes
In the bivariate analysis, using investigators’ assessment, patients with MRSA-BSI had higher 30 day attributable mortality compared with patients with MSSA-BSI; 25.0% (78 of 312) versus 13.2% (48 of 363), P < 0.001, Table 2. Using a generalized linear model, the difference in mortality remained significant after adjusting for covariates that included age, sex, presence of comorbidity, Charlson comorbidity score, source of bacteraemia, Pittsburgh bacteraemia score, complicated bacteraemia, place of acquisition, previous MRSA infection, previous hospitalization, previous antimicrobial therapy, severity of sepsis, clinical information 48 h before sampling and hospital (RR: 1.94, 95% CI 1.38–2.73, P = 0.019). The difference in attributable mortality was also observed at all time periods from 7–84 days.
Table 2.
Bivariate and multivariate analysis for the primary outcome of the 30 day attributable mortality based on investigators’ assessment
| Relative risk (95% CI) |
||
|---|---|---|
| Characteristic | bivariate analysis | multivariate analysisa |
| MRSA | 1.89 (1.40–2.56) | 1.94 (1.38–2.73) |
| Age, years | 1.03 (1.01–1.04) | 1.02 (1.01–1.03) |
| Male gender | 0.87 (0.67–1.13) | |
| Charlson comorbidity score | 1.14 (1.07–1.22) | 1.10 (1.03–1.19) |
| Pittsburgh bacteraemia score | 1.20 (1.16–1.25) | |
| Source of secondary bacteraemia | 1.28 (0.78–2.04) | |
| Complicated bacteraemia | 1.97 (1.18–3.28) | 1.62 (1.13–2.32) |
| Place of acquisition | ||
| community | (ref)b | (ref)b |
| healthcare associated | 0.63 (0.38–1.04) | 0.58 (0.40–0.83) |
| hospital acquired | 0.69 (0.50–0.92) | 0.64 (0.48–0.86) |
| Comorbidities | ||
| peripheral vascular disease | 1.11 (0.72–1.72) | |
| neurologic disease | 1.64 (0.97–2.77) | |
| surgery | 0.78 (0.56–1.09) | |
| immunosuppressive therapy | 1.26 (0.83–1.91) | |
| Previous MRSA infection | 1.29 (0.63–2.64) | |
| Previous hospitalization | 1.20 (0.85–1.71) | |
| Previous antimicrobial therapy | 1.52 (1.06–2.18) | |
| Clinical information within 48 h before blood sampling | ||
| mechanical ventilation | 1.55 (1.04–2.31) | |
| CVC in place | 0.98 (0.70–1.38) | |
| surgical procedure | 1.05 (0.50–2.22) | |
| Severity of sepsis | ||
| sepsis | (refb) | (refb) |
| multi-organ failure | 3.42 (1.57–7.45) | 2.09 (0.88–4.94) |
| severe sepsis | 2.26 (1.57–2.25) | 1.6 (1.19–2.22) |
| septic shock | 5.46 (3.67–8.14) | 3.60 (2.77–4.69) |
Adjusted for clustering on hospital and other significant variables identified in the bivariate analysis.
ref, indicates reference for comparison.
Subsequently, a per-protocol analysis was performed in this analysis we used the pre-defined definitions for attributable mortality (Table 3). MRSA-BSI was associated with higher 30 day attributable mortality in the bivariate analysis: 13% (35 of 270) versus 8.1% (28 of 347), crude RR: 1.61 (1.01–2.54), but this difference disappeared in the multivariate analysis, adjusted RR: 1.10, 95% CI: 0.75–1.60, P = 0.616. Indeed, parameters of severity of illness prior to identification of the pathogen were associated with mortality, including Pittsburgh bacteraemia score and severity of sepsis. In addition, we performed a sensitivity analysis evaluating differences in 30 day all-cause mortality between MRSA- and MSSA-BSIs. MRSA was associated with higher mortality than MSSA in the bivariate analysis: 36% (132 of 367) versus 27.8% (123 of 442), crude RR: 1.28, 95% CI: 1.06–1.55), P = 0.010, but the difference was diluted in the multivariate analysis, adjusted RR: 1.09 (95% CI: 0.96–1.23, P = 0.182). Moreover, the difference in mortality disappeared when severity of sepsis and the Pittsburgh score were added into the model (Table S1 and S2, available as Supplementary information at JAC Online). A subset analysis excluding patients with a primary source of BSI did not also find a difference in 30 day mortality (Table S3).
Table 3.
Bivariate and multivariate analysis for the primary outcome of the 30 day attributable mortality using per-protocol definitions
| Relative risk (95% CI) |
||
|---|---|---|
| Characteristic | bivariate analysis | multivariate analysisa |
| MRSA | 1.61 (1.01–2.54) | 1.10 (0.75–1.60) |
| Age in years | 1.00 (0.99–1.02) | |
| Male gender | 1.03 (0.65–1.63) | |
| Charlson comorbidity score | 1.11 (0.98–1.25) | |
| Pittsburgh bacteraemia score | 1.31 (1.26–1.36) | 1.21 (1.08–1.36) |
| Source of secondary bacteraemia | 0.93 (0.61–1.42) | |
| Complicated bacteraemia | 1.15 (0.78–1.70) | |
| Place of acquisition | ||
| community | (ref)b | |
| healthcare associated | 0.50 (0.25–0.99) | |
| hospital acquired | 0.92 (0.58–1.46) | |
| Comorbidities | ||
| peripheral vascular disease | 1.18 (0.54–2.57) | |
| neurologic disease | 1.94 (1.20–3.13) | |
| surgery | 0.83 (0.43–1.59) | |
| immunosuppressive therapy | 0.91 (0.52–1.59) | |
| Previous MRSA infection | 0.50 (0.07–3.31) | |
| Previous hospitalization | 0.85 (0.50–1.44) | |
| Previous antimicrobial therapy | 1.72 (1.18–2.49) | |
| Clinical information within 48 h before blood sampling | ||
| mechanical ventilation | 3.50 (2.30–5.32) | |
| CVC in place | 1.32 (0.88–1.99) | |
| surgical procedure | 1.48 (0.69–3.19) | |
| Severity of sepsis | ||
| sepsis | (refb) | (refb) |
| multi-organ failure | 5.67 (2.37–13.59) | 3.69 (1.98–6.87) |
| severe sepsis | 3.01 (1.81–5.03) | 2.11 (1.30–3.41) |
| septic shock | 8.94 (5.45–14.66) | 3.23 (1.47–7.08) |
Adjusted for clustering on hospital and other significant variables identified in the bivariate analysis.
ref, reference for comparisons.
MRSA-BSI were associated with increased parameters of healthcare utilization compared with MSSA-BSIs (Table 4). Indeed, patients with MRSA-BSI remained in the hospital and in the ICU longer with an excess of 10 and 4 days, respectively, compared with patients with MSSA-BSI. However, MRSA was not associated with longer length of hospital stay than MSSA in a multivariate analysis that adjusted for significant covariates (difference in mean duration of hospital stay of 1.5 days, P = 0.248; Table S4). No difference was observed in the rate of admission to ICU and hospital readmission rates, including early readmissions (within 30 days of discharge). However, early readmissions were more common in MRSA-infected patients: 51.9% (28 of 54) versus 23.8% (10 of 42), P = 0.006. We evaluated the management of MRSA- and MSSA-BSI by assessing rates of catheter removal; follow-up blood cultures; use of echocardiogram and targeted antimicrobial therapy using β-lactams and glycopeptides (or other anti-MRSA antibiotic) for MSSA and MRSA, respectively. No difference was observed in the rates of removal of CVCs, follow-up blood cultures during treatment and recommended duration of treatment for complicated and uncomplicated bacteraemia between MRSA- versus MSSA-BSI. Patients with MSSA-BSI underwent more transthoracic echocardiographic evaluations. Interestingly, a higher number of patients with MRSA-BSI received appropriate antimicrobial therapy (glycopeptides or other anti-MRSA antibiotics) compared with patients with MSSA-BSIs. However, a striking finding was that a change in empirical regimen (usually vancomycin) when susceptibility results were available, occurred only in 49.2% of patients with MSSA-BSI. Vancomycin was continued (instead of a recommended antistaphylococcal β-lactam) as definitive therapy for MSSA in almost half of patients with MSSA-BSI. Thus, we evaluated the effect of appropriate definitive therapy for MSSA-BSI on 30 day all-cause mortality in a bivariate and multivariate analysis. A reduction of 30% in 30 day all-cause mortality was observed when appropriate definitive antimicrobial treatment was offered for patients with MSSA-BSI (crude RR: 0.70, 95% CI: 0.52–0.95, P = 0.024), but the difference was not evident after adjusting for comorbidities (Charlson score) and Pittsburgh score, adjusted RR: 0.93, 95% CI: 0.70–1.23, P = 0.602.
Table 4.
Healthcare resource utilization parameters and management of S. aureus-BSIs
| Patient characteristics | MRSA | MSSA | P valuea |
|---|---|---|---|
| No. (%) | 409 (44.7) | 506 (55.3) | |
| Healthcare resource utilization parameters | |||
| length of hospital stay, mean (SD), days | 39.1 (37.6) | 28.6 (31.2) | <0.001 |
| admission to an ICU | 149 (36.3) | 159 (31.2) | 0.107 |
| length of stay in ICU, mean (SD), days | 17.2 (16.6) | 13.6 (14.8) | 0.042 |
| re-admission in the 84 day period | 38 (14.6) | 58 (15.6) | 0.737 |
| S. aureus episode in readmission | 10 (27.0) | 17 (32.1) | 0.392 |
| Management of S. aureus-BSI | |||
| CVC removed in first 72 h in bacteraemia related to CVC | 123 (68.0) | 118 (60.0) | 0.104 |
| subsequent blood cultures taken after baseline | 228 (57.6) | 258 (52.0) | 0.184 |
| trans-thoracic echocardiography performed | 162 (39.9) | 243 (48.5) | 0.015 |
| duration of antibiotic treatment ≥14 days for uncomplicated bacteraemia | 256 (79.0) | 307 (75.8) | 0.329 |
| duration of antibiotic treatment ≥28 days for complicated bacteraemia | 49 (79.0) | 64 (81.0) | 0.833 |
| regimen modified based on susceptibility test results | 103 (25.2) | 249 (49.2) | <0.001 |
| appropriate empiric antimicrobial therapy | 284 (74.5) | 202 (51.9) | <0.001 |
| appropriate definitive antimicrobial therapy | 304 (79.6) | 290 (60.0) | <0.001 |
Values are n (%) unless noted otherwise.
χ2 or Fisher’s exact test for categorical variables, Student’s t-test when means are displayed and Kruskal–Wallis test when medians are displayed.
Discussion
Our results derived from this multinational comprehensive study of S. aureus-BSI in Latin America indicate that MRSA continues to cause a high burden of disease with almost half (44.7%) of episodes caused by MRSA, although important regional variations were observed. Importantly, MRSA-BSI were not associated with higher 30 day attributable mortality when a per-protocol analysis was performed. In addition, a sensitivity analysis evaluating 30 day all-cause mortality did not find that MRSA-BSIs were associated with higher mortality or longer hospital stay than those caused by MSSA.
Several studies have previously documented that MRSA-BSI was associated with higher mortality rates than MSSA-BSI.3–8 More recent studies have provided additional support to this association. A study conducted in Taiwan in a single large hospital over a period of 15 years recruited 1148 patients with HA S. aureus-BSI; MRSA-BSI was associated with 1.78-fold higher mortality rates than MSSA-BSI, after adjusting for covariates in a multivariate analysis.18 Another study conducted among 13 hospitals in Europe over a period of 2 years analysed 248 MRSA-BSI and 618 MSSA-BSI episodes in a matched case–control design.19 MRSA-BSI was associated with excess 30 day mortality compared with MSSA-BSI (OR: 1.8, 95% CI: 1.04–3.20) in the multivariate analysis. An additional 3 year retrospective study conducted in Taiwan evaluated inhospital mortality among 353 patients with S. aureus-BSI, and found higher crude mortality rates for MRSA-BSI than for MSSA-BSI: 47.5% for HA-MRSA, 30.5% for community-associated MRSA and 23.3% for MSSA.20 Older studies have been criticized for not adjusting properly for significant confounding variables, and this may be the case for the above-mentioned studies.21 In contrast to these studies, an observational cohort study conducted in a single hospital in Australia, which included 185 episodes of MRSA-BSI and 397 episodes of MSSA-BSI, found no differences in long-term all-cause mortality and in infection-related mortality after adjusting for 13 covariates in the multivariate analysis.22 Several caveats should be taken into consideration in interpreting the results of this study. The study followed a retrospective design; the number of MRSA infections was low and the setting had a low MRSA rate (18%); overadjustment cannot be ruled out, and causes of death were obtained from death certificates rather than from clinical charts.22–24 Despite these drawbacks, the study demonstrated that MRSA itself may not be associated with higher mortality, which can be explained by comorbidities and proper management. Our results, using per-protocol and all-cause mortality analysis, are in agreement with these conclusions. In the per-protocol analysis, severity of sepsis and Pittsburgh bacteraemia score (indicative of risk of death) were independently associated with the 30 day attributable mortality but not MRSA by itself. For the 30 day all-cause mortality analysis, again, severity of sepsis, Pittsburgh bacteraemia and Charlson comorbidity score were independently associated with mortality, but MRSA was not. Taking all these results together, we can conclude that MRSA was not associated with 30 day mortality in the hospitals included in this study and mortality was more influenced by severity of illness and comorbidities.
MRSA-BSI was not associated with longer length of hospital stay, which correlated more with complications of BSI rather than with MRSA itself. Overall, the management of S. aureus-BSI in the region was comparable to that reported in developed countries for four parameters including removal of CVC, performance of echocardiography, repeated blood cultures taken during treatment and duration of treatment for complicated and uncomplicated BSI.15 One of the most striking findings was the low modification rate of empirical regimens containing vancomycin or teicoplanin in MSSA-BSI when susceptibility test results were available (49%). Not surprisingly, appropriate definitive therapy for MSSA-BSI, which included a β-lactam antimicrobial, was associated with an almost 30% reduction in 30 day all-cause mortality in our study, and such findings have been reported previously.16 However, our study had limited power to detect differences in 30 day all-cause mortality in the multivariate analysis (conducted among 433 subjects). A large study involving 5633 subjects who received definitive therapy for MSSA-BSI found 35% lower mortality among β-lactam recipients compared with those who received a glycopeptide.16 Therefore, based on our findings we consider that infectious disease consultation should be mandatory in the region to guide properly the antimicrobial therapy and de-escalation. A recent study and a systematic review of the role of infectious disease consultation in the management of S. aureus-BSI showed that the involvement of infectious diseases consultants improves management and survival.15,25
Our study has several limitations. First, the analysis of the primary outcome was restricted to 675 patients. However, the sample size was large enough to test the primary hypothesis of 8% difference in the 30 day attributable mortality between MRSA and MSSA. Second, participating hospitals were all major hospitals in capital cities of Latin America. These hospitals were selected based on good performance in laboratory identification of pathogens, as determined by a previous evaluation carried-out by the Pan-American Health Organization, and may not reflect the current situation in smaller hospitals of less developed regions in Latin America. Third, we could only get information on removal of CVC for primary bacteraemia related to CVC; no information was obtained on removal of other foci. Fourth, investigators overestimated 30 day attributable mortality in our study. This may be explained by the fact that they were not blinded to the susceptibility results. Two subsequent analyses, including both per-protocol 30 day attributable and 30 day all-cause mortality provided more accurate information on the actual association between MRSA-BSIs and the primary outcome. Finally, treating physicians used diverse dosing regimens; and we did not collect information on involvement of infectious disease consultation and of evaluation of pharmacokinetic parameters when vancomycin was used. The strengths of the study include the prospective design, multinational nature of participants from all over Latin America and large sample size. A large-scale study has not previously been attempted in the region. In conclusion, MRSA-BSIs are an important healthcare burden in Latin America but may not be associated with higher mortality or length of hospital stay compared with MSSA. Management of S. aureus-BSI should be improved in the region focusing on the involvement of infectious diseases consultants and strengthening antibiotic stewardship efforts.
Supplementary Material
Acknowledgements
We dedicate the manuscript to the memory of Carlos Mejía-Villatoro.
Members of the Latin America Working Group on Bacterial Resistance
Argentina: Didier Bruno, Hospital de Clinicas, Buenos Aires; Ernesto Efron, Hospital Británico, Buenos Aires; Marcelo Del Castillo, Sanatorio Mater Dei, Buenos Aires. Brazil: Thaís Guimarães, Hospital do Servidor Publico Estadual de São Paulo, São Paulo. Chile: María Elena Ceballos, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago; Isabel Domínguez, Daniela Beltrán, Hospital Sótero del Río, Santiago; Gisela Riedel, Hospital Guillermo Grant Benavente, Concepción. Colombia: Sandra Liliana Valderrama, Unidad de Enfermedades Infecciosas, Hospital Universitario San Ignacio, Pontificia Universidad Javeriana, Bogota, Colombia; Sandra Milena Gualtero, Hospital Universitario San Ignacio; Clínica Shaio, Carlos Humberto Saavedra, Unidad Infectologia, Hospital Universitario Clínica San Rafael, Facultad de Medicina, Universidad Nacional de Colombia, Bogotá, Colombia. Ecuador: Betzabé Tello, Hospital Vozandes, Quito; Juan Carlos Aragón, Hospital General de las Fuerzas Armadas, Quito; Fausto Guerrero, Hospital Carlos Andrade Marín, Quito. Guatemala: María Mónica Silvestre, Hospital Roosevelt, Guatemala. Mexico: Rayo Morfin-Otero, Hospital Civil de Guadalajara; Fray Antonio Alcalde, Centro Universitario Ciencias de la Salud, Universidad de Guadalajara, Guadalajara. Peru: Jose Hidalgo, Hospital Guillermo Almenara, Lima; Luis Hercilla, Hospital Alberto Sabogal, Lima. Venezuela: Ana María Cáceres Hernández, Clinica La Floresta, Caracas; Marisela Silva, Hospital Universitario de Caracas, Caracas; Alfonso José Guzmán, Centro Médico de Caracas, Caracas.
Funding
This work was supported by Pfizer. C. A. A. is supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) (grants K24-AI114818, R01-AI093749, R21-AI114961, R21/R33 AI121519).
Transparency declarations
C. A. A. has received grant support, consulted for or provided lectures for Pfizer, Cubist, Bayer, Forest Pharmaceuticals, Novartis, and Theravance. M. J. S. has received grant support from Pfizer and Novartis; and lectures and consulting fees from Pfizer, Novartis, Bayer, MSD, Sanofi-Aventis, and Astra-Zeneca. E. R. N. has received lecture fees and consulting fees from Pfizer. C. S. has received fees and grant support from Pfizer and grant support from GlaxoSmithKline and Bristol Myers Squibb. All other authors: none to declare.
Supplementary data
Definitions and Tables S1 to S4 appear as Supplementary data at JAC Online.
Contributor Information
Members of the Latin America Working Group on Bacterial Resistance:
Didier Bruno, Ernesto Efron, Marcelo Del Castillo, Sanatorio Mater Dei, Thaís Guimarães, María Elena Ceballos, Escuela de Medicina, Isabel Domínguez, Daniela Beltrán, Gisela Riedel, Sandra Liliana Valderrama, Sandra Milena Gualtero, Clínica Shaio, Carlos Humberto Saavedra, Facultad de Medicina, Juan Carlos Aragón, Fausto Guerrero, María Mónica Silvestre, Rayo Morfin-Otero, Fray Antonio Alcalde, Jose Hidalgo, Luis Hercilla, Ana María Cáceres Hernández, Marisela Silva, and Alfonso José Guzmán
References
- 1. Jenkins TC, Price CS, Sabel A. et al. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46: 1000–8. [DOI] [PubMed] [Google Scholar]
- 2. Guzmán-Blanco M, Mejía C, Isturiz R. et al. Epidemiology of methicillin resistant Staphylococcus aureus (MRSA) in Latin America. Int J Antimicrob Agents 2009; 34: 304–8. [DOI] [PubMed] [Google Scholar]
- 3. Allard C, Carignan A, Bergevin M. et al. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteremia in Quebec, Canada, 1991-2005. Clin Microbiol Infect 2008; 14: 421–8. [DOI] [PubMed] [Google Scholar]
- 4. Whitby M, McLaws ML, Berry G.. Risk of death from methicillin resistant Staphylococcus aureus bacteremia: a meta-analysis. Med J Aust 2001; 175: 264–7. [DOI] [PubMed] [Google Scholar]
- 5. Blot SI, Vandewoude KH, Hoste EA. et al. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162: 2229–5. [DOI] [PubMed] [Google Scholar]
- 6. Kang CI, Song JH, Chung DR. et al. Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect 2010; 61: 299–306. [DOI] [PubMed] [Google Scholar]
- 7. Cosgrove SE, Sakoulas G, Perencevich EN. et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36: 53–9. [DOI] [PubMed] [Google Scholar]
- 8. Wyllie DH, Crook DW, Peto TE.. Mortality after Staphylococcus aureus bacteremia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 2006; 331: 982.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosgrove SE, Qi Y, Kaye KS. et al. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 2005; 26: 166–74. [DOI] [PubMed] [Google Scholar]
- 10. Chu VH, Crosslin DR, Friedman JY. et al. Staphylococcus aureus bacteremia in patients with prosthetic devices: costs and outcomes. Am J Med 2005; 118: 1416. [DOI] [PubMed] [Google Scholar]
- 11. Rubio-Terrés C, Garau J, Grau S. et al. Cost of bacteremia caused by methicillin-resistant vs. methicillin-susceptible Staphylococcus aureus in Spain: a retrospective cohort study. Clin Microbiol Infect 2010; 16: 722–8. [DOI] [PubMed] [Google Scholar]
- 12. Reyes J, Rincón S, Díaz L. et al. Dissemination of methicillin-resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin Infect Dis 2009; 49: 1861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egea AL, Gagetti P, Lamberghini R. et al. New patterns of methicillin-resistant Staphylococcus aureus (MRSA) clones, community-associated MRSA genotypes behave like healthcare-associated MRSA genotypes within hospitals, Argentina. Int J Med Microbiol 2014; 304: 1086–99. [DOI] [PubMed] [Google Scholar]
- 14. Gelatti LC, Bonamigo RR, Inoue FM. et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying SCCmec type IV in southern Brazil. Rev Soc Bras Med Trop 2013; 46: 34–8. [DOI] [PubMed] [Google Scholar]
- 15. Bai AD, Showler A, Burry L. et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60: 1451–61. [DOI] [PubMed] [Google Scholar]
- 16. McDanel JS, Perencevich EN, Diekema DJ. et al. Comparative effectiveness of β-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis 2015; 61: 361–7. [DOI] [PubMed] [Google Scholar]
- 17. Arias CA, Reyes J, Carvajal LP. et al. Molecular epidemiology and phylogenetics of Staphylococcus aureus bacteremia in Latin America: a prospective cohort multicenter study in nine countries. Antimicrob Agents Chemother 2017; doi:10.1128/AAC.00816-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang FD, Chen YY, Chen TL. et al. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control 2008; 36: 118–22. [DOI] [PubMed] [Google Scholar]
- 19. de Kraker MEA, Wolkewitz M, Davey PG. et al. Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2011; 55: 1598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang JT, Hsu LY, Lauderdale TL. et al. Comparison of outcomes among adult patients with nosocomial bacteremia caused by methicillin-susceptible and methicillin-resistant Staphylococcus aureus: a retrospective cohort study. PLoS One 2015; 10: e0144710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Hal SJ, Jensen SO, Vaska VL. et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25: 362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yaw LK, Robinson JO, Ho KM.. A comparison of long-term outcomes of methicillin-resistant and methicillin-sensitive Staphylococcus aureus bacteraemia. An observational cohort study. Lancet Infect Dis 2014; 14: 967–75. [DOI] [PubMed] [Google Scholar]
- 23. Lawes T, Edwards B, Gould IM.. Are all Staphylococcus aureus equal? Lancet Infect Dis 2015; 15: 762. [DOI] [PubMed] [Google Scholar]
- 24. Fätkenheuer G, Kaasch AJ.. How deadly is methicillin-resistant Staphylococcus aureus? Lancet Infect Dis 2014; 14: 905–7. [DOI] [PubMed] [Google Scholar]
- 25. Paulsen J, Solligård E, Damås JK. et al. The impact of infectious disease specialist consultation for Staphylococcus aureus bloodstream infections: a systematic review. Open Forum Infect Dis 2016; 3: ofw048.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.