Abstract
Objectives
Staphylococcus aureus native efflux pump Tet38 confers resistance to tetracycline when overexpressed. tet38 expression is selectively upregulated in infection sites. This study evaluated the effect of Tet38 on tetracycline response in a murine subcutaneous abscess model.
Methods
S. aureus MW2 and its tet38 mutant were injected subcutaneously on the opposite flanks of each mouse. The infected mice were treated with tetracycline (10 mg/kg) or PBS (control) intraperitoneally every 12 h. The efficacy of tetracycline against S. aureus was measured by the relative change in viable bacteria in the abscesses 24 h after infection compared with the initial inoculum. Plasmid-based tet38-complemented strains were created and used to infect the mice followed by tetracycline or PBS treatment.
Results
The increased bacterial loads of S. aureus MW2 and its tet38 mutant in the abscess after 24 h were similar. Tetracycline produced significant decreases of both MW2 and the tet38 mutant compared with control. Although the tetracycline MICs for MW2 and the tet38 mutant did not differ in vitro, the antibacterial effect of tetracycline was significantly 2-fold greater in the tet38 mutant compared with the MW2 parental strain in vivo with a decrease of 0.67 ± 0.21 and 0.35 ± 0.19 log10 cfu/abscess, respectively (P < 0.05). The increased tetracycline activity in the tet38 mutant was complemented by plasmid-encoded tet38.
Conclusions
This study demonstrated that selective increased expression of tet38 in an abscess can affect tetracycline efficacy against S. aureus in vivo, highlighting an effect of native efflux pumps on response to therapy not reflected by testing in vitro.
Introduction
Staphylococcus aureus is an important human pathogen that commonly causes skin and soft-tissue infections with subcutaneous abscess formation.1 Tetracyclines have been used as empirical antibiotic therapies for S. aureus skin infections, including those due to strains resistant to methicillin,2 but resistance to tetracyclines has emerged.3 Resistance to tetracyclines is often associated with plasmid-mediated genes encoding active efflux pumps or proteins that protect ribosomes from drug action.4 Tet38, in contrast, is a chromosomally encoded native efflux pump in S. aureus that when overexpressed from a plasmid confers a 32-fold increase and 4–8-fold increase in MICs of tetracycline and antibacterial fatty acids, respectively.5,6 Expression of tet38 is up-regulated in sites of infection, such as murine abscesses and endocardial vegetations,7,8 and thus may have greater effects in vivo than under laboratory conditions with limited expression. Although the physiological roles of Tet38 overexpression, independent of its protection from tetracycline action, may relate to its ability to confer resistance to antibacterial fatty acids, the trigger for overexpression in the abscess environment has not been fully evaluated. Various efflux pumps have been well characterized in S. aureus, few studies have examined the effect of these efflux pumps on response to therapy in infection models.9,10 The association of upregulated expression of efflux pumps with clinically significant antibiotic resistance has been well established in Gram-negative organisms.11,12 These transporters can act synergistically with the permeability barrier of the outer membrane, which enhances multiple drug resistances and can result in antibiotic treatment failure.13 Examples of the well-studied efflux systems include Escherichia coli AcrAB–TolC14 and Pseudomonas aeruginosa MexAB–OprM and related systems.15,16 Because Gram-positive pathogens lack the outer membrane of Gram-negative bacteria that can enhance the effect of efflux pumps, we assessed whether the chromosomally encoded Tet38 efflux pump, which is selectively overexpressed in vivo could affect the response to antibiotic treatment of S. aureus infection. In the present study, we assessed the in vivo activity of tetracycline against MRSA strain MW2 and an otherwise isogenic tet38 mutant in a murine subcutaneous abscess model, which represents a common site of infection with S. aureus.
Materials and methods
Strains, plasmids and primers
The bacterial strains, plasmids and primers used in this study are listed in Table 1. The MW2 tet38 mutant was generated in previous study.7 To construct tet38-complemented strains, the tet38 gene including its promoter region was amplified by PCR, then cloned into the pMSP3535 vector. Plasmids pMSP3535 and pMSP3535::tet38 (pTet38) were transformed into S. aureus RN4220 and then into MW2 and the MW2 tet38 mutant following selection with 10 mg/L erythromycin. The presence of tet38 was confirmed by PCR and DNA sequencing.
Table 1.
Bacterial strains, plasmids and primers used in this study
| Genotype, characteristic(s) or sequences | Reference or source | |
|---|---|---|
| S. aureus strains | ||
| RN4220 | restriction-deficient mutagenized RN450 | 7 |
| MW2 | CA-MRSA (USA 400 lineage) | 7 |
| MW2 tet38 | tet38 partial deletion mutant with cat gene insertion | 7 |
| MW2 (pMSP) | MW2 carrying empty plasmid vector pMSP3535 | this study |
| MW2 tet38 (pMSP) | MW2 tet38 carrying empty plasmid vector pMSP3535 | this study |
| MW2 tet38 (pTet38) | tet38-complemented strain | this study |
| E. coli strains | ||
| E. coli DH5a | general host for pMSP3535 vector | Invitrogen |
| Plasmids | ||
| pMSP3535 | Gram-positive bacterial shuttle vector, ERYRa | 20 |
| pMSP::tet38 (pTet38) | pMSP3535 with tet38 gene, ERYR | this study |
| PCR primers (5′–3′) | ||
| tet38 XhoI-Forb | GCTACTCGAGTGGATGCGTATGGGTATTTTAG | this study |
| tet38 BamHI-Rev | GCTAGGATCCTTATTTTTCAGATTGTGTCCAACG | this study |
| pMSP-For | AATGCAGGTTAACCTGGCTTATC | this study |
| pMSP-Rev | TGCATCACCACGCATTACAA | this study |
| qRT–PCR primers (5′–3′) | ||
| gmk-RT For | ACTAGGGATGCGTTTGAAGC | 7 |
| gmk-RT Rev | TCATGACCTTCGTCCATTGT | 7 |
| tet38-RT For | TGACAGGTGTGGCTATTGGT | this study |
| tet38-RT Rev | TTGCCTGGGAAATTTAATGC | this study |
ERY, erythromycin. Plasmid selection: 300 mg/L erythromycin for E. coli and 10 mg/L erythromycin for S. aureus.
Restriction sites are indicated by underlining.
Ethics
All animal studies were approved by Institutional Animal Care and Use Committees, Office of Laboratory Animal Welfare and followed the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care.
Mouse abscess model and tetracycline treatment in vivo
Swiss Webster male mice aged 6–8 weeks (weight 25–30 g, Charles River Laboratories Wilmington, MA, USA) were housed in biosafety level 2 facilities located at Massachusetts General Hospital (Boston, MA, USA). The mouse subcutaneous abscesses model was as previously described.6 Briefly, S. aureus strains MW2 and its isogenic tet38 mutant (∼1 × 106 cfu in a volume of 0.2 mL) were injected subcutaneously on the opposite flanks of each mouse after anaesthesia. Infected mice were randomly allocated to the tetracycline treatment group or PBS control group (nine mice per group). Tetracycline (10 mg/kg) or PBS was injected intraperitoneally immediately and 12 h after infection. Twenty-four hours after infection (12 h after the last treatment), mice were euthanized and the abscesses were excised and homogenized. The viable bacterial burden was determined by counting cfu. The relative fold change (log10 cfu/abscess) was measured by comparing the viable bacteria recovered from the abscesses with the initial inoculum. All the experiments were performed at least three times. Statistical differences were analysed using the non-parametric Wilcoxon signed-rank test and P < 0.05 was considered significant.
RNA isolation and real-time quantitative RT–PCR
Total S. aureus RNA was isolated using the Qiagen RNeasy mini kit following the manufacturer’s instructions. tet38 expression was measured by quantitative RT–PCR (qRT–PCR) with the housekeeping gene gmk as internal control.7 The primers are listed in Table 1. All the experiments were performed in triplicate, with three independent biological samples. The difference in relative expression was analysed using the Mann–Whitney U-test and P < 0.05 was considered significant.
Results and discussion
Tet38 selectively reduces tetracycline activity in vivo
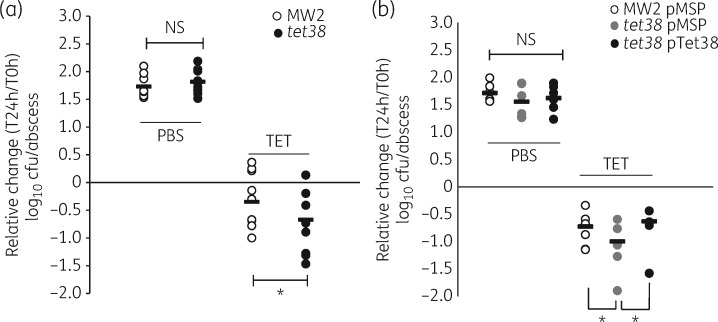
The MICs of tetracycline in vitro did not differ between MW2 and its tet38 mutant (0.25 mg/L). Because we had also shown previously that tet38 was selectively overexpressed in an abscess environment,7 we sought to evaluate whether tet38 has an effect on response to tetracycline in vivo using an abscess model. The viable bacteria recovered from either MW2- or MW2 tet38-infected abscesses in the PBS group after 24 h were similar, with 1.73 ± 0.07 and 1.82 ± 0.07 log10 cfu/abscess increase compared with the inoculum, respectively (P = 0.192) (Figure 1a). Tetracycline at 10 mg/kg had in vivo activity in mice infected with MW2 and those infected with the tet38 mutant compared with the PBS-treated mice. Importantly, the recovered bacterial load of the tet38 mutant was ∼2-fold less than that of the MW2 parental strain (with decrease of 0.35 ± 0.19 versus 0.67 ± 0.21 log10 cfu/abscess, P = 0.028) (Figure 1a). The tet38 mutant also demonstrated a bacterial load 2-fold lower than that of MW2 when treated with tetracycline at 5 mg/kg, but the difference did not reach statistical significance (P = 0.066, data not shown). Thus, the tet38 mutant was more sensitive to tetracycline treatment in vivo, indicating that the presence of Tet38 improved the survival of S. aureus in the abscesses of mice treated with tetracycline, an effect that is not apparent with in vitro testing.
Figure 1.
Tetracycline effect on MW2, the tet38 mutant and tet38-complemented strains in a mouse subcutaneous abscess model. (a) Relative change in log10 cfu/abscess of MW2 and the tet38 mutant in the control group (PBS) and the TET. (b) Relative change in log10 cfu/abscess of tet38-complemented strains in the control group (PBS) and the TET. Mice were infected with ∼6 log10 cfu of bacteria in each abscess; tetracycline at 10 mg/kg or PBS was administered intraperitoneally every 12 h. Each circle represents the relative change in bacterial load in each abscess (log10 cfu/abscess) at 24 h after infection compared with the initial inoculum (T = 0 h). Horizontal bars represent the means of each group. NS, not significant (P > 0.05). *P < 0.05. TET, tetracycline-treated group.
To validate further that the decreased activity of tetracycline against the tet38 mutant was due to the disruption of tet38, we compared tetracycline in vivo activity against a tet38-complemented strain (tet38 pTet38) with MW2 and the tet38 mutant carrying the empty vector. The expression of tet38 in tet38 pTet38 was stable with a 6.3 ± 1.5-fold increase compared with tet38 pMSP3535 as evaluated by qRT–PCR (P < 0.05). The plasmids were stable in abscesses as determined by plating the strains recovered from the abscesses on tryptone soya agar plates containing erythromycin. Twenty-four hours after infection, the recovered viable bacteria of MW2 pMSP, tet38 pMSP and tet38 pTet38 from abscesses in PBS-treated mice were similar with an increase of 1.72 ± 0.07, 1.56 ± 0.12 and 1.62 ± 0.1 log10 cfu/abscess, respectively (P > 0.05) (Figure 1b). In tetracycline-treated mice, tet38 pMSP exhibited a higher reduction in bacterial burden in abscesses (1 ± 0.16 log10 cfu/abscess) than WT MW2 pMSP (0.72 ± 0.13 log10 cfu/abscess, P < 0.05) and tet38-complemented strain tet38 pTet38 (0.63 ± 0.16 log10 cfu/abscess, P < 0.05) (Figure 1b), further validating the effect of Tet38 on the response to tetracycline in vivo.
It has been shown that expression levels of efflux pumps influenced antibiotic efficacy against infections caused by Gram-negative pathogens.14–16 The possible clinical relevance of pump-related drug resistance in S. aureus is supported by observations revealing augmented expression of various pumps such as NorA and NorB at sites of infection17 and carriage of plasmid-borne efflux pumps QacA/B.18 There have, however, been no data assessing the direct association of chromosomally encoded efflux pump overexpression with treatment response in vivo in a Gram-positive pathogen that lacks the outer membrane barrier that can enhance the effect of efflux pumps. This study demonstrated that the chromosomally encoded Tet38 efflux pump, which is selectively upregulated in the infection sites, reduces the tetracycline efficacy against S. aureus in subcutaneous abscesses, whereas no difference in MICs was observed in vitro. The findings support the concept that the selective expression of resistance genes in an abscess or other infection environment can affect response to therapy in a manner that may not be readily detected by routine susceptibility testing in vitro, reflecting a perhaps under-appreciated insidious effect of efflux pumps on response to therapy. As in all animal studies, the extrapolation of the results to responses to human infections is uncertain.
Triggers for tet38 upregulation in abscesses
Efflux pumps not only confer antibiotic resistance but also have physiological roles in response to the host environment.19 Our previous study showed that tet38 was selectively upregulated by 24-fold in the murine abscess model compared with in vitro.7 To look for those conditions present in the abscess environment that may be signals for tet38 overexpression, we evaluated tet38 expression by exposure in vitro to a variety of signals mimicking those in an abscess environment, including starvation (growth in PBS), reduced oxygen tension, acid stress (pH 5.5) and free iron restriction. None of these conditions, however, induced the up-regulation of tet38 transcription (data not shown). We have previously showed that tet38 expression can be induced by certain fatty acids, which are also present in abscesses.6 Therefore, the upregulation of tet38 in abscesses may relate to its exposure to antibacterial fatty acids or yet undefined host factors that exist in the abscess environment. The additional effects of efflux pumps with natural substrates on bacterial fitness in vivo further highlight their importance as a challenge to antimicrobial therapy.
Acknowledgments
Funding
This work was supported in part by a grant to D. C. H. from the National Institutes of Health, United States Public Health Service, R01-AI057576.
Transparency declarations
None to declare.
References
- 1. Moran GJ, Krishnadasan A, Gorwitz RJ. et al. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355: 666–74. [DOI] [PubMed] [Google Scholar]
- 2. Singer AJ, Talan DA.. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med 2014; 370: 1039–47. [DOI] [PubMed] [Google Scholar]
- 3. Han LL, McDougal LK, Gorwitz RJ. et al. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J Clin Microbiol 2007; 45: 1350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med 2016; 6: a025387.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Truong-Bolduc QC, Dunman PM, Strahilevitz J. et al. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J Bacteriol 2005; 187: 2395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Truong-Bolduc QC, Villet RA, Estabrooks ZA. et al. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. J Infect Dis 2014; 209: 1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Y, Onodera Y, Lee JC. et al. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol 2008; 190: 7123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanses F, Roux C, Dunman PM. et al. Staphylococcus aureus gene expression in a rat model of infective endocarditis. Genome Med 2014; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schindler BD, Kaatz GW.. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist Updat 2016; 27: 1–13. [DOI] [PubMed] [Google Scholar]
- 10. Jang S. Multidrug efflux pumps in Staphylococcus aureus and their clinical implications. J Microbiol 2016; 54: 1–8. [DOI] [PubMed] [Google Scholar]
- 11. Hernando-Amado S, Blanco P, Alcalde-Rico M. et al. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist Updat 2016; 28: 13–27. [DOI] [PubMed] [Google Scholar]
- 12. Li XZ, Plésiat P, Nikaido H.. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev 2015; 28: 337–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masi M, Réfregiers M, Pos KM. et al. Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat Microbiol 2017; 2: 17001.. [DOI] [PubMed] [Google Scholar]
- 14. Swick MC, Morgan-Linnell SK, Carlson KM. et al. Expression of multidrug efflux pump genes acrAB-tolC, mdfA, and norE in Escherichia coli clinical isolates as a function of fluoroquinolone and multidrug resistance. Antimicrob Agents Chemother 2011; 55: 921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adamson DH, Krikstopaityte V, Coote PJ.. Enhanced efficacy of putative efflux pump inhibitor/antibiotic combination treatments versus MDR strains of Pseudomonas aeruginosa in a Galleria mellonella in vivo infection model. J Antimicrob Chemother 2015; 70: 2271.. [DOI] [PubMed] [Google Scholar]
- 16. Lister P, Wolter D, Hanson N.. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22: 582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosmidis C, Schindler BD, Jacinto PL. et al. Expression of multidrug resistance efflux pump genes in clinical and environmental isolates of Staphylococcus aureus. Int J Antimicrob Agents 2012; 40: 204–9. [DOI] [PubMed] [Google Scholar]
- 18. Wassenaar TM, Ussery D, Nielsen LN. et al. Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species. Eur J Microbiol Immunol 2015; 5: 44–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blanco P, Hernando-Amado S, Reales-Calderon JA. et al. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms 2016; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryan EM, Bae T, Kleerebezem M. et al. Improved vectors for nisin-controlled expression in Gram-positive bacteria. Plasmid 2000; 44: 183–90. [DOI] [PubMed] [Google Scholar]