Abstract
Background: International data on the molecular epidemiology of Enterobacteriaceae with VIM carbapenemases are limited.
Methods: We performed short read (Illumina) WGS on a global collection of 89 VIM-producing clinical Enterobacteriaceae (2008–14).
Results: VIM-producing (11 varieties within 21 different integrons) isolates were mostly obtained from Europe. Certain integrons with blaVIM were specific to a country in different species and clonal complexes (CCs) (In87, In624, In916 and In1323), while others had spread globally among various Enterobacteriaceae species (In110 and In1209). Klebsiella pneumoniae was the most common species (n = 45); CC147 from Greece was the most prevalent clone and contained In590-like integrons with four different blaVIMs. Enterobacter cloacae complex was the second most common species and mainly consisted of Enterobacter hormaechei (Enterobacter xiangfangensis, subsp. steigerwaltii and Hoffmann cluster III). CC200 (from Croatia and Turkey), CC114 (Croatia, Greece, Italy and the USA) and CC78 (from Greece, Italy and Spain) containing blaVIM-1 were the most common clones among the E. cloacae complex.
Conclusions: This study highlights the importance of surveillance programmes using the latest molecular techniques in providing insight into the characteristics and global distribution of Enterobacteriaceae with blaVIMs.
Introduction
Carbapenems are often the last line of effective therapy available for the treatment of serious infections due to multidrug-resistant bacteria. The rapid evolution of carbapenem resistance in Enterobacteriaceae during the last decade is an emerging global threat.1,2 Enzymes that hydrolyse the carbapenems, known as carbapenemases, are the most important causes of carbapenem resistance. Carbapenemase-producing Enterobacteriaceae (CPE) have acquired multiple resistance genes making therapy for infections due to these bacteria challenging.1,2
The most common carbapenemases among CPE are KPCs (Amber class A), IMPs, VIMs, NDMs (class B lactamases or MBLs) and OXA-48-like (class D) enzymes.1 MBLs hydrolyse all β-lactams except aztreonam although resistance levels may vary according to different subtypes. After the initial discovery of VIM-1 in Italy during 1997, bacteria with VIM enzymes have been detected worldwide.1 VIMs are common among MBL-producing Pseudomonas aeruginosa, but remain relatively rare among members of the Enterobacteriaceae.3 VIM-producing Enterobacteriaceae are mainly found in Europe, particularly Greece, Spain, Hungary and Italy.1,4 The most common species associated with VIMs among the Enterobacteriaceae include Klebsiella pneumoniae, Escherichia coli and Enterobacter spp.2,3 VIM genes are often situated within class 1 integrons harboured on broad-host range plasmids.2,3 These mobile genetic elements play an important role in the interspecies distribution of VIM types of carbapenemases.5
Comprehensive global data regarding the molecular epidemiology of CPE with blaVIM are currently limited. We designed a study that utilized short read WGS to describe the molecular characteristics and international distribution of blaVIM among Enterobacteriaceae obtained from two global surveillance systems.
Methods
Bacterial isolates
We included 89 VIM-producing clinical, non-repeat Enterobacteriaceae collected from two global surveillance programmes namely the Merck Study for Monitoring Antimicrobial Resistance Trends (SMART) (2008–14) and the AstraZeneca global surveillance study of antimicrobial resistance (2012–13) (Dataset S1, available as Supplementary data at JAC Online).
The SMART programme included isolates from intra-abdominal and urinary tract infections from the following countries: Morocco, South Africa and Tunisia (Africa); China, Malaysia, Singapore, South Korea, Taiwan, Thailand and Vietnam (Asia); the Czech Republic, Estonia, France, Georgia, Greece, Germany, Hungary, Italy, Latvia, Lithuania, Portugal, Romania, Slovenia, Spain, Turkey and the UK (Europe); Argentina, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Puerto Rico, Panama, Uruguay and Venezuela (Latin America); Jordan, Lebanon, Israel, Saudi Arabia and UAE (Middle East); Canada and the USA (North America); and Australia, New Zealand, the Philippines and Japan (South Pacific).
The AstraZeneca programme included isolates from skin and soft tissue and lower respiratory tract infections from the following countries: Egypt, Kenya, Nigeria and South Africa (Africa); China, South Korea, Taiwan and Thailand (Asia); Austria, Belgium, Bulgaria, Greece, the Czech Republic, Denmark, France, Germany, Hungary, Italy, Macedonia, Portugal, Poland, Russia, Romania, Slovakia, Spain, Turkey and the UK (Europe); Argentina, Brazil, Chile, Colombia, Mexico, Uruguay and Venezuela (Latin America); Lebanon, Israel, Syria and Kuwait (Middle East); the USA (North America); and Australia, the Philippines and Japan (South Pacific).
Both programmes collected consecutive clinically relevant Gram-negative aerobes in each institution. These isolates initially underwent micro-dilution panel susceptibility testing and molecular screening for blaVIM as described previously.6 Overall 107 366 isolates were obtained from 2008 to 2014; of these 755 were positive for blaKPC, 281 for blaOX-48-like, 271 for blaNDM, 89 for blaVIM and 38 for blaIMP.
WGS
We used the Nextera XT DNA sample preparation kit (Illumina, San Diego, CA, USA) to prepare libraries for sequencing. Samples were multiplexed and sequenced on an Illumina NextSeq500 for 300 cycles (151 bp paired-end).
Genomic analysis
Draft genomes were obtained using SPAdes version 3.8.1.7 Species identification was performed using SILVA 16s rRNA gene database release 123.8 In addition, we used a whole genome-based phylogenetic tree including type strains for identification of Klebsiella spp., Enterobacter spp.9 and Citrobacter spp. (Dataset S2). Average nucleotide identity (ANI) was calculated using JSpecies.10
To define presence of genes and their alleles, we used SRST211 and BLAST+12 in combination with following databases or typing schemes: NCBI BLAST database (http://blast.ncbi.nlm.nih.gov/Blast/), NCBI Beta-Lactamase Data Resources (http://www.ncbi.nlm.nih.gov/pathogens/beta-lactamase-data-resources/), ARG-ANNOT,13 PlasmidFinder,14 plasmid addiction systems15 and MLST (http://bigsdb.pasteur.fr/klebsiella/, http://pubmlst.org/ecloacae/, http://pubmlst.org/cfreundii/, http://mlst.ucc.ie/mlst/dbs/Ecoli/).
The goeBURST algorithm implemented in PHYLOViZ software16 was used to demonstrate relationships between STs and to define the founder of a clonal complex (CC). We defined CCs at the single-locus variant level. Integrons were classified according to INTEGRALL (http://integrall.bio.ua.pt/) and promoters of gene cassettes were characterized according to a previous study.17 For Klebsiella isolates, we performed in silico detection of K capsular type based on wzi alleles,18 virulence genes (http://bigsdb.pasteur.fr/klebsiella) and promoters and coding sequences of ompK35/K36.19,20 For E. coli isolates, we performed in silico phylogenetic grouping.21
Phylogenetic analysis
We used a core genome SNP-based approach to create a phylogenetic tree for each Enterobacteriaceae genus. SNPs were identified using trimmed reads mapping to a genus-specific reference genome (Dataset S2) followed by GATK Best Practices workflow22 and SAMtools23 (depth of sequencing >10 and Phred-score >20). Draft or complete genomes downloaded from the NCBI database (Dataset S2) were aligned against the reference genome of the genus using ProgressiveMauve to obtain pseudo-chromosomes that contained only SNPs.24 The SNP-only core genome was identified as the common blocks of >500 bp to all of the study isolates. Maximum-likelihood tree was built using RAxML25 and visualized using FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Sequence data accession numbers
We deposited the sequencing data in the DDBJ and NCBI databases (accession no. DRA004879 and SRP046977). The sequences of new integrons described in this study ranged from accession number LC169570 to LC169586.
Results and discussion
Geographical distribution showed VIM-producing Enterobacteriaceae mostly in Europe
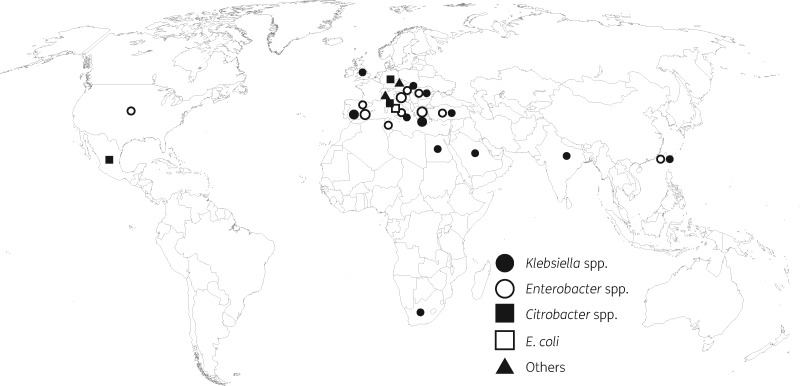
The 89 VIM-producing Enterobacteriaceae were present in 17 countries, mostly from Europe (n = 79) followed by Africa (n = 4) (Figure 1 and Dataset S1). The common sources were intra-abdominal specimens (n = 59) and urines (n = 28). The isolates include the following microorganisms: Klebsiella pneumoniae subsp. pneumoniae (n = 45), Klebsiella variicola (n = 2), Enterobacter cloacae complex (n = 33), Citrobacter spp. (n = 6), E. coli (n = 1), Proteus mirabilis (n = 1) and Serratia marcescens (n = 1) (Figure 1 and Table 1).
Figure 1.
Global distribution of VIM-producing Enterobacteriaceae isolates in this study.
Table 1.
VIM subtypes and integrons of the Enterobacteriaceae isolates
| Carbapenemase (n) | Species, country (n) |
Defined integron numbers (species, n) | ||||
|---|---|---|---|---|---|---|
| Klebsiella spp. (KP) | E. cloacae complex (Ecl) | Citrobacter spp. (CI) | E. coli (EC) | P. mirabilis (PM), S. marcescens (SM) | ||
| VIM-1 (67) | Greece (14), Spain (12), Italy (4), South Africa (2), Egypt (1), Taiwan (1) | Greece (8), Croatia (7), Spain (6), Italy (4), Taiwan (1), Tunisia (1), the USA (1) | Italy (2), Germany (1) | Italy (1) | PM, Italy (1) | In916a (KP, 4; Ecl, 2; CI, 1; PM 1), In591b (KP, 8), In1209b (KP, 5; Ecl 1), In87a (Ecl, 4; KP, 1), In110b (KP, 1; Ecl, 4; CI, 1), In624a (Ecl, 4; KP, 1), In237 (Ecl, 2), In1315 (Ecl, 1), In1318 (Ecl, 1), In1322 (CI, 1), In3103 (Ecl, 1), In4873 (Ecl, 1) |
| VIM-2 (2) | Mexico (1), Spain (1) | In339 (CI, 1) | ||||
| VIM-4 (7) | Hungary (2), Romania (1) | Romania (2), Hungary (1) | SM, the Czech Republic (1) | In1323a (Ecl, 2; KP, 1), In238 (SM, 1) | ||
| VIM-5 (2) | Turkey (1) | Turkey (1) | In1316 (Ecl, 1) | |||
| VIM-19 (2) | Greece (2) | In4863 (KP, 2) | ||||
| VIM-23 (1) | Mexico (1) | In1320 (CI, 1) | ||||
| VIM-26 (2) | Greece (2) | In1157 (KP, 2) | ||||
| VIM-27 (1) | Greece (1) | undefined | ||||
| VIM-29 (2) | Saudi Arabia (1), the UK (1) | undefined | ||||
| VIM-31 (1) | Turkey (1) | In669 (KP, 1) | ||||
| VIM-33 (2) | Greece (2) | In1317 (KP, 2) | ||||
In1315 to In1318, In1320, In1322 and In1323 were novel integrons found in this study.
Same integron was found in isolates from only one country: Greece (In87, In237), Italy (In916), Spain (In624) and Romania (In1323).
Same integron was found in isolates from multiple countries: In110, Croatia (Ecl), South Africa (KP), Spain (Ecl) and Germany (CI); In591, Greece and Egypt (KP); In1209, Greece (KP) and the USA (Ecl).
The 89 genomes were sequenced at an average depth of 167 [standard deviation (SD) 87.9] (Dataset S1). Assembled genomes had an average number of contigs of 101 (SD 50.4) and N50 value of 265 210 bp (SD 98 928 bp). We confirmed the presence of blaVIM in the draft genomes of all the isolates.
The presence of resistance genes, antibiotic resistance profiles, plasmid replicons and plasmid addiction systems is shown in Figure S1. Table 1 shows the geographical distribution of the different species, types of carbapenemases and integrons. We identified 11 blaVIM variants namely: blaVIM-1 (n = 67), blaVIM-2 (n = 2), blaVIM-4 (n = 7), blaVIM-5 (n = 2), blaVIM-19 (n = 2), blaVIM-23 (n = 1), blaVIM-26 (n = 2), blaVIM-27 (n = 1), blaVIM-29 (n = 2), blaVIM-31 (n = 1) and blaVIM-33 (n = 2). VIM-1, -4 and -5 were present in different microorganisms (Table 1). The distribution of the different blaVIM subtypes was similar to previously published data.2,26,27 Our results show that VIM-1 has a global distribution, VIM-2 was present in Mexico and Spain, VIM-4 in Europe, VIM-5 and -31 in Turkey, VIM-19, -26, -27 and -33 were limited to Greece, VIM-23 in Mexico and VIM-29 was present in Saudi Arabia and the UK (Table 1). Enterobacteriaceae (most often K. pneumoniae) with blaVIM-1 were previously responsible for nosocomial outbreaks throughout Greece and Italy during the early–mid 2000s28,29 and since then sporadic outbreaks had been described from different parts of the world.1,30 Apart from blaVIM-1, Enterobacteriaceae with the following blaVIMs have been reported: blaVIM-2 in Austria,31 Mexico32 and Venezuela33; and blaVIM-4 in the Czech Republic,34 Egypt,35 Hungary,36 Italy37 and Kuwait.38 In addition, a recent global surveillance study from 2012 to 2014 reported Enterobacteriaceae with the following blaVIMs: blaVIM-5 in Turkey and Nigeria; blaVIM-23 in Mexico; blaVIM-26 in Greece; blaVIM-32 in the USA; and blaVIM-42 from Italy.39
Characterization of class 1 integrons identified 21 different integron types, including seven novel cassette combinations
All of the blaVIMs were situated within class 1 integrons. We were unable to sequence the complete integron-associated gene cassettes in 30 isolates due to the limitations associated with short-read sequencing. We were able to characterize partially 27 of 30 additional integrons (Figures 2 and 3, and Dataset S3).
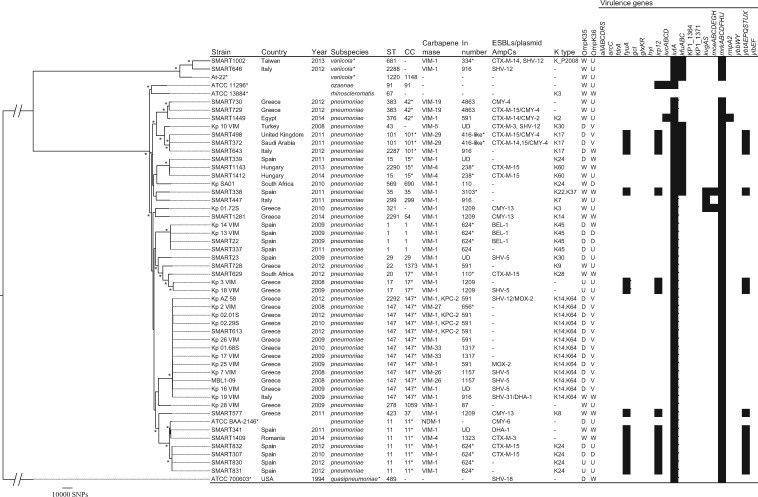
Figure 2.
Phylogenetic tree of VIM-producing Klebsiella spp. This maximum-likelihood phylogram is based on a 3737 806 bp core genome and a total of 369 829 SNPs. Core genome was identified using K. pneumoniae subsp. pneumoniae ATCC BAA-2146 as a reference genome. Tree includes 47 study isolates and five reference strains (marked with asterisks). Tree is rooted by using the outgroup of K. quasipneumoniae ATCC 700603 and asterisks indicate bootstrap support >90% from 100 replicates. In the ‘Subspecies’ column, K. variicola and K. quasipneumoniae (marked with asterisks) are not subspecies of K. pneumoniae, but distinct species. STs 2287–2292 were novel types found in this study. A CC marked with an asterisk was distributed internationally. Integron numbers with asterisks were partially characterized (Dataset S3). ‘OmpK35’ and ‘OmpK36’ columns indicate predicted mutation of porins: W, WT; D, deficient (due to premature stop codon); V, variant associated with increased MIC of carbapenems; U, variant with unknown significance. Virulence genes of clbA-R (colibactin), iroBCDN (salmochelin) and rmpA were sought, but not found. UD, undetermined.
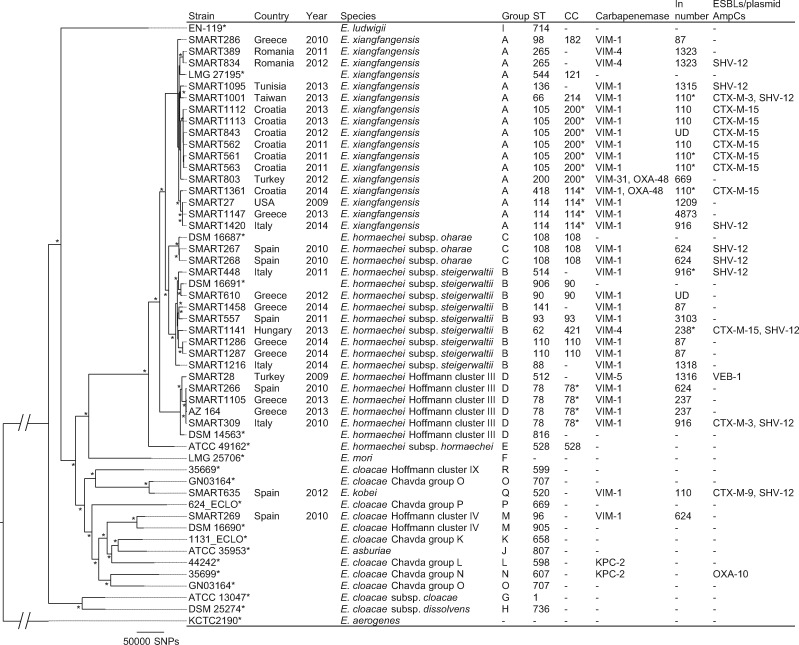
Figure 3.
Phylogenetic tree of VIM-producing Enterobacter spp. This maximum-likelihood phylogram is based on a 1738 728 bp core genome and a total of 511 679 SNPs. Core genome was identified using E. cloacae subsp. cloacae ATCC 13047 as a reference genome. Tree includes 33 study isolates and 19 reference strains (marked with asterisks). Tree is rooted by using the outgroup of E. aerogenes KCTC 2190 and asterisks indicate bootstrap support >90% from 100 replicates. ‘Group’ column indicates E. cloacae complex groups defined by Chavda et al.9 ST512, ST514 and ST520 were novel types found in this study. CC marked with an asterisk was distributed internationally. Integron numbers with asterisks were partially characterized (Dataset S3). UD, undetermined.
We identified 21 different integron types containing blaVIM, including seven novel combinations (Table 2). In110 and In1209, that contain blaVIM-1 had international, intercontinental and inter-genus distribution [In110, Croatia (Enterobacterxiangfangensis), South Africa (K. pneumoniae), Spain (Enterobacter kobei) and Germany (Citrobacter freundii); In1209, Greece (K. pneumoniae) and the USA (E. xiangfangensis)]. In87, In624, In916 and In1323 were present in different species from the same country (Tables 1 and 2). The international and inter-genus distribution of blaVIM-1 was similar to integrons and their variants previously reported, including In590-like (In-e541-like) reported from Greece, In416-like from Greece, In110 from Spain, Italy and Latvia, In476-like (originally In113, corresponding to In624 in this study) from Spain and In916 from Italy, France and Spain.28,40
Table 2.
Details of class 1 integrons with blaVIM
| Integron number |
n | Gene cassettes | Promoter type (n) | Downstream of gene cassettes (n) | Accession number of the integron | |
|---|---|---|---|---|---|---|
| Major type | variant | |||||
| In87 | 5 | blaVIM-1-aacA27 | PcS (1), UD (4) | qacEΔ1-sul1-orf5-orf6-IS26 (1), UD (4) | AY648125 | |
| In110 | 6 | blaVIM-1-aacA4-aadA1 | PcH2 (6) | qacEΔ1-sul1-ISCR1 (2), qacEΔ1-sul1-orf5-orf6-IS6100 (1), qacEΔ1-sul1-orf5-ΔtniB-tniA-IRt (1), qacEΔ1-sul1-ISCR1-sapA-orf2-qnrB2-ΔqacEΔ1-sul1-orf5-orf6-IRt (1), UD (1) | LC169583 | |
| In237 | In237a | 2 | aacA4-blaVIM-1 | PcS (1), UD (1) | qacEΔ1-sul1-orf5-IS1326-ΔtniB-tniA-IRt (1), UD (1) | LC169571 |
| In238a | 1 | aacA4-blaVIM-4 | PcS (1) | qacEΔ1-sul1-orf5-orf6-IS6100 (1) | LC169580 | |
| In339 | 1 | blaVIM-2-aacA7 | UD (1) | UD (1) | FJ627181 | |
| In416 | In416 | 0b | blaVIM-4-aacA7-dfrA1-ΔaadA1-smr | PcS | ISPa21-like-arsR | AJ704863 |
| In4863 | 2 | blaVIM-19-aacA7-dfrA1-ΔaadA1-smr | PcH2 (1), UD (1) | ISPa21-like-arsR (2) | LC169563 | |
| In4873 | 1 | blaVIM-1-aacA7-dfrA1-ΔaadA1-smr | PcS (1) | ISPa21-like-qacEΔ1-sul1-orf5-ΔIS1326-IS1353-ΔIS1326-ΔtniB-ΔtniA-IS26 (1) | LC169572 | |
| In590 (In-e541) | In590 | 0b | blaVIM-1-aacA7-dfrA1-aadA1c | PcS | qacEΔ1-sul1-orf5-IS26 | AY339625 |
| In591 | 8 | blaVIM-1-aacA7-dfrA1-ΔaadA1 | PcS (8) | qacEΔ1-sul1-Δorf5-IS26 (6), qacEΔ1-sul1-orf5-ΔIS1326-ΔIS1353-IS26 (1), UD (1) | LC169574, LC169576, LC169577 | |
| In1157 | 2 | blaVIM-26-aacA7-dfrA1-ΔaadA1 | PcS (2) | qacEΔ1-sul1-Δorf5-IS26 (1), ΔqacEΔ1-IS10 (1) | LC169582 | |
| In1209 | 6 | blaVIM-1-aacA7-dfrA1-aadA1c | UD (6) | IS1R (5), IS1R-like (1) | LC169573 | |
| In1317 | 2 | blaVIM-33-aacA7-dfrA1-ΔaadA1 | PcS (2) | qacEΔ1-sul1-Δorf5-IS26 (2) | LC169581 | |
| In624 | 5 | blaVIM-1-aacA4-dfrB1-aadA1, catB2 | PcH1TTN-10 (2), UD (3) | qacEΔ1-sul1-orf5-ΔIS1326-IS26 (2), UD (3) | GQ422827 | |
| In669 | 1 | blaVIM-31-aacA4 | PcWTGN-10 (1) | qacEΔ1-sul1-orf5-Δorf6-IS6100 (1) | JN982330 | |
| In916 | 8 | blaVIM-1-aacA4-aphA15-aadA1-catB2 | PcS (1), UD (7) | qacEΔ1-sul1-orf5-ΔtniB-tniA-IS26 (2), qacEΔ1-sul1-Δorf5-chrA-padR-IS6100 (2), UD (4) | KF856617 | |
| In1315 | 1 | blaVIM-1-aacA7-smr | UD (1) | ISPa21-like-3′-CSd (1) | LC169570 | |
| In1316 | 1 | blaVIM-5-gcuD-aacA4-blaOXA-2-gcuD | PcWTGN-10 (1) | UD (1) | LC169578 | |
| In1318 | 1 | blaVIM-1, aadA1e | PcS (1) | qacEΔ1-sul1-orf5-IS26 (1) | LC169584 | |
| In1320 | 1 | blaVIM-23-gcu172-aacA7 | UD (1) | UD (1) | LC169586 | |
| In1322 | 1 | blaVIM-1-aadA7-ΔgcuDf | UD (1) | UD (1) | LC169574 | |
| In1323 | 3 | blaVIM-4-aacA27 | PcW-P2 (1), UD (2) | qacEΔ1-sul1-orf5-ΔtniB-tniA-IRt (1), qacEΔ1-sul1-orf5-ΔtniB-ΔtniA-IS26 (1), UD (1) | LC169579 | |
| In3103 | 1 | blaVIM-1-aacA4-dfrB1-aadA1 | UD (1) | UD (1) | LC169588 | |
UD, undetermined due to a contig break in 5′-CS or 3′-CS; IRt, inverted repeat of Tn402-like transposon.
These integrons lacked the duplication of the ΔblaVIM regions which was present in the original sequences of In237 (GenBank accession no. EF690695) and In238 (EU581706).
This type was not identified in this study, but is presented here for comparison.
In590 and In1209 have a different aadA1 allele (aadA1a and aadA1b, respectively).
Contig break in the nucleotide position 123 of 3′-CS.
Between blaVIM-1 and aadA1, putative group II intron reverse transcriptase, which has 93% nucleotide identity to the reverse transcriptase gene found in GenBank accession no. CP002811.1, was present disrupting the attC site.
C to A mutation at nucleotide position 279 created premature stop codon.
Integrons with strong promoters (i.e. PcS and PcH2) were common whereas weak promoters (i.e. PcW and PcH1) were rare (Tables 2 and S1). We were able to characterize the downstream structures in 16 blaVIM-containing integrons (Tables 2 and S2). The majority contained 3′-CS structures immediately downstream of the gene cassettes. Of these, variants of a typical class 1 integron structure, 3′-CS-IS1326-ΔtniB-tniA-IRt,41 with disruption by IS26, were prevalent. Non-3′-CS variants included ISPa21-like or IS1R-like ISs downstream in four integrons with blaVIM-1 and blaVIM-19 (Table 2).
Klebsiella spp. consisted mostly of K. pneumoniae subsp. pneumoniae with three dominant CCs
The phylogenetic relationships of 46 K. pneumoniae (including 1 reference strain) and 3 K. variicola isolates (including 1 reference strain) are shown in Figure 2. Genome analyses revealed that ‘K. pneumoniae’ includes three distinct phylogroups of KpI (K. pneumoniae), KpII (K. quasipneumoniae) and KpIII (K. variicola).42K. variicola was previously identified among 11% and 24% of clinical ‘K. pneumoniae’ isolates43,44 and patients with bloodstream infection due to K. variicola had higher mortality than those due to K. pneumoniae.44
K. pneumoniae subsp. pneumoniae from our study comprised 23 different STs (Figure 2). The most prevalent CCs (with ≥5 isolates) included CC147 (n = 13) (from Italy and Greece) and CC11 (n = 6) (from Spain and Romania); CC147 was dominated by ST147 and CC11 consisted only of ST11. CC147 accommodated four different integron types (the most common being In590-like) and were associated with the PcS strong promoter and the IS26 insertion variant that formed part of the 3′-CS downstream structures. CC147 with In590-like integrons is endemic in Greece and is currently emerging globally with different carbapenemases, including KPCs, OXA-181 and NDMs.28,30,45 ST11 is a successful global, multidrug-resistant clone and is a single-locus variant of ST258.5 Some CCs in our study had an international distribution (i.e. present in at least two countries on different continents): CC17 (n = 3) in South Africa and Greece; CC42 (n = 3) in Greece and Egypt; and CC101 (n = 3) in Saudi Arabia, the UK and Italy.
OmpK35 and OmpK36 deficiencies and variants are responsible for alterations in porins that contribute to increased MICs of the carbapenems.30 The majority of the study isolates had OmpK35 deficiency due to premature stop codons and OmpK36 deficiency or variants (Figure 2). Only 17% of the isolates had WT OmpK35 and OmpK36.
Hypervirulent K. pneumoniae strains often possess siderophore clusters (i.e. yersiniabactin, aerobactin, colibactin and salmochelin) as well as rmpA or rmpA2.42 Yersiniabactin, which is encoded by a pathogenicity island that includes ybt, irp12 and fyuA genes,42 was present in isolates from this study belonging to CCs 11, 17, 35, 37 and 101 (Figure 2).
E. cloacae complex consisted mostly of Enterobacter hormaechei with three dominant CCs
The latest WGS-based phylogenomic study revealed that the E. cloacae complex is made up of 18 groups, which are difficult to distinguish using phenotypic or conventional molecular methods.9 That study proposed that E. hormaechei included two more subspecies of E. xiangfangensis and Hoffmann cluster III, in addition to the three original subspecies (hormaechei, oharae and steigerwaltii) defined by Hoffmann et al.46E. xiangfangensis was the most common Enterobacter group associated with blaKPC.9 Other recent studies showed that E. hormaechei subsp. steigerwaltii and E. hormaechei Hoffmann cluster III are the most prevalent clinical species among the E. cloacae complex.47,48
The E. cloacae complex (n = 33) was the second most common microorganism in our study and consisted mainly of E. hormaechei: E. xiangfangensis (n = 16), subsp. steigerwaltii (n = 8) and Hoffmann cluster III (n = 5), and subsp. oharae (n = 2) (Figure 3). In silico MLST analysis identified 11 CCs and 24 STs among the E. cloacae complex (Figure 3). E. xiangfangensis CC200 (with blaVIM-1 from Croatia and Turkey), E. xiangfangensis CC114 (with blaVIM-1 from Croatia, Greece, Italy and the USA) and E. hormaechei Hoffmann cluster III CC78 (with blaVIM-1 from Greece, Italy and Spain) were the most common CCs among the E. cloacae complex. Previous molecular epidemiology studies have shown that CC200 (more specifically ST105) with blaVIM-1 are common in Croatia,49 while CC78 and CC114 are global clones associated with blaCTX-M-15 or blaVIM-1 particularly among European countries.50 None of the study isolates belonged to ST171.
Citrobacter spp. and E. coli
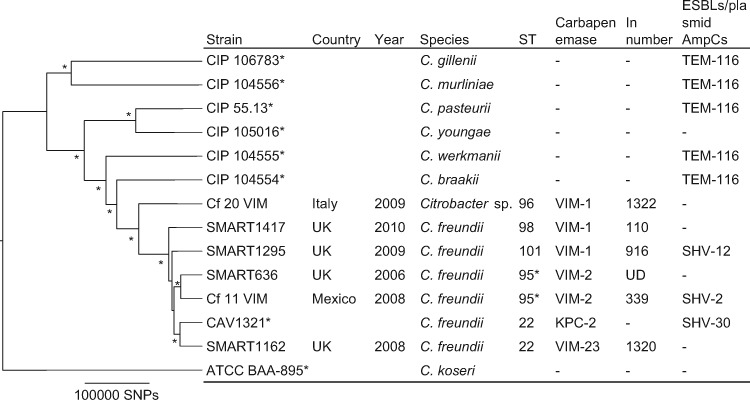
Citrobacter spp. isolates (n = 6) included in our study belonged to ST22, ST95, ST96, ST98 and ST101 (Figure 4). One isolate (Cf 20 VIM) was classified as Citrobacter spp. based on the phylogenetic tree constructed with type strains (Figure 4).51 The ANI values between this isolate and the three most closely related Citrobacter species (i.e. C. freundii, Citrobacter braakii and Citrobacter werkmanii) were <95% (i.e. is the cut-off value of species definition) (Table S3). ANI is a promising method of defining species using WGS replacing DNA–DNA hybridization.10
Figure 4.
Phylogenetic tree of VIM-producing Citrobacter spp. This maximum-likelihood phylogram is based on a 2 406 029 bp core genome and a total of 594 405 SNPs. Core genome was identified using C. freundii CAV1321 as a reference genome. Tree includes six study isolates and eight reference strains (marked with asterisks). Tree is rooted by using the outgroup of Citrobacter koseri ATCC BAA-895 and asterisks indicate bootstrap support >90% from 100 replicates. An ST marked with an asterisk was distributed internationally. STs 95, 96, 98 and 101 were novel types found in this study. UD, undetermined.
The phylogenetic relationship of one E. coli isolate with blaVIM-1 belonged to phylogenetic group E and ST1955.
This study has some limitations. Our collection may not represent the global prevalence of VIM and integron subtypes. We were unable to determine all of the integron structures due to the limitation of short-read sequencing. Long-read sequencing techniques, including the detailed analysis of plasmids, would provide more knowledge on location, mobile elements and plasmid backbones of these carbapenemases.
Summary
To the best of our knowledge, this is the first study to elucidate the global epidemiology on a large scale of blaVIM-containing Enterobacteriaceae using WGS with comprehensive molecular analysis. The distribution of blaVIM-containing integrons showed distinctive patterns. (i) Certain integrons were present in specific countries, but in different species (i.e. In87 with blaVIM-1 from Greece, In624 with blaVIM-1 from Spain, In916 with blaVIM-1 from Italy and In1323 with blaVIM-4 from Romania were present in different species from that country). This suggested the circulation of the same integron among different bacteria within the same country. (ii) The same integron was present globally in different species. We identified In110 with blaVIM-1 in K. pneumoniae, E. xiangfangensis, E. kobei and C. freundii from Croatia, Germany, South Africa and Spain. In1209 with blaVIM-1 was present in different K. pneumoniae CCs from Greece and E. xiangfangensis from the USA. (iii) The remaining blaVIM containing integrons were limited to one country within a single species.
The association of certain high-risk clones with specific integrons showed that K. pneumoniae CC147 from Greece was associated with In590-like integrons that only differ because of the VIM subtypes (i.e. In591 with blaVIM-1; In1157 with blaVIM-26; and In1317 with blaVIM-33). This had previously been described.28E. xiangfangensis ST105 from Croatia was associated with In110 containing blaVIM-1.
This study highlights the importance of surveillance programmes using the latest molecular techniques in providing insight into the characteristics, global distribution of CCs and their association with integrons containing blaVIMs.
Supplementary Material
Acknowledgements
This research was in part supported by WestGrid (www.westgrid.ca) and Compute Canada Calcul Canada (www.computecanada.ca). We thank the team of curators of the Institut Pasteur MLST and whole genome MLST databases for curating the data and making them publicly available at http://bigsdb.web.pasteur.fr/. We thank Thomas Jové from INTEGRALL for curating integrons.
Funding
This work was supported by the John Mung Program from Kyoto University, Japan (Y. M.), a research grant from the Calgary Laboratory Services (no. 10015169; J. D. D. P.) and federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under Award Numbers U19AI110819 (M. D. A.) and R01AI090155 (to B. K.).
The funding organizations had no role in study design, data collection and interpretation, or the decision to submit the work for publication
Transparency declarations
P. A. B. is an employee of AstraZeneca and M. R. M. is an employee of Merck. J. D. D. P. had previously received research funds from Merck and AstraZeneca. All other authors: none to declare.
Supplementary data
Datasets S1 to S3, Figure S1 and Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Nordmann P, Naas T, Poirel L.. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 2011; 17: 1791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tzouvelekis LS, Markogiannakis A, Psichogiou M. et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25: 682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh TR, Toleman MA, Poirel L. et al. Metallo-β-lactamases: the quiet before the storm? Clin Microbiol Rev 2005; 18: 306–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albiger B, Glasner C, Struelens MJ. et al. Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 2015; 20: pii=30062. [DOI] [PubMed] [Google Scholar]
- 5. Mathers AJ, Peirano G, Pitout JD.. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 2015; 28: 565–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peirano G, Bradford PA, Kazmierczak KM. et al. Global incidence of carbapenemase-producing Escherichia coli ST131. Emerg Infect Dis 2014; 20: 1928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nurk S, Bankevich A, Antipov D. et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol 2013; 20: 714–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quast C, Pruesse E, Yilmaz P. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41: D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chavda KD, Chen L, Fouts DE. et al. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. MBio 2016; 7: e02093–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richter M, Rosselló-Móra R.. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 2009; 106: 19126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inouye M, Dashnow H, Raven LA. et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 2014; 6: 90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camacho C, Coulouris G, Avagyan V. et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10: 421.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta SK, Padmanabhan BR, Diene SM. et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 2014; 58: 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carattoli A, Zankari E, García-Fernández A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mnif B, Vimont S, Boyd A. et al. Molecular characterization of addiction systems of plasmids encoding extended-spectrum β-lactamases in Escherichia coli. J Antimicrob Chemother 2010; 65: 1599–603. [DOI] [PubMed] [Google Scholar]
- 16. Francisco AP, Vaz C, Monteiro PT. et al. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics 2012; 13: 87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jové T, Da Re S, Denis F. et al. Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet 2010; 6: e1000793.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brisse S, Passet V, Haugaard AB. et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 2013; 51: 4073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Papagiannitsis CC, Giakkoupi P, Kotsakis SD. et al. OmpK35 and OmpK36 porin variants associated with specific sequence types of Klebsiella pneumoniae. J Chemother 2013; 25: 250–4. [DOI] [PubMed] [Google Scholar]
- 20. Matsumura Y, Tanaka M, Yamamoto M. et al. High prevalence of carbapenem resistance among plasmid-mediated AmpC β-lactamase-producing Klebsiella pneumoniae during outbreaks in liver transplantation units. Int J Antimicrob Agents 2015; 45: 33–40. [DOI] [PubMed] [Google Scholar]
- 21. Clermont O, Christenson JK, Denamur E. et al. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 2013; 5: 58–65. [DOI] [PubMed] [Google Scholar]
- 22. McKenna A, Hanna M, Banks E. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010; 20: 1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Handsaker B, Wysoker A. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009; 25: 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darling AE, Mau B, Perna NT.. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 2010; 5: e11147.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30: 1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Queenan AM, Bush K.. Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 2007; 20: 440–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lascols C, Peirano G, Hackel M. et al. Surveillance and molecular epidemiology of Klebsiella pneumoniae isolates that produce carbapenemases: first report of OXA-48-like enzymes in North America. Antimicrob Agents Chemother 2013; 57: 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papagiannitsis CC, Izdebski R, Baraniak A. et al. Survey of metallo-β-lactamase-producing Enterobacteriaceae colonizing patients in European ICUs and rehabilitation units, 2008-11. J Antimicrob Chemother 2015; 70: 1981–8. [DOI] [PubMed] [Google Scholar]
- 29. Gaibani P, Ambretti S, Farruggia P. et al. Outbreak of Citrobacter freundii carrying VIM-1 in an Italian Hospital, identified during the carbapenemases screening actions, June 2012. Int J Infect Dis 2013; 17: e714–7. [DOI] [PubMed] [Google Scholar]
- 30. Pitout JD, Nordmann P, Poirel L.. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 2015; 59: 5873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duljasz W, Gniadkowski M, Sitter S. et al. First organisms with acquired metallo-β-lactamases (IMP-13, IMP-22, and VIM-2) reported in Austria. Antimicrob Agents Chemother 2009; 53: 2221–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morfin-Otero R, Rodriguez-Noriega E, Deshpande LM. et al. Dissemination of a blaVIM-2-carrying integron among Enterobacteriaceae species in Mexico: report from the SENTRY Antimicrobial Surveillance Program. Microb Drug Resist 2009; 15: 33–5. [DOI] [PubMed] [Google Scholar]
- 33. Falco A, Ramos Y, Franco E. et al. A cluster of KPC-2 and VIM-2-producing Klebsiella pneumoniae ST833 isolates from the pediatric service of a Venezuelan Hospital. BMC Infect Dis 2016; 16: 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hrabák J, Papagiannitsis CC, Študentová V. et al. Carbapenemase-producing Klebsiella pneumoniae in the Czech Republic in 2011. Euro Surveill 2013; 18: pii=20626. [DOI] [PubMed] [Google Scholar]
- 35. Dimude JU, Amyes SG.. Molecular characterisation and diversity in Enterobacter cloacae from Edinburgh and Egypt carrying blaCTX-M-14 and blaVIM-4 β-lactamase genes. Int J Antimicrob Agents 2013; 41: 574–7. [DOI] [PubMed] [Google Scholar]
- 36. Melegh S, Kovács K, Gám T. et al. Emergence of VIM-4 metallo-β-lactamase-producing Klebsiella pneumoniae ST15 clone in the Clinical Centre University of Pécs, Hungary. Clin Microbiol Infect 2014; 20: O27–9. [DOI] [PubMed] [Google Scholar]
- 37. Luzzaro F, Docquier JD, Colinon C. et al. Emergence in Klebsiella pneumoniae and Enterobacter cloacae clinical isolates of the VIM-4 metallo-β-lactamase encoded by a conjugative plasmid. Antimicrob Agents Chemother 2004; 48: 648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jamal W, Rotimi VO, Albert MJ. et al. High prevalence of VIM-4 and NDM-1 metallo-β-lactamase among carbapenem-resistant Enterobacteriaceae. J Med Microbiol 2013; 62: 1239–44. [DOI] [PubMed] [Google Scholar]
- 39. Kazmierczak KM, Rabine S, Hackel M. et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2016; 60: 1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tato M, Coque TM, Baquero F. et al. Dispersal of carbapenemase blaVIM-1 gene associated with different Tn402 variants, mercury transposons, and conjugative plasmids in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2010; 54: 320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Partridge SR, Tsafnat G, Coiera E. et al. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol Rev 2009; 33: 757–84. [DOI] [PubMed] [Google Scholar]
- 42. Holt KE, Wertheim H, Zadoks RN. et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA 2015; 112: E3574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brisse S, van Himbergen T, Kusters K. et al. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin Microbiol Infect 2004; 10: 942–5. [DOI] [PubMed] [Google Scholar]
- 44. Maatallah M, Vading M, Kabir MH. et al. Klebsiella variicola is a frequent cause of bloodstream infection in the Stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One 2014; 9: e113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasan CM, Turlej-Rogacka A, Vatopoulos AC. et al. Dissemination of blaVIM in Greece at the peak of the epidemic of 2005-2006: clonal expansion of Klebsiella pneumoniae clonal complex 147. Clin Microbiol Infect 2014; 20: 34–7. [DOI] [PubMed] [Google Scholar]
- 46. Hoffmann H, Stindl S, Ludwig W. et al. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. J Clin Microbiol 2005; 43: 3297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kremer A, Hoffmann H.. Prevalences of the Enterobacter cloacae complex and its phylogenetic derivatives in the nosocomial environment. Eur J Clin Microbiol Infect Dis 2012; 31: 2951–5. [DOI] [PubMed] [Google Scholar]
- 48. Ohad S, Block C, Kravitz V. et al. Rapid identification of Enterobacter hormaechei and Enterobacter cloacae genetic cluster III. J Appl Microbiol 2014; 116: 1315–21. [DOI] [PubMed] [Google Scholar]
- 49. Bedenić B, Sardelić S, Luxner J. et al. Molecular characterization of class b carbapenemases in advanced stage of dissemination and emergence of class d carbapenemases in Enterobacteriaceae from Croatia. Infect Genet Evol 2016; 43: 74–82. [DOI] [PubMed] [Google Scholar]
- 50. Izdebski R, Baraniak A, Herda M. et al. MLST reveals potentially high-risk international clones of Enterobacter cloacae. J Antimicrob Chemother 2015; 70: 48–56. [DOI] [PubMed] [Google Scholar]
- 51. Clermont D, Motreff L, Passet V. et al. Multilocus sequence analysis of the genus Citrobacter and description of Citrobacter pasteurii sp. nov. Int J Syst Evol Microbiol 2015; 65: 1486–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.